Quantitative volumetric assessment of pulmonary involvement in patients with systemic sclerosis
Introduction
Systemic sclerosis (SSc) is a chronic progressive autoimmune disease that may involve the pulmonary, gastrointestinal, cardiac, musculoskeletal, and renal systems, in addition to skin lesions. In SSc patients, lung involvement is high, with previously reported rates of 50–92% (1-3). In such patients, pulmonary hypertension (PH) results in fibrotic lung parenchyma, often with concomitant cardiac involvement, which shortens the lifespan (4-8). SSc has a 50% mortality rate and cardiac and pulmonary complications are the main causes of death (7,9). In SSc patients, lung involvement is prominent in the basal segments, primarily in the form of reticular honeycomb areas and ground-glass densities accompanied by interstitial lung disease (ILD) (3). In addition, chronic obstructive pulmonary disease symptoms, such as emphysema and pulmonary nodules, may be observed (10-12).
To assess the severity of lung involvement, high-resolution computed tomography (HRCT), pulmonary function tests (PFT) and diffusing capacity of the lung for carbon monoxide (DLCO) tests are used (13-15). However, DLCO and spirometric measures may not be conclusive in the early stages of SSC. In HRCT measurements, fibrosis scales are used to determine the severity of lung involvement (3,16). However, these methods are subjective and do not provide quantitative results for determining severity.
Radiological quantitative parameters are used to evaluate the severity of ILD in SSc patients. Quantitative studies assessing ILD in SSc patients have been performed previously (13,17). However, to the best of our knowledge, studies of volume reduction in the lower lobes of patients with SSc have not been performed. Because lung involvement in SSc patients is more severe in basal segments, we aimed to show quantitatively that volume loss is a result of fibrosis in the lower lobe.
Materials and methods
Patients
The records of 45 patients diagnosed with SSc, who presented to our clinic between July 2008 and June 2014, were reviewed retrospectively. The study was approved by the Ethics Committee of our institution. The patients were diagnosed with SSc and evaluated according to the criteria of the American Rheumatism Association (18). All patients satisfied the 2013 American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) criteria skin thickening of the fingers of both hands extending proximal to the metacarpophalangeal joints (sufficient criterion) (weighted 9), skin thickening of the fingers, (only count the higher score); puffy fingers (weighted 2), sclerodactyly of the fingers (weighted 4), fingertip lesions, (only count the higher score); digital tip ulcers (weighted 2) or fingertip pitting scars (weighted 3), telangiectasia (weighted 2), abnormal nail fold capillary pattern (weighted 2), pulmonary arterial hypertension and/or ILD; (maximum score is 2) pulmonary arterial hypertension (weighted 2) ILD (weighted 2), Raynaud’s phenomenon (weighted 3), SSc-related autoantibodies (maximum score is 3): anticentromere antibody, anti-topoisomerase I antibody (also known as anti-Scl-70 antibody), or anti-RNA polymerase III antibody. The maximum possible score is 19, and patients with a score of 9 or higher are classified as having SSc (19). HRCT examinations of patients diagnosed with SSc were evaluated. Seven cases were excluded from the study; two patients could not be evaluated due to motion artifacts, two had emphysema, which caused volume increase in the upper lobe lung, two had a previous tuberculosis infection, and one patient was previously exposed to asbestos, which caused volume loss in both lungs. In total, 38 patients were included in the study; 2 patients were male (5.3%) and 36 were female (94.7%). The HRCT scan was evaluated for GGO and ILD patern including reticular intra-interlobular thickening, honeycomb cysts, traction bronchiectasis, bronchiolectasis. HRCT scans the distribution of ILD was categorized as upper, middle, or lower zone predominant. Patients were divided into two groups according to the results of HRCT examination: there were 23 patients with ILD and 15 patients without ILD. The median age of patients with ILD (43 years) was not significantly different from that of patients without ILD (37 years; P=0.698). The demographic data and echocardiograms of all patients were obtained from the archives of our hospital (Table 1).

Full table
HRCT scanning protocol
All views were obtained with patients in the supine position using Toshiba Activion 16 equipment (Toshiba Medical Systems, Tokyo, Japan) with the following parameters: Kv, 120; mAs, 300; slice thickness, 5 mm; and detector collimation, 16×1 mm2. HRCT images were created using 1 mm reconstructions.
HRCT measurement
All HRCT measurements were performed by a thoracic radiology specialist with 5 years of experience (MG. Ç), using OsiriX 4.0 software (OsiriX Foundation, Geneva, Switzerland) with our picture archiving and communication system (PACS) on an IMac27 Mid 2011 (Apple Inc., Cupertino, CA, USA) workstation. All measurements were confirmed by a thoracic radiology specialist with 7 years of experience (Goya C). Measurements for each lobe were performed at 5 mm intervals, forming a complete series. On axial images, the parietal pleura (and fissure) were used as the border and measurements were then obtained (Figure 1). Lobe volume was calculated automatically by combining the areas in all sections using the OsiriX program. The following areas were measured in each patient: the right upper and middle lobes, lower lobe of the right lung, upper lobe of the left lung and lower lobe of the left lung. The percentage of lower lobe volume (PLLV) was calculated as follows:
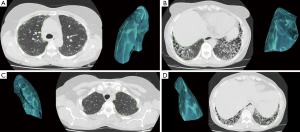
PLLV of the right lung = [lower lobe volume/(upper lobe + middle lobe + lower lobe volume)] ×100%
PLLV of the left lung = [lower lobe volume/(upper lobe + lower lobe volume)] ×100%
Total PLLV of the lung = [right +left lower lobe volume/(right lung + left lung volume)] ×100%
PFT and carbon monoxide (co) tests
All spirometric measurements were performed using a Vmax 22 Series spirometer (Viasys Healthcare, Yorba Linda, CA, USA) according to the American Thoracic Society (ATS) standards (20), and forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1) and the FEV1/FVC ratio were recorded. Predicted values of FVC, FEV1 and the FEV1/FVC ratio according to gender and height were used to determine respiratory function.
Each patient’s total lung function was measured using a body box plethysmograph (Vmax 22 Series, Viasys Healthcare) and ATS/European Respiratory Society (ERS) standard criteria. The lung volumes and predicted CO diffusing capacity of each patient were measured using the single breath wash-out technique and by adjusting blood hemoglobin values, respectively (21).
Statistics
Patient data, including the number of cases, were recorded using SPSS for Windows (ver. 18.0; SPSS Inc., Chicago, IL, USA) and Microsoft Excel software (Microsoft Corp., Redmond, WA, USA). Because the PLLVs of the two groups were not normally distributed, the Mann-Whitney U test was used. In all patients, correlations between the PLLV values of the left and right lungs were assessed using the Spearman correlation test. Spearman correlation analysis of the PLLV of the right lung, age, DLCO and PFT results of patients with ILD was performed. A P value <0.05 was considered to indicate statistical significance.
Results
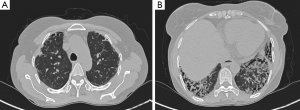
In SSc patients, lung involvement is prominent in the basal segments, primarily in the form of reticular honeycomb areas and ground-glass densities accompanied by ILD (Figure 2). Table 1 shows the characteristics of the study population, including the median values of the demographic data, PLLV rate, PFT results and DLCO measurements. ILD was seen in 23 patients, and GGO was seen in 3 patients. ILD distribution was seen lower lobe predominant in the all patients with ILD (Figure 2). The PLLV of the right lung was correlated with the PLLV of the left lung and total lung (Spearman correlation coefficient, 0.846; P<0.001 and 0.977; P<0.001, respectively). Based on HRCT, the media PLLV of patients with ILD (41.47%) was significantly reduced compared with patients without ILD (47.07%; P=0.041). A negative correlation between the PLLV of the lung and age was observed in the patients with ILD (Spearman correlation coefficient, −0.527; P=0.01). A positive correlation between the PLLV of the lung and FVC was also observed in the patients with ILD (Spearman correlation coefficient, 0.536; P=0.012). The FEV1/FVC ratio and predicted DLCO results did not demonstrate a significant correlation with the PLLV of the affected lung (Table 2).

Full table
Discussion
According to our results, the PLLV was decreased in SSc patients with ILD compared to those without ILD. Moreover, in patients with lung involvement, the PLLV rate decreased as age increased. Pulmonary fibrosis indicates irreversible damage in patients with SSc. In the literature, a decrease in lower lobe volume due to SSc is considered as a prognostic factor (16,22). A number of techniques have been developed to measure pulmonary fibrosis, including visual inspection, and Goh, Wells and Warrick scores, which are all semi-quantitative methods (16,23,24). The major limitation of these methods concerns inter-observer discrepancies. The PLLV can provide a simple, quantitative parameter for assessing pulmonary sclerosis in patients with SSc.
In our study, the PLLV of the lung and FVC were correlated. A decrease in FVC is expected in restrictive lung disease, and in our study was correlated with the PLLV of the lung. According to the literature, FVC values are decreased with fibrosis. FLV can be graded using pulmonary fibrosis scales in patients with SSc (3,25). A 70% reduction of FVC is considered as a critical limit. Patients with an FVC reduction of more than 70% have a decreased likelihood of survival (15,16). In our study, the PLLV of the lung was not correlated with the FEV1/FVC ratio; a lack of correlation between these parameters might be due to poor patient compliance with the FEV1/FVC ratio measurement test.
In this study, the predicted DLCO and PLLV values were not correlated. In the literature, differences in the degree of interstitial lung involvement and predicted DLCO results have been reported in patients with SSc. DLCO results that differ from predictions may be explained by the influence of numerous factors, including the prevention of obstructive disease in accordance with CO distribution, emphysema, chest deformity, obesity, PH, anemia-polycythemia and alveolar-capillary thickness (21,26,27). Although Goh and colleagues (16) found that DLCO values were associated with PH, in other studies pulmonary fibrosis and PH were found to be related (22,28).
The most important limitation of our study was we did not measure sclerosis in the upper and middle lobes. The small study population, small number of male participants, manual drawing of the lung border and long duration required for volume calculation (although automatic segmentation programs may also be used) are additional further limitations.
Conclusions
In summary, PLLV values were significantly lower in SSc patients with ILD than in those without ILD. PLLV may be a quantitative parameter indicating damage in the lung. We believe the method described herein can be used for future large-scale studies on this issue.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Hashimoto A, Endo H, Kondo H, Hirohata S. Clinical features of 405 Japanese patients with systemic sclerosis. Mod Rheumatol 2012;22:272-9. [PubMed]
- Steele R, Hudson M, Lo E, Baron M; Canadian Scleroderma Research Group. Clinical decision rule to predict the presence of interstitial lung disease in systemic sclerosis. Arthritis Care Res (Hoboken) 2012;64:519-24. [PubMed]
- Goldin JG, Lynch DA, Strollo DC, Suh RD, Schraufnagel DE, Clements PJ, Elashoff RM, Furst DE, Vasunilashorn S, McNitt-Gray MF, Brown MS, Roth MD, Tashkin DP; Scleroderma Lung Study Research Group. High-resolution CT scan findings in patients with symptomatic scleroderma-related interstitial lung disease. Chest 2008;134:358-67. [PubMed]
- Hassoun PM. Lung involvement in systemic sclerosis. Presse Med 2011;40:e3-e17. [PubMed]
- Hachulla E, Gressin V, Guillevin L, Carpentier P, Diot E, Sibilia J, Kahan A, Cabane J, Francès C, Launay D, Mouthon L, Allanore Y, Tiev KP, Clerson P, de Groote P, Humbert M. Early detection of pulmonary arterial hypertension in systemic sclerosis: a French nationwide prospective multicenter study. Arthritis Rheum 2005;52:3792-800. [PubMed]
- Wells AU. High-resolution computed tomography and scleroderma lung disease. Rheumatology (Oxford) 2008;47 Suppl 5:v59-61. [PubMed]
- Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972-2002. Ann Rheum Dis 2007;66:940-4. [PubMed]
- Hassoun PM. The right ventricle in scleroderma (2013 Grover Conference Series). Pulm Circ 2015;5:3-14. [PubMed]
- Rubio-Rivas M, Royo C, Simeón CP, Corbella X, Fonollosa V. Mortality and survival in systemic sclerosis: systematic review and meta-analysis. Semin Arthritis Rheum 2014;44:208-19. [PubMed]
- Solomon JJ, Olson AL, Fischer A, Bull T, Brown KK, Raghu G. Scleroderma lung disease. Eur Respir Rev 2013;22:6-19. [PubMed]
- Ferguson EC, Berkowitz EA, Lung CT. Part 2, The interstitial pneumonias--clinical, histologic, and CT manifestations. AJR Am J Roentgenol 2012;199:W464-76. [PubMed]
- Desai SR, Veeraraghavan S, Hansell DM, Nikolakopolou A, Goh NS, Nicholson AG, Colby TV, Denton CP, Black CM, du Bois RM, Wells AU. CT features of lung disease in patients with systemic sclerosis: comparison with idiopathic pulmonary fibrosis and nonspecific interstitial pneumonia. Radiology 2004;232:560-7. [PubMed]
- Kim HJ, Brown MS, Elashoff R, Li G, Gjertson DW, Lynch DA, Strollo DC, Kleerup E, Chong D, Shah SK, Ahmad S, Abtin F, Tashkin DP, Goldin JG. Quantitative texture-based assessment of one-year changes in fibrotic reticular patterns on HRCT in scleroderma lung disease treated with oral cyclophosphamide. Eur Radiol 2011;21:2455-65. [PubMed]
- Bussone G, Mouthon L. Interstitial lung disease in systemic sclerosis. Autoimmun Rev 2011;10:248-55. [PubMed]
- Hoffmann-Vold AM, Aaløkken TM, Lund MB, Garen T, Midtvedt Ø, Brunborg C, Gran JT, Molberg Ø. Predictive value of serial high-resolution computed tomography analyses and concurrent lung function tests in systemic sclerosis. Arthritis Rheumatol 2015;67:2205-12. [PubMed]
- Goh NS, Desai SR, Veeraraghavan S, Hansell DM, Copley SJ, Maher TM, Corte TJ, Sander CR, Ratoff J, Devaraj A, Bozovic G, Denton CP, Black CM, du Bois RM, Wells AU. Interstitial lung disease in systemic sclerosis: a simple staging system. Am J Respir Crit Care Med 2008;177:1248-54. [PubMed]
- Yabuuchi H, Matsuo Y, Tsukamoto H, Horiuchi T, Sunami S, Kamitani T, Jinnouchi M, Nagao M, Akashi K, Honda H. Evaluation of the extent of ground-glass opacity on high-resolution CT in patients with interstitial pneumonia associated with systemic sclerosis: comparison between quantitative and qualitative analysis. Clin Radiol 2014;69:758-64. [PubMed]
- Preliminary criteria for the classification of systemic sclerosis (scleroderma). Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum 1980;23:581-90. [PubMed]
- van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, Matucci-Cerinic M, Naden RP, Medsger TA Jr, Carreira PE, Riemekasten G, Clements PJ, Denton CP, Distler O, Allanore Y, Furst DE, Gabrielli A, Mayes MD, van Laar JM, Seibold JR, Czirjak L, Steen VD, Inanc M, Kowal-Bielecka O, Müller-Ladner U, Valentini G, Veale DJ, Vonk MC, Walker UA, Chung L, Collier DH, Csuka ME, Fessler BJ, Guiducci S, Herrick A, Hsu VM, Jimenez S, Kahaleh B, Merkel PA, Sierakowski S, Silver RM, Simms RW, Varga J, Pope JE. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum 2013;65:2737-47. [PubMed]
- Standardization of spirometry--1987 update. Statement of the American Thoracic Society. Am Rev Respir Dis 1987;136:1285-98. [PubMed]
- Macintyre N, Crapo RO, Viegi G, Johnson DC, van der Grinten CP, Brusasco V, Burgos F, Casaburi R, Coates A, Enright P, Gustafsson P, Hankinson J, Jensen R, McKay R, Miller MR, Navajas D, Pedersen OF, Pellegrino R, Wanger J. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J 2005;26:720-35. [PubMed]
- Goldin J, Elashoff R, Kim HJ, Yan X, Lynch D, Strollo D, Roth MD, Clements P, Furst DE, Khanna D, Vasunilashorn S, Li G, Tashkin DP. Treatment of scleroderma-interstitial lung disease with cyclophosphamide is associated with less progressive fibrosis on serial thoracic high-resolution CT scan than placebo: findings from the scleroderma lung study. Chest 2009;136:1333-40. [PubMed]
- Wells AU, Desai SR, Rubens MB, Goh NS, Cramer D, Nicholson AG, Colby TV, du Bois RM, Hansell DM. Idiopathic pulmonary fibrosis: a composite physiologic index derived from disease extent observed by computed tomography. Am J Respir Crit Care Med 2003;167:962-9. [PubMed]
- Bellia M, Cannizzaro F, Scichilone N, Riili M, Triolo G, Midiri M, Lagalla R. HRCT and scleroderma: semiquantitative evaluation of lung damage and functional abnormalities. Radiol Med 2009;114:190-203. [PubMed]
- Clements PJ, Roth MD, Elashoff R, Tashkin DP, Goldin J, Silver RM, Sterz M, Seibold JR, Schraufnagel D, Simms RW, Bolster M, Wise RA, Steen V, Mayes MD, Connelly K, Metersky M, Furst DE; Scleroderma Lung Study Group. Scleroderma lung study (SLS): differences in the presentation and course of patients with limited versus diffuse systemic sclerosis. Ann Rheum Dis 2007;66:1641-7. [PubMed]
- Olson TP, Denzer DL, Sinnett WL, Wilson T, Johnson BD. Prognostic value of resting pulmonary function in heart failure. Clin Med Insights Circ Respir Pulm Med 2013;7:35-43. [PubMed]
- Frans A, Nemery B, Veriter C, Lacquet L, Francis C. Effect of alveolar volume on the interpretation of single breath DLCO. Respir Med 1997;91:263-73. [PubMed]
- Hudson M, Assayag D, Caron M, Fox BD, Hirsch A, Steele R, Gaudreau-Taillefer R, Tatibouet S. Canadian Scleroderma Research Group (CSRG), Rudski L, Baron M. Comparison of different measures of diffusing capacity for carbon monoxide (DLCO) in systemic sclerosis. Clin Rheumatol 2013;32:1467-74. [PubMed]


