A higher aneurysmal subarachnoid hemorrhage incidence in women prior to menopause: a retrospective analysis of 4,895 cases from eight hospitals in China
Introduction
Subarachnoid hemorrhage (SAH) from a ruptured cerebral aneurysm is devastating. Its overall incidence is approximately 9 per 100,000 person-years (1-3). Incidences are higher in Japan and Finland, indicating a partial genetic role (2). Current medical management options in patients with unruptured cerebral aneurysms are limited, consisting largely of smoking cessation, blood pressure control, and neurosurgical or endovascular interventions (3). Decisions regarding optimum of unruptured cerebral aneurysm management are made on the basis of careful comparison of the short-term and long-term risks of aneurysmal rupture with the risk associated with the intervention, whether it would be surgical clipping or endovascular management. Controversy remains regarding optimum management, and thorough assessments of the risks and benefits of contemporary management options, specific to aneurysm size, location, and many other aneurysm and patient factors, are needed. Endovascular management options continue to improve as micro-catheter technology advances and newer devices and embolic materials are developed. The most commonly used endovascular option is the Guglielmi detachable coil system. This system allows delivery of soft platinum coils into intracranial aneurysms. Overall, clinical trial data showed better recovery data with patients treated endovascularly than treated surgically (3). Findings from several studies also suggested that cerebral aneurysm rupture risk is reduced in patients taking aspirin (4). Recent data support long-term serial screening in individuals with a family history of aneurysmal SAH (5).
Despite the risk factors, including hypertension, cigarette smoking and alcohol use, are more common in men, aneurysmal SAH belongs to a few diseases which the incidence is higher in women than in men (1-3,6,7). Women have a significantly higher risk for de novo cerebral aneurysm formation than men in a long-term follow-up study, being female is a significant independent risk factor for aneurysm growth; and women are more likely than men to have multiple cerebral aneurysms (7-9). It has been noted that the incidence was higher in younger men, whereas after the age of 55, the incidence was higher in women (2). Furthermore, earlier age at menopause may be associated with a greater risk of cerebral aneurysm (10). Therefore, a sex-specific hormonal factor may play a role in the pathogenesis of aneurysm formation and rupture.
Sex hormones, especially estrogen, might be protective against cerebral aneurysm rupture (11). Men are at less risk because they do not experience a dramatic estrogen withdrawal as women do. Among women still menstruating, it was suggested the risk for hemorrhage was greatest in the perimenstrual period (11). Clinical trials show hormone replacement therapy (HRT) seems to be associated with a reduced risk for aneurysmal SAH (12,13). Experimental study also demonstrated a sex-specific hormonal factor may play a role in the pathogenesis of aneurysm formation and rupture (14,15). Because it is a relatively common cause of stroke in women under age 65 and because of its high morbidity and mortality, the excess of aneurysmal SAH in women remains a pressing question in stroke research.
The average menopause age in Chinese women has been confirmed to be around 49 years (16,17). A systematic review by de Rooij et al. suggested that at younger ages the aneurysmal SAH incidence is higher in men, whereas, after the age of 55, the incidence is higher in women. Recently it was suggested that, while disc degeneration is more common in young men than young women, it is more advanced in elderly women than in elderly men, and women start to show more severe lumbar disc degeneration in their fifties with relatively clear onset time point (18-20). We aimed to see whether such a time point can be detected for aneurysmal SAH, and we hypothesized women’s aneurysmal SAH incidence will be higher after middle fifties. The answer to this question may provide some clues about the pathophysiology or even prevention of this deadly condition.
Materials and methods
Aneurysmal SAH cases were collected retrospectively from the archives of eight medical centers in Mainland China, including (I) Nanjing University Medical School Nanjing Drum Tower Hospital, Nanjing (female: 994, male: 684); (II) The Second Hospital of Hebei Medical University (female: 936, male: 561); (III) The Affiliated Hospital of Zunyi Medical University (female: 233, male: 143); (IV) The First Affiliated Hospital of Nanchang University (female: 335, male: 182); (V) North Sichuan Medical College Hospital (female: 359, male: 179); (VI) The First Affiliated Hospital of Xi’an Jiao Tong University (female: 29, male: 19); (VII) The General Hospital of Guangzhou Military Command (female: 50, male: 48); (VIII) Capital Medical University Beijing Friendship Hospital (female: 143, male: 63). All the cases collected were from September 2015 and backward consecutively for a period of time up to 7 years. SAH was initially diagnosed by brain computed tomography, and digital subtraction angiography (DSA) was followed and SAH was confirmed to be due to cerebral aneurysm, and the cases were restricted to first-ever SAH.
Results
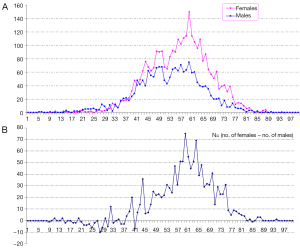
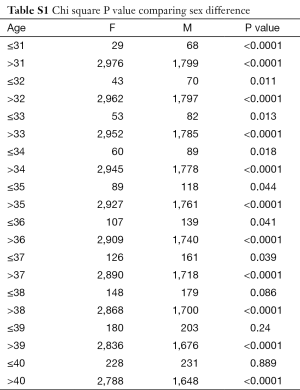
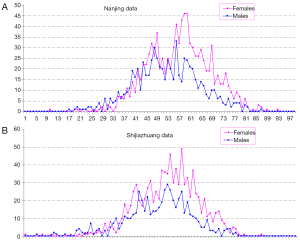
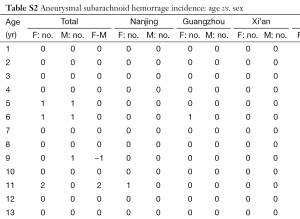
In total there were 4,895 cases, with 3,016 females and 1,879 males, and their age distribution is shown in Figure 1. Stepwise Chi square test showed there were significantly more male cases of aneurysmal SAH before 37-year-old (Table S1). Women started to have a higher incidence of aneurysmal SAH than men after late thirties. In addition, the higher incident of aneurysmal SAH in women after their later sixties might be due by a small portion to that that women had longer life expectancy than men (20). Our further subgroup analysis according to different centers did not differ from this observation (Figure 2, and Table S2).
Full table
Full table
Discussion
Estrogen has a multitude of biological effects that may account for its cardiovascular benefits, including favorable effects on the lipid profile, antioxidant activity, enhanced fibrinolysis (21). Estrogens have been shown to enhance endothelial-dependent relaxation in arterial rings from different vascular beds, including cerebral arteries. Estrogen replacement treatment increases coronary flow and decreases both coronary resistance and peripheral vascular tone (21). Local delivery of 17ß-estradiol during percutaneous transluminal coronary angioplasty improved endothelial function, enhanced re-endothelialization and endothelial NOS expression and decreased neointima formation (21). Estrogen deficiency in postmenopausal women may have a significant impact on the pathophysiology of cerebral aneurysm and SAH. The arteries could undergo postmenopausal connective tissue changes (22). The collagen wasting commonly observed in bone and skin in the postmenopausal period due to decreased estrogen levels could possibly be responsible for the formation of cerebral aneurysms (23-26). Estrogen has been found to improve lipid profiles and thus may reduce the risk for arteriosclerosis, which has been considered a risk factor for aneurysm formation (27,28). Low-dose estrogens are also associated with a reduced blood pressure (29).
Pathology-based therapies for patients harboring unruptured aneurysms may be a reasonable starting point for a disease with otherwise few treatment options. Estrogen might play an important role in vascular and aneurysmal integrity through the control of collagen content and wall thickness (29). Kadasi et al. reported that the proportion of super-thin translucent tissue at the aneurysm dome was significantly greater in women compared with men, suggestive of the susceptibility for rupture (30). The high quality prospective clinical trials by Longstreth et al. and Mhurchu et al. demonstrated HRT seems to be associated with a reduced risk for aneurysmal SAH (12,13).
While initial clinical studies showed HRT is associated with beneficial cardiovascular effects in postmenopausal women (31,32). The Women’s Health Initiative (WHI) hormone trials shows oral estrogens increase the risk of venous thromboembolism among postmenopausal women and increase the risk of breast cancer (33,34). However, the expert views of HRT evolved during the last 10 years since the publication of WHI trials (35). Dose regimen, combination of estrogen with progestins versus estrogen alone, the administration route and duration of treatment such as the choice of repetitive or periodic administration simulating the menstrual cycle are some of the factors that may be involved in the benefit discrepancies. The Estrogen and Thromboembolism Risk (ESTHER) study confirmed that oral estrogens increased venous thromboembolism risk, whereas transdermal estrogens had little or no impact on the development of thrombosis (36). Recent Korean data do not support HRT history for the risk of breast cancer in women (37). It has also been suggested that the presence of gene polymorphisms has also been implicated. If this is the case, estrogen replacement therapy may be useful to prevent cardiovascular disease in a large number of postmenopausal women, but not in a subset of women who are at high risk for cardiovascular and thrombotic complications (21,38). A number of selective estrogen receptor modulators (SERMs) have also been developed. SERMs could be selective in targeting vascular estrogen receptors while having few undesirable effects, such as cardiovascular disease, and breast cancer. It is conceivable that one could design SERMs that would retain the desired effects of estrogen to targeted tissues but devoid of the undesirable effects (39). In one study SERM attenuated the development of experimental aneurysms (40). Phytoestrogens are estrogenic compounds of plant origin classified into different groups with structural similarities to estrogen that allow them to mimic the effects of estrogen, but less likely have harmful effects (11,35).
There are a number of limitations of our study. This is a retrospective analysis of archived data. There were heterogeneities in different hospital, and the severity of the aneurysmal SAH of individual cases could not be pooled for comparison analysis. On the other hand, the relative large number of cases would likely eliminate potential selection biases.
There is growing interest in the pathogenesis of cerebral aneurysm focused on the development of drug therapies to decrease the incidence of aneurysm growth and rupture, particularly for screening detected female subjects. While our study further suggest that the pathology, etiology, and essential effector of cerebral aneurysm formation and progression are different in men and women and may also differ by age.
Our results disputed our initial hypothesis. Menopause and low estrogen relevant may be relevant in post-menopausal women, but may not be the only dominant factor causing higher incidence of aneurysmal SAH in women. Other possible pathophysiological causes should be further actively explored. Our results may have practical implications for the on-going efforts to development of using estrogen, either alone or in combination with progestogen, to reduce the risk of screening detected cerebral aneurysms from rupture (11-14).
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- van Gijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. Lancet 2007;369:306-18. [Crossref] [PubMed]
- de Rooij NK, Linn FH, van der Plas JA, Algra A, Rinkel GJ. Incidence of subarachnoid haemorrhage: a systematic review with emphasis on region, age, gender and time trends. J Neurol Neurosurg Psychiatry 2007;78:1365-72. [Crossref] [PubMed]
- Brown RD Jr, Broderick JP. Unruptured intracranial aneurysms: epidemiology, natural history, management options, and familial screening. Lancet Neurol 2014;13:393-404. [Crossref] [PubMed]
- Hasan DM, Mahaney KB, Brown RD Jr, Meissner I, Piepgras DG, Huston J, Capuano AW, Torner JC; International Study of Unruptured Intracranial Aneurysms Investigators. Aspirin as a promising agent for decreasing incidence of cerebral aneurysm rupture. Stroke 2011;42:3156-62. [Crossref] [PubMed]
- Bor AS, Rinkel GJ, van Norden J, Wermer MJ. Long-term, serial screening for intracranial aneurysms in individuals with a family history of aneurysmal subarachnoid haemorrhage: a cohort study. Lancet Neurol 2014;13:385-92. [Crossref] [PubMed]
- Zhao L, Zhang L, Zhang X, Li Z, Tian L, Wang YX. An analysis of 1256 cases of sporadic ruptured cerebral aneurysm in a single Chinese institution. PLoS One 2014;9:e85668. [Crossref] [PubMed]
- Liu H, Zhang T, Jiao S, Li B, Guan J, Wang YX. Epidemiological investigation of 264 sporadic cases of ruptured cerebral aneurysm at a single institution in southwest China. Neuropsychiatr Dis Treat 2015;11:1609-14. [PubMed]
- Juvela S, Poussa K, Porras M. Factors affecting formation and growth of intracranial aneurysms: a long-term follow-up study. Stroke 2001;32:485-91. [Crossref] [PubMed]
- Ostergaard JR, Høg E. Incidence of multiple intracranial aneurysms. Influence of arterial hypertension and gender. J Neurosurg 1985;63:49-55. [Crossref] [PubMed]
- Ding C, Toll V, Ouyang B, Chen M. Younger age of menopause in women with cerebral aneurysms. J Neurointerv Surg 2013;5:327-31. [Crossref] [PubMed]
- Tabuchi S. Relationship between Postmenopausal Estrogen Deficiency and Aneurysmal Subarachnoid Hemorrhage. Behav Neurol 2015;2015:720141.
- Longstreth WT, Nelson LM, Koepsell TD, van Belle G. Subarachnoid hemorrhage and hormonal factors in women. A population-based case-control study. Ann Intern Med 1994;121:168-73. [Crossref] [PubMed]
- Mhurchu CN, Anderson C, Jamrozik K, Hankey G, Dunbabin D; Australasian Cooperative Research on Subarachnoid Hemorrhage Study (ACROSS) Group. Hormonal factors and risk of aneurysmal subarachnoid hemorrhage: an international population-based, case-control study. Stroke 2001;32:606-12. [Crossref] [PubMed]
- Tada Y, Makino H, Furukawa H, Shimada K, Wada K, Liang EI, Murakami S, Kudo M, Kung DK, Hasan DM, Kitazato KT, Nagahiro S, Lawton MT, Hashimoto T. Roles of estrogen in the formation of intracranial aneurysms in ovariectomized female mice. Neurosurgery 2014;75:690-5; discussion 695. [Crossref] [PubMed]
- Tada Y, Wada K, Shimada K, Makino H, Liang EI, Murakami S, Kudo M, Shikata F, Pena Silva RA, Kitazato KT, Hasan DM, Kanematsu Y, Nagahiro S, Hashimoto T. Estrogen protects against intracranial aneurysm rupture in ovariectomized mice. Hypertension 2014;63:1339-44. [Crossref] [PubMed]
- Chow SN, Huang CC, Lee YT. Demographic characteristics and medical aspects of menopausal women in Taiwan. J Formos Med Assoc 1997;96:806-11. [PubMed]
- Yang D, Haines CJ, Pan P, Zhang Q, Sun Y, Hong S, Tian F, Bai B, Peng X, Chen W, Yang X, Chen Y, Feng H, Zhao S, Lei H, Jiang Z, Ma X, Liao W. Menopausal symptoms in mid-life women in southern China. Climacteric 2008;11:329-36. [Crossref] [PubMed]
- Wang YX, Griffith JF. Effect of menopause on lumbar disk degeneration: potential etiology. Radiology 2010;257:318-20. [Crossref] [PubMed]
- Wáng YX. Postmenopausal Chinese women show accelerated lumbar disc degeneration compared with Chinese men. J Orthop Transl 2015;3:205-11. [Crossref]
- Wáng YX. Advance modern medicine with clinical case reports. Quant Imaging Med Surg 2014;4:439-43. [PubMed]
- Tostes RC, Nigro D, Fortes ZB, Carvalho MH. Effects of estrogen on the vascular system. Braz J Med Biol Res 2003;36:1143-58. [Crossref] [PubMed]
- Calleja-Agius J, Brincat M. The effect of menopause on the skin and other connective tissues. Gynecol Endocrinol 2012;28:273-7. [Crossref] [PubMed]
- Stober T, Sen S, Anstätt T, Freier G, Schimrigk K. Direct evidence of hypertension and the possible role of post-menopause oestrogen deficiency in the pathogenesis of berry aneurysms. J Neurol 1985;232:67-72. [Crossref] [PubMed]
- Brincat M, Moniz CF, Studd JW, Darby AJ, Magos A, Cooper D. Sex hormones and skin collagen content in postmenopausal women. Br Med J (Clin Res Ed) 1983;287:1337-8. [Crossref] [PubMed]
- Bolognia JL, Braverman IM, Rousseau ME, Sarrel PM. Skin changes in menopause. Maturitas 1989;11:295-304. [Crossref] [PubMed]
- Robinson JD, Judd HL, Young PE, Jones OW, Yen SS. Amniotic fluid androgens and estrogens in midgestation. J Clin Endocrinol Metab 1977;45:755-61. [Crossref] [PubMed]
- Nabulsi AA, Folsom AR, White A, Patsch W, Heiss G, Wu KK, Szklo M. Association of hormone-replacement therapy with various cardiovascular risk factors in postmenopausal women. The Atherosclerosis Risk in Communities Study Investigators. N Engl J Med 1993;328:1069-75. [Crossref] [PubMed]
- Lind T, Cameron EC, Hunter WM, Leon C, Moran PF, Oxley A, Gerrard J, Lind UC. A prospective, controlled trial of six forms of hormone replacement therapy given to postmenopausal women. Br J Obstet Gynaecol 1979;86 Suppl 3:1-29. [Crossref] [PubMed]
- Baron YM, Galea R, Brincat M. Carotid artery wall changes in estrogen-treated and -untreated postmenopausal women. Obstet Gynecol 1998;91:982-6. [PubMed]
- Kadasi LM, Dent WC, Malek AM. Cerebral aneurysm wall thickness analysis using intraoperative microscopy: effect of size and gender on thin translucent regions. J Neurointerv Surg 2013;5:201-6. [Crossref] [PubMed]
- Barrett-Connor E, Bush TL. Estrogen and coronary heart disease in women. JAMA 1991;265:1861-7. [Crossref] [PubMed]
- Kannel WB, Hjortland MC, McNamara PM, Gordon T. Menopause and risk of cardiovascular disease: the Framingham study. Ann Intern Med 1976;85:447-52. [Crossref] [PubMed]
- Rossouw JE. Effect of postmenopausal hormone therapy on cardiovascular risk. J Hypertens Suppl 2002;20:S62-5. [PubMed]
- Gadducci A, Biglia N, Cosio S, Sismondi P, Genazzani AR. Progestagen component in combined hormone replacement therapy in postmenopausal women and breast cancer risk: a debated clinical issue. Gynecol Endocrinol 2009;25:807-15. [Crossref] [PubMed]
- Ghazal S, Pal L. Perspective on hormone therapy 10 years after the WHI. Maturitas 2013;76:208-12. [Crossref] [PubMed]
- Scarabin PY, Oger E, Plu-Bureau G. EStrogen and THromboEmbolism Risk Study Group. Differential association of oral and transdermal oestrogen-replacement therapy with venous thromboembolism risk. Lancet 2003;362:428-32. [Crossref] [PubMed]
- Bae JM, Kim EH. Hormone Replacement Therapy and Risk of Breast Cancer in Korean Women: A Quantitative Systematic Review. J Prev Med Public Health 2015;48:225-30. [Crossref] [PubMed]
- Herrington DM, Klein KP. Invited review: Pharmacogenetics of estrogen replacement therapy. J Appl Physiol (1985) 2001;91:2776-84. [PubMed]
- Mirkin S, Pickar JH. Selective estrogen receptor modulators (SERMs): a review of clinical data. Maturitas 2015;80:52-7. [Crossref] [PubMed]
- Grigoryants V, Hannawa KK, Pearce CG, Sinha I, Roelofs KJ, Ailawadi G, Deatrick KB, Woodrum DT, Cho BS, Henke PK, Stanley JC, Eagleton MJ, Upchurch GR. Tamoxifen up-regulates catalase production, inhibits vessel wall neutrophil infiltration, and attenuates development of experimental abdominal aortic aneurysms. J Vasc Surg 2005;41:108-14. [Crossref] [PubMed]


