Liver fat content is negatively associated with atherosclerotic carotid plaque in type 2 diabetic patients
Introduction
Nonalcoholic fatty liver disease (NAFLD) is currently the most common cause of chronic liver disease among adults in Western countries (1,2). The spectrum of NAFLD ranges from simple steatosis to nonalcoholic steatohepatitis (NASH), which can progress to end-stage liver disease. NAFLD is commonly associated with obesity, metabolic syndrome, and type 2 diabetes (1-3). In the last few years, several studies assessed the association between hepatic steatosis and carotid atherosclerosis. Their results suggest that NAFLD in nondiabetic individuals may be associated with an increased cardiovascular risk and, in particular, with an increased prevalence of carotid lesions (4-9). In patients with type 2 diabetes, the link between fatty liver and atherosclerosis is more controversial, as studies have produced conflicting results (10-14). Reasons for the discrepancies across studies may include the lack of standardization of measurement methods and the detection of hepatic steatosis using ultrasound, which allows only a qualitative assessment of liver fat content (15).
Here, our objective was to assess whether liver fat content was associated with prevalent carotid plaque in patients with type 2 diabetes. We quantitated liver fat content using 1H-magnetic resonance spectroscopy (1H-MRS), a highly accurate imaging technique. We planned to evaluate whether any link between liver fat content and carotid plaque was independent from conventional cardiovascular risk factors.
Materials and methods
Study population
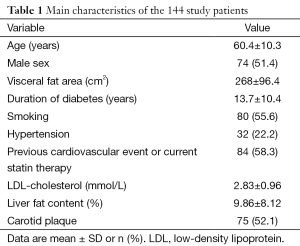
The GEnetic Polymorphisms, Steatosis and Diabetes (GEPSAD) study is a prospective single-center study conducted in 144 consecutive type 2 diabetic patients (74 men; 70 women; mean age 60.4±10.3 years; range, 36-84 years) recruited prospectively at an endocrinology department. Inclusion criteria were type 2 diabetes for at least 2 years; age 18 years or older; body weight no greater than 150 kg; no concurrent acute or chronic disease, physical examination, blood cell counts, and electrolyte concentrations; alcohol consumption less than 20 grams per day; and no evidence of liver disease such as viral hepatitis. Patients were not eligible if they had causes of liver steatosis [alcohol use ≥20 g/day, hepatitis B or C virus infection, or use of drugs known to precipitate steatosis (thiazolidinediones, corticosteroids, and immunosuppressants)] or contraindications to magnetic resonance imaging (pacemaker, metallic implants, claustrophobia, or body weight greater than 150 kg). Patient characteristics are reported in Table 1. The study protocol was approved by our local research ethics committee and all patients gave written informed consent before study inclusion.
Full table
Measurements
Clinical characteristics
Sociodemographic characteristics and medical histories were assessed by computer-aided face-to-face interview. Diabetes was diagnosed according to American Diabetes Association criteria (symptoms of diabetes and casual plasma glucose level greater than or equal to 11.1 mmol/L or fasting plasma glucose level greater than or equal to 7.0 mmol/L) (16). Mean daily alcohol consumption was calculated using beverage-specific percentages of pure ethanol by volume. Smokers were defined as former or current smokers. Systolic and diastolic blood pressures were measured and arterial hypertension defined as mean systolic blood pressure ≥140 mmHg, means diastolic blood pressure ≥90 mmHg, or self-reported use of antihypertensive medication. We recorded previous cardiovascular events (myocardial infarction, angina, coronary revascularization, ischemic stroke, and symptomatic peripheral arterial disease).
Laboratory tests
Plasma glucose, fasting serum low-density lipoprotein (LDL) cholesterol, plasma liver enzymes, and markers for hepatitis B virus (HBV) and hepatitis C virus (HCV) infections were determined using standard laboratory procedures.
1H-magnetic resonance spectroscopy (1H-MRS)
Liver fat content was measured using a 3.0-Tesla Magnetom Trio A TIM whole-body system (Siemens, Erlangen, Germany) as previously described (17). Briefly, sagittal, coronal, and axial slices through the right lobe of the liver were acquired, and a 27-cm3 spectroscopic volume of interest was positioned on segment VII. To measure T2 relaxation times of water and methylene, single-voxel 1H-MRS data were acquired using seven breath-hold PRESS sequences (repetition time, 5,000 msec; 3 acquisitions; and 2,048 data points over 1,250 Hz spectral width) with echo times of 30, 40, 50, 60, 80, 100, and 135 msec. For each of the two voxel placements, automated optimization of gradient shimming followed by manual adjustment of central frequency was performed, and water line widths of 40-50 Hz were obtained.
The Java-based MR user interface (jMRUI) spectroscopic analysis package was used for the time-domain analysis. Metabolite signals were analyzed using the Advanced Magnetic Resonance (AMARES) fitting algorithm within jMRUI, which enables the inclusion of a large amount of prior knowledge. We measured the water peak at 4.7 ppm and the methylene peak at 1.3 ppm. Spectra were used only if homogeneity after shimming was better than 0.45 ppm, measured as the full width at 50% peak height. Peak integrals were quantified by fitting to a Gaussian line shape. Finally, liver fat content was calculated as previously reported (17). Hepatic steatosis was defined as hepatic triglyceride content greater than 5.5% (2).
Visceral fat measurement
Visceral fat was measured using a single-slice axial T1-weighted image at the level of the L4-L5 intervertebral disc. Images were analyzed for cross-sectional area of visceral fat (18).
Carotid ultrasound
Carotid plaque assessment and intima-media thickness (IMT) measurement were performed by a single experienced sonographer who was blind to all clinical and laboratory data. High-resolution real-time B-mode ultrasound scanning of the extracranial carotid arteries was performed using a 7.5-MHz linear transducer (Philips HD 11, Best, The Netherlands), with the patient lying supine. Longitudinal projections of both carotid arteries were examined.
Carotid IMT (c-IMT) was measured off-line using specially designed software (Philips Qlab 6.0) at the level of the common carotid artery far wall, in the 15-mm segment proximal to the carotid bulb and in plaque-free segments, as previously described (19,20), as the distance between the leading edges of the lumen-intima (first echogenic line) and media-adventitia (second echogenic line) interfaces. The mean of three measurements at each artery was used for the analysis.
Plaque was defined as focal widening of the vessel wall relative to adjacent segments (with protrusion into the lumen and/or localized roughness with increased echogenicity and/or focal IMT ≥1.2 mm) (5). Plaque was considered present when one or more plaques were seen in any of 12 carotid segments (near and far walls of the right and left common carotid arteries, bifurcation, and internal and external carotid arteries) (6,21).
Statistical analysis
The patients were divided into two groups based on presence or absence of carotid plaque. We investigated whether presence of carotid plaque was associated with any of the following variables: age, sex, visceral fat area, diabetes duration, smoking, hypertension, previous cardiovascular events or current statin therapy, LDL-cholesterol, and liver fat content. Continuous variables were expressed as mean ± SD and compared using the Student test, whereas categorical variables were expressed as number (percentage) and compared between groups using the chi-square test. All variables were included in a multivariate logistic regression model to identify factors independently associated with carotid plaque. The odds ratios (ORs) were calculated, with the model-based lower and upper 95% confidence intervals (CIs). P values smaller than 0.05 were considered statistically significant. All statistical analyses were performed using Stata 11 software (StataCorp 2009, Stata Statistical Software, Release 11, College Station, TX, USA).
Results
Table 1 reports the main features in the 144 patients at study inclusion. No patients had positive HIV serology, inflammatory bowel disease, or current parenteral nutrition. Previous cardiovascular events or current statin therapy was noted in 84 (58.3%) patients. Mean ± SD liver fat content was 9.86±8.12%, ranging from 0.03% to 30.45%. Carotid plaque was found in 75 (52.1%) patients.
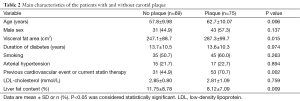
The main characteristics of patients with and without carotid plaque are reported in Table 2. Patients with carotid plaque were older and had a higher visceral fat area values and a higher reported prevalence of previous cardiovascular events or current statin therapy. Liver fat content was significantly higher in patients without plaque than in those with plaque in the univariate analysis (P=0.009). No differences between the two groups were found for sex distribution, diabetes duration, prevalence of smoking, prevalence of hypertension, or LDL-cholesterol levels (Table 2).
Full table
In the multivariable analysis, age, visceral fat area, and prevalence of previous cardiovascular events or current statin therapy were positively associated with the presence of plaque. In contrast, liver fat content was negatively associated with presence of plaque (OR, 0.94; 95% CI, 0.89-0.99; P=0.017). Presence of plaque was not predicted by sex, diabetes duration, smoking, hypertension, or LDL-cholesterol levels (Table 3).

Full table
Discussion
The main finding from this study is that a higher fat liver fat content, as assessed using 1H-MRS, was strongly associated with absence of carotid plaque, independently from conventional cardiovascular risk factors, in patients with type 2 diabetes mellitus. To our knowledge, this is the first cross-sectional study demonstrating an independent relationship between the amount of liver fat and a decreased risk of carotid atherosclerosis in a population of type 2 diabetic patients.
Whether liver fat content is an independent risk factor for cardiovascular morbidity and mortality is an important issue. Liver steatosis is now considered the hepatic manifestation of metabolic syndrome (22). Recent data indicate that the severity of NAFLD is associated with higher c-IMT values (7), higher carotid plaque prevalence (6,21), and lower endothelial flow-mediated vasodilation (23), independently from the underlying metabolic abnormalities. Furthermore, in nondiabetic individuals, NAFLD is associated with higher all-cause mortality (24,25) and higher prevalence (4,6) and incidence (25) of cardiovascular disease. Several studies consistently demonstrated a link between hepatic steatosis and a pro-atherogenic pro-inflammatory biomarker profile (low HDL, high triglycerides, high C-reactive protein, and low adiponectin) (12). However, nearly all the studies showing independent associations between fatty liver and atherosclerosis were done in nondiabetic individuals and involved no adequate evaluation of visceral fat or insulin sensitivity (13). Furthermore, liver fat was generally assessed using ultrasonography, which has two technical limitations. First, ultrasonography detects steatosis only when the amount of fat by liver biopsy is greater than 33% (15,26). Second, ultrasonography allows qualitative scoring of steatosis severity but does not provide quantitative measurements (27,28).
In patients with type 2 diabetes, the relationship between carotid atherosclerosis and fatty liver is unclear. Conflicting results were obtained from the only two studies investigating whether hepatic steatosis in type 2 diabetic patients was independently associated with cardiovascular disease, defined as cerebrovascular event prevalence (11) or increased c-IMT (12). Targher et al. (11) found that patients with NAFLD had a considerably higher prevalence of cerebrovascular disease compared to patients without NAFLD (20.0% vs. 13.3%, P<0.001). The main limitation of this study is that NAFLD was diagnosed using ultrasonography. In contrast, McKimmie et al. (12) found no significant association between hepatic steatosis evaluated by computed tomography and c-IMT or carotid calcium deposition and concluded that hepatic steatosis was probably not a direct mediator of cardiovascular disease. In our study, we evaluated liver fat content using 1H-MR spectroscopy, which is considered the most accurate noninvasive method (17,26). Contrary to previous studies, we found that liver fat content was negatively associated with the presence of carotid plaque. The 1H-MRS method measures the percentage of fat in the liver and, unlike other noninvasive methods such as ultrasonography or computed tomography, provides highly accurate quantitative measurements of the amount of liver fat, allowing grading of disease severity (17,29,30).
Our study has several limitations. First, the data from our study do not provide an explanation for the negative correlation between liver fat and carotid plaque. Furthermore, the cross-sectional design does not allow us to determine whether liver fat and absence of carotid plaque were causally related. Second, c-IMT is only a marker for atherosclerosis and not atherosclerosis itself. The mechanisms and disorders that cause carotid wall hypertrophy may increase c-IMT without increasing the risk of clinical atherosclerotic events. However, a strong positive association has been reported between carotid plaque prevalence and stroke (31). Third, the reference standard for evaluating hepatic steatosis is liver biopsy. However, liver biopsy was not ethically acceptable in our population and 1H-MRS is highly accurate (17). Fourth, we included only patients with type 2 diabetes. The distribution of many pro-atherogenic variables differs between type 2 diabetic patients and the general population. We do not know whether our findings apply to other populations. However, type 2 diabetic patients are of special concern, as they are at high risk for metabolic syndrome and subclinical cardiovascular disease. Despite this high risk, we found that higher liver fat content was associated with absence of carotid plaque.
Conclusions
In conclusion, in our study the severity of hepatic steatosis was strongly associated with the absence of carotid plaque in type 2 diabetic patients, independently from conventional cardiovascular risk factors and metabolic syndrome components. These findings suggest that increased liver fat content may be associated with relative protection against carotid atherosclerosis and with a decreased risk of future cardiovascular events in patients with type 2 diabetes, though there is probably an upper limit hepatic steatosis to find above which liver fat content is more detrimental. Longitudinal studies are needed to assess this hypothesis and to elucidate the molecular mechanisms linking liver steatosis and cardiovascular disease.
Acknowledgements
The authors are deeply grateful to Prof. Bonithon-Kopp from the Clinical Investigation Center of the University Hospital Center of Dijon, France, for assistance with this study.
Funding: This study was supported by a grant (NCT02045563) from the University Hospital Center of Dijon (PHRC 2007).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Angulo P. Nonalcoholic fatty liver disease. N Engl J Med 2002;346:1221-31. [PubMed]
- Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 2004;40:1387-95. [PubMed]
- Younossi ZM, Gramlich T, Matteoni CA, Boparai N, McCullough AJ. Nonalcoholic fatty liver disease in patients with type 2 diabetes. Clin Gastroenterol Hepatol 2004;2:262-5. [PubMed]
- Targher G, Bertolini L, Padovani R, Zenari L, Zoppini G, Falezza G. Relation of nonalcoholic hepatic steatosis to early carotid atherosclerosis in healthy men: role of visceral fat accumulation. Diabetes Care 2004;27:2498-500. [PubMed]
- Brea A, Mosquera D, Martín E, Arizti A, Cordero JL, Ros E. Nonalcoholic fatty liver disease is associated with carotid atherosclerosis: a case-control study. Arterioscler Thromb Vasc Biol 2005;25:1045-50. [PubMed]
- Volzke H, Robinson DM, Kleine V, Deutscher R, Hoffmann W, Ludemann J, Schminke U, Kessler C, John U. Hepatic steatosis is associated with an increased risk of carotid atherosclerosis. World J Gastroenterol 2005;11:1848-53. [PubMed]
- Targher G, Bertolini L, Padovani R, Rodella S, Zoppini G, Zenari L, Cigolini M, Falezza G, Arcaro G. Relations between carotid artery wall thickness and liver histology in subjects with nonalcoholic fatty liver disease. Diabetes Care 2006;29:1325-30. [PubMed]
- Sookoian S, Pirola CJ. Non-alcoholic fatty liver disease is strongly associated with carotid atherosclerosis: a systematic review. J Hepatol 2008;49:600-7. [PubMed]
- Kim HC, Kim DJ, Huh KB. Association between nonalcoholic fatty liver disease and carotid intima-media thickness according to the presence of metabolic syndrome. Atherosclerosis 2009;204:521-5. [PubMed]
- Targher G, Bertolini L, Padovani R, Poli F, Scala L, Zenari L, Zoppini G, Falezza G. Non-alcoholic fatty liver disease is associated with carotid artery wall thickness in diet-controlled type 2 diabetic patients. J Endocrinol Invest 2006;29:55-60. [PubMed]
- Targher G, Bertolini L, Rodella S, Tessari R, Zenari L, Lippi G, Arcaro G. Nonalcoholic fatty liver disease is independently associated with an increased incidence of cardiovascular events in type 2 diabetic patients. Diabetes Care 2007;30:2119-21. [PubMed]
- McKimmie RL, Daniel KR, Carr JJ, Bowden DW, Freedman BI, Register TC, Hsu FC, Lohman KK, Weinberg RB, Wagenknecht LE. Hepatic steatosis and subclinical cardiovascular disease in a cohort enriched for type 2 diabetes: the Diabetes Heart Study. Am J Gastroenterol 2008;103:3029-35. [PubMed]
- Picardi A, Vespasiani-Gentilucci U. Association between non-alcoholic fatty liver disease and cardiovascular disease: a first message should pass. Am J Gastroenterol 2008;103:3036-8. [PubMed]
- Petit JM, Guiu B, Terriat B, Loffroy R, Robin I, Petit V, Bouillet B, Brindisi MC, Duvillard L, Hillon P, Cercueil JP, Verges B. Nonalcoholic fatty liver is not associated with carotid intima-media thickness in type 2 diabetic patients. J Clin Endocrinol Metab 2009;94:4103-6. [PubMed]
- McCullough AJ. The clinical features, diagnosis and natural history of nonalcoholic fatty liver disease. Clin Liver Dis 2004;8:521-33. viii. [PubMed]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2004;27 Suppl 1:S5-S10. [PubMed]
- Guiu B, Petit JM, Loffroy R, Ben Salem D, Aho S, Masson D, Hillon P, Krause D, Cercueil JP. Quantification of liver fat content: comparison of triple-echo chemical shift gradient-echo imaging and in vivo proton MR spectroscopy. Radiology 2009;250:95-102. [PubMed]
- Ross R, Léger L, Morris D, de Guise J, Guardo R. Quantification of adipose tissue by MRI: relationship with anthropometric variables. J Appl Physiol (1985) 1992;72:787-95. [PubMed]
- Touboul PJ, Prati P, Scarabin PY, Adrai V, Thibout E, Ducimetière P. Use of monitoring software to improve the measurement of carotid wall thickness by B-mode imaging. J Hypertens Suppl 1992;10:S37-41. [PubMed]
- Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, Csiba L, Desvarieux M, Ebrahim S, Fatar M, Hernandez Hernandez R, Jaff M, Kownator S, Prati P, Rundek T, Sitzer M, Schminke U, Tardif JC, Taylor A, Vicaut E, Woo KS, Zannad F, Zureik M. Mannheim carotid intima-media thickness consensus (2004-2006). An update on behalf of the Advisory Board of the 3rd and 4th Watching the Risk Symposium, 13th and 15th European Stroke Conferences, Mannheim, Germany, 2004, and Brussels, Belgium, 2006. Cerebrovasc Dis 2007;23:75-80. [PubMed]
- Ramilli S, Pretolani S, Muscari A, Pacelli B, Arienti V. Carotid lesions in outpatients with nonalcoholic fatty liver disease. World J Gastroenterol 2009;15:4770-4. [PubMed]
- Kotronen A, Yki-Järvinen H. Fatty liver: a novel component of the metabolic syndrome. Arterioscler Thromb Vasc Biol 2008;28:27-38. [PubMed]
- Villanova N, Moscatiello S, Ramilli S, Bugianesi E, Magalotti D, Vanni E, Zoli M, Marchesini G. Endothelial dysfunction and cardiovascular risk profile in nonalcoholic fatty liver disease. Hepatology 2005;42:473-80. [PubMed]
- Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology 2005;129:113-21. [PubMed]
- Ekstedt M, Franzén LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, Kechagias S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 2006;44:865-73. [PubMed]
- Mazhar SM, Shiehmorteza M, Sirlin CB. Noninvasive assessment of hepatic steatosis. Clin Gastroenterol Hepatol 2009;7:135-40. [PubMed]
- Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M, Mullen KD, Cooper JN, Sheridan MJ. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology 2002;123:745-50. [PubMed]
- Bohte AE, van Werven JR, Bipat S, Stoker J. The diagnostic accuracy of US, CT, MRI and 1H-MRS for the evaluation of hepatic steatosis compared with liver biopsy: a meta-analysis. Eur Radiol 2011;21:87-97. [PubMed]
- d'Assignies G, Ruel M, Khiat A, Lepanto L, Chagnon M, Kauffmann C, Tang A, Gaboury L, Boulanger Y. Noninvasive quantitation of human liver steatosis using magnetic resonance and bioassay methods. Eur Radiol 2009;19:2033-40. [PubMed]
- Ligabue G, Besutti G, Scaglioni R, Stentarelli C, Guaraldi G. MR quantitative biomarkers of non-alcoholic fatty liver disease: technical evolutions and future trends. Quant Imaging Med Surg 2013;3:192-5. [PubMed]
- Ebrahim S, Papacosta O, Whincup P, Wannamethee G, Walker M, Nicolaides AN, Dhanjil S, Griffin M, Belcaro G, Rumley A, Lowe GD. Carotid plaque, intima media thickness, cardiovascular risk factors, and prevalent cardiovascular disease in men and women: the British Regional Heart Study. Stroke 1999;30:841-50. [PubMed]


