Intraoperative image-guided surgery for ovarian cancer
Abstract
Approximately two thirds of the ovarian cancer is diagnosed at late stage with peritoneal seeding. The most important prognostic factor is the residual tumor size after surgery. The better survival outcome from minimizing tumor has been established well from several institutional reports, clinical trials for chemotherapy, and meta-analyses. The current standard treatment for such advanced ovarian cancer is to resect all visible and palpable ovarian cancer as much as possible and postoperative chemotherapy. But, even after complete resection of all visible and palpable tumors, a significant percentage of the patients have recurrence usually in the peritoneal cavity in advanced ovarian cancer. Recurrence likely stems from microscopic residual tumor not resected at time of the initial surgery. Currently, advanced technology for intraoperative tumor detection has been reported. Although the current clinical outcome is at an early stage definitively, clinical benefit could be expected from the future in-depth basic research and clinical trials in this area. If all residual microscopic disease, especially chemotherapy-resistant cancer cells, could be removed or treated using new technology, groundbreaking advance for treating ovarian cancer will be realized.
Key words
Ovarian cancer; surgery; cytoreductive surgery; image; fluorescent
Ovarian cancer: Best candidate for image-guiding surgery
The critical problem in ovarian cancer is the lack of a specific screening method and nonexistent symptoms suggestive of early carcinogenesis. Although serum tumor markers such as CA-125 and transvaginal ultrasonography can detect ovarian cancer, critical problems including high rate of false positivity still exist. Usual symptoms related to ovarian cancer including abdominal distension, bloating, or indigestion and are signs of peritoneal seeding. However, such symptoms are quite vague and not disease specific (1,2).
These challenging problem result in delayed diagnosis of ovarian cancer. Approximately two third of ovarian cancer patients were diagnosed at advanced stage with peritoneal cancer. For advanced ovarian cancer, prognostic markers are age, stage, histology, grade, family history of ovarian or breast cancer, ascites, albumin level, and postoperative residual tumor. At the time of diagnosis, all factors except postoperative residual tumor are fixed and not modifiable. Residual tumor size could be decreased with maximum surgical efforts (3-5). Survival benefit is estimated as 5.5% in primary ovarian cancer and 1.9% in recurrent ovarian cancer with 10% increase following optimal debulking surgery (6,7). Survival benefit from resection of peritoneal seeding is generally limited to some intra-abdominal cancer such as ovarian cancer, primary peritoneal cancer, tubal cancer, mesothelioma, and pseudomyxoma peritonei. Clinically, primary peritoneal cancer and tubal cancer are treated in the same fashion as ovarian cancer in terms of diagnosis, surgical treatment, postoperative chemotherapy, and prognosis.
Primary surgery or primary chemotherapy
Currently, preoperative resectability of tumor in the peritoneal cavity is usually determined based on imaging studies, where computed tomography is conventionally used. The most challenging area to provide complete resection of tumor is metastases on small bowel mesentery, supra-renal lymph node including superior mesenteric artery and celiac axis, and porta hepatis (8). When tumor on computed tomography appears fully resectable (no visible residual tumor or residual tumor <1 cm), primary surgery is planned. If the suboptimal debulking (residual tumor >1 cm) is estimated based on computed tomography, primary chemotherapy is considered as the first line of treatment. After chemotherapy, interval debulking surgery is usually attempted. Minimizing residual tumor after surgery (cytoreductive surgery) is the most important to improve ovarian cancer survival.
Surgical procedures
After completely exposing the whole peritoneal cavity (Figure 1), all tumors were inspected and palpated. All visible and/or palpable tumors were removed as much as possible. Usually the most difficult tumor to be resected is located in the upper abdomen, where the surgery typically begins. First, resection of omentum is tried. If the tumors at omentum are extended to the splenic hilum, the omentum and spleen are resected together: en bloc resection. Second, the surgery for right upper quadrant is tried: full liver mobilization, right diaphragmatic tumor resection (diaphragmatic stripping and/or resection), peritonectomy at hepatorenal recess of subhepatic space (Morrison’s pouch), and rarely right adrenalectomy. Third, the surgery for upper middle abdomen is attempted: tumor resection on porta hepatis, lesser omentum, and lesser sac or partial gastrectomy. Fourth, the surgery for left upper quadrant is tried: left diaphragmatic tumor resection (diaphragmatic stripping and/or resection) or distal pancreatectomy. Fifth, the surgery for pelvis is tried: en bloc resection of tumor at uterus, ovaries, and rectum (posterior pelvic exenteration). Tumors at small bowel, colon, and paracolic gutter could be resected individually or together with other surgical procedures.
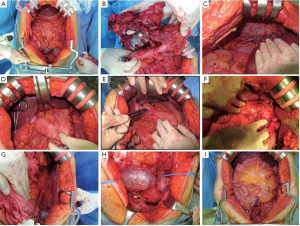
Challenges for current surgical management of ovarian cancer
Surgical resection of ovarian cancer and peritoneal seeding are based on direct inspection and palpation during surgery. However, even after complete removal of all visible and palpable tumors, more than two thirds of the women with advanced epithelial ovarian cancer have recurrence (3-8), where microscopic tumor seems to be the source of peritoneal recurrence. If microscopic tumor could be detected and resected and/or treated completely during surgery, recurrence could be significantly decreased.
In early stage ovarian cancer, the treatment options must be carefully evaluated. The current standard treatment for early ovarian cancer is to performing staging surgery. The staging surgery is composed of hysterectomy, resection of ovaries and tubes bilaterally, resection of omentum, small tissue sampling of both diaphragmatic peritoneum and both paracolic gutter (between colon mesenteric peritoneum and parietal peritoneum), resection of retroperitoneal lymph node at pelvis and aorta. Since approximately one third of the patients with early stage ovarian cancer based on intraoperative findings have more advanced disease in final pathological examination (9), the staging surgery has been performed until now. In other words, two thirds of patients undergo unnecessary surgery. If exact disease status could be determined during surgery, the current staging surgery can be individually performed. Finally, surgical extent could decrease which might result in medical cost reduction, decreased complication and improved quality of life.
Open surgery (laparotomy), provides tactile sense to the surgeon, although does not afford the advantages to the patient of laparoscopic or robotic surgery. As mentioned earlier, ovarian cancer surgery is performed based on visual and palpable tumor extent. Without tactile sense, tumor at peritoneal folding such as ileocecum or paracolic gutter can be easily missed during surgery. Additionally, tumor at hepato-phrenic junction is difficult area to reach using laparoscopic or robotic camera. The loss of tactile sense during laparoscopic or robotic surgery in detecting tumors might be overcome by various advanced technologies such as nanotechnology, fluorescence image-guided surgery, and radioactive tracers.
Current efforts using fluorescence image-guided surgery
Recently, intraoperative tumor identification using tumor specific fluorescence imaging has been reported (11). This report suggests that new imaging technology might leads surgeons in the operating room to better surgical decisions and approaches in terms of surgical extent.
Several issues should be considered before introducing clinical application of such imaging techniques as standard treatment modalities. First, invisible and non-palpable tumors should be identified using this new imaging technique. Furthermore, minimal surgical approaches such as laparoscopic surgery and robotic surgery might show increased resection completion if assisted by imaging technology. Second, in the case of ovarian cancer, histology is quite heterogeneous. Generally histologically, ovarian cancer is designated as an epithelial ovarian cancer. In epithelial ovarian cancer, there are serous, endometrioid, mucinous, clear, transitional cell, as well as other cell types. Except epithelial ovarian cancer, sex-cord stromal tumor and germ cell tumor form parts of ovarian cancer. Therefore, new markers compatible and specific for each histological type of ovarian cancer should be developed. Third, treatment including surgical resection/destruction or using medication should be effective for the uncovered lesions. Considering the feasibility in clinical practice, medical treatment using new technology seems to be more applicable than surgical resection/destruction. The reason is that the difficult lesions are usually in upper abdomen. Although ovarian cancer is one of the gynecologic malignancies, the most difficult surgical procedures are belonging to surgical oncology of hepatobiliary department and so on. Fourth, survival benefit should be confirmed in the setting of clinical trials. The survival benefit comes from not only surgery but also chemotherapy. Currently, targeted therapy has been introduced in the clinical field of cancer therapy. Therefore, identifying and resection of chemotherapy-resistant and potentially targeted therapy-resistant cancer cells can lead to survival benefit, taken as a whole. These suggestions are the final pursuit.
Smaller tumors, which are not palpable and not visible could be removed using advanced intraoperative imaging technique in the near future. Ovarian cancer and related seeding are usually limited to the intra-peritoneum (abdominal cavity). Recurrence and cause of deaths are related with peritoneal disease in most cases. Therefore, removing all intraperitoneal tumors using new image techniques could result in improved survival outcomes.
Conclusions
New technology for image guiding surgery is quite promising considering disease characteristics of ovarian cancer. The feasibility of such technique has been demonstrated in a pilot study in ovarian cancer (11). Basic research that could be applicable to broad spectrum of ovarian cancer is still needed. Robust outcomes from clinical trials using new image technique are expected.
Acknowledgements
This report was supported by a grant of the Korea Healthcare technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A100059), and a grant from National Cancer Center Korea (1010112).
Disclosure: The authors declare no conflict of interest.
References
- Lim MC, Lee DO, Kang S, et al. Clinical manifestations in patients with ovarian clear cell carcinoma with or without co-existing endometriosis. Gynecol Endocrinol 2009;25:435-40.
- Lim MC, Chun KC, Shin SJ, et al. Clinical presentation of endometrioid epithelial ovarian cancer with concurrent endometriosis: a multicenter retrospective study. Cancer Epidemiol Biomarkers Prev 2010;19:398-404.
- Lim MC, Kang S, Lee KS, et al. The clinical significance of hepatic parenchymal metastasis in patients with primary epithelial ovarian cancer. Gynecol Oncol 2009;112:28-34.
- Song YJ, Lim MC, Kang S, et al. Total colectomy as part of primary cytoreductive surgery in advanced Müllerian cancer. Gynecol Oncol 2009;114:183-7.
- Chi DS, Eisenhauer EL, Zivanovic O, et al. Improved progression-free and overall survival in advanced ovarian cancer as a result of a change in surgical paradigm. Gynecol Oncol 2009;114:26-31.
- Bristow RE, Tomacruz RS, Armstrong DK, et al. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol l 2002;20:1248-59.
- Bristow RE, Chi DS. Platinum-based neoadjuvant chemotherapy and interval surgical cytoreduction for advanced ovarian cancer: a meta-analysis. Gynecol Oncol 2006;103:1070-6.
- Song YJ, Lim MC, Kang S, et al. Extended cytoreduction of tumor at the porta hepatis by an interdisciplinary team approach in patients with epithelial ovarian cancer. Gynecol Oncol 2011;121:253-7.
- Young RC, Decker DG, Wharton JT, et al. Staging laparotomy in early ovarian cancer. JAMA 1983;250:3072-6.
- van Dam GM, Themelis G, Crane LM, et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-α targeting: first in-human results. Nat Med 2011;17:1315-9.
- Themelis G, Harlaar NJ, Kelder W, et al. Enhancing surgical vision by using real-time imaging of αvβ3-integrin targeted near-infrared fluorescent agent. Ann Surg Oncol 2011;18:3506-13.


