What does diffusion tensor imaging (DTI) tell us about cognitive networks in temporal lobe epilepsy?
Introduction
With greater appreciation of epilepsy as a network disorder, diffusion tensor imaging (DTI) has emerged as one of the most popular imaging modalities for studying network dysfunction associated with seizures in both children and adults. DTI has a broad range of applications in studying epilepsy-related networks, including lateralization of the seizure focus (1-5), delineating the location of key white matter pathways for surgical planning (e.g., optic radiations) [see (6) for a recent review; (7)], and understanding total white matter burden associated with different epilepsy syndromes (8-13). Emerging DTI literature also describes how disruptions in white matter networks contribute to cognitive morbidity and post-operative cognitive decline in patients with epilepsy, as well as how adaptive changes in white matter networks may allow for inter- and intra-hemispheric reorganization of function.
In this article, we review the current literature demonstrating the unique contributions of DTI to understanding cognitive dysfunction in patients with temporal lobe epilepsy (TLE). Since the majority of work to date has surrounded language and memory networks in TLE this topic will be the primary focus of our review. We also survey the extant literature on how white matter microstructure can probe networks that underlie aspects of executive functioning and global intelligence in epilepsy. Finally, we address limitations of the tensor model and discuss how advanced diffusion techniques may improve white matter-cognitive associations in TLE.
What can DTI tell us about language networks in TLE?
Perhaps the area that has received the most attention surrounds the use of DTI for mapping the structure and localization of language networks in healthy controls and patients with TLE. Several white matter tracts, including the arcuate fasciculus (ARC), uncinate fasciculus (UNC), inferior longitudinal fasciculus (ILF), and the inferior frontal occipital fasciculus (IFOF) have emerged as important fibers in the study of language networks (see Figure 1). In particular the ARC, which includes the frontotemporal portion of the superior longitudinal fasciculus (SLF), is a fiber long known to be important in language functioning as it connects areas involved in language comprehension (i.e., Wernicke’s area) in the posterior superior temporal gyrus to areas involved in language expression (i.e., Broca’s area) in the inferior frontal gyrus (14). Another commonly studied fiber is the UNC, a curved fiber that connects the anterior temporal lobes (TL) to the frontal lobes (15). The ILF connects the occipital lobe to the anterior TL running laterally and inferiorly above optic radiation fibers (16). Finally, the IFOF is a relatively long fiber running from occipital cortex passing through temporal/basal areas and the superior parietal lobe to the frontal lobe (17). The ARC, UNC, ILF and IFOF have all been implicated as facets of a complex network of fibers involved in language performance, where damage to one or more of these fiber tracts can lead to impairment in specific language skills.
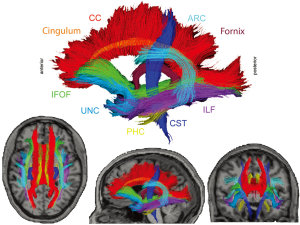
Research using DTI to probe language networks in patients with TLE has used two basic approaches: either examining how DTI-derived metrics, typically fractional anisotropy (FA) or mean diffusivity (MD), correlate with impairment on neuropsychological measures of language (e.g., naming, fluency), or investigating how FA/MD laterality within frontotemporal fiber tracts corresponds to language laterality as determined by functional MRI (fMRI) or the intracarotid amobarbital procedure (IAP). With regard to the former, our lab applied probabilistic tractography to a group of 17 patients with TLE and 17 controls and demonstrated that increased MD of the ARC and UNC, along with decreased FA of bilateral ARC, UNC, and left IFOF were related to poorer naming performances in patients with TLE, but not controls (18). Not surprisingly, the strongest correlation was observed between FA of the left ARC and naming (r=0.80, P<0.0001) and this association held even after controlling for left hippocampal volume. We also found a strong correlation between integrity of the right ARC and visual naming in our patients. These data are commensurate with studies demonstrating right hemisphere contributions to language processing (3), and with studies suggesting partial reorganization of language to the right hemisphere in some patients with left TLE [LTLE; (19)]. Despite frontotemporal white matter correlations with naming, we did not find an association between fiber tract compromise and phonemic fluency impairments in our patient cohort. Conversely, Wang et al. (20) used a voxel-based approach and found that lower FA in regions of the left frontal lobe and right occipital lobe were associated with poorer semantic fluency in patients with TLE. Although the reason for these divergent findings is unclear, it is possible that the use of different fluency measures (i.e., phonemic versus semantic) or the use of different DTI approaches (tract-based versus voxel-based) may explain these disparate outcomes.
Beyond the examination of DTI alone, Kucukboyaci et al. (21) took a multimodal imaging approach to understanding language impairment in TLE by examining the relative contributions of DTI-based versus structural MRI-based measures to predicting visual and auditory naming performances. Based on previous research implicating a network of frontotemporal fiber tracts in language performance (18), principal components analysis (PCA) was used to leverage the shared variance among multiple frontotemporal fiber tracts to evaluate language functioning in patients with TLE. This analysis showed that after controlling for the effects of left hippocampal volume, reduced frontotemporal FA was associated with poorer visual naming while increased MD was associated with poorer auditory naming. Neither cortical thickness nor gray-white contrast within frontotemporal regions explained language performances in TLE. These data supported our previous results demonstrating the value of DTI for understanding language performances and showed that measures of microstructural white matter integrity may be more sensitive than measures obtained from structural MRI for understanding language impairment in TLE.
A second approach to probing language networks in patients with TLE has been to examine the degree to which asymmetries in frontotemporal fiber tract FA/MD values are associated with asymmetries in fMRI language activations and results from the IAP. This approach is based on research demonstrating that in healthy individuals, a leftward asymmetry exists in frontotemporal FA and tract volumes (including the ARC, UNC, and IFOF), which correlates with typical leftward asymmetries in fMRI language activations (22,23). Thus, deviations from this expected asymmetry may imply language reorganization in TLE. Powell et al. (3) combined fMRI and probabilistic tractography in a cohort of patients with TLE to study language reorganization. Using fMRI tasks of verb generation and reading comprehension, they demonstrated that patients with LTLE had less lateralized language activation patterns relative to patients with right TLE (RTLE) and healthy controls. Reductions in language asymmetry were coupled with reductions in FA asymmetry within frontotemporal fiber tracts. The authors interpreted this finding as evidence of both structural and functional reorganization of language in patients with LTLE. Rodrigo et al. (12) used a similar approach and found a leftward asymmetry in FA of the ARC that was associated with leftward fMRI language activations in patients with RTLE and controls. However, this relationship did not hold for patients with LTLE. Despite reduced microstructural and functional asymmetry of the ARC in patients with LTLE, there was a lack of coherence between functional and DTI measures, which underlines the complexity of language reorganization in patients with LTLE. In a third multimodal study, Ellmore et al. (24) examined whether white matter microstructure of several frontotemporal fiber tracts, including the ARC, UNC, and ILF, could be used to predict asymmetric language representation, as determined by the IAP. They found that laterality indices computed from asymmetry of the ARC, but not other pathways, correctly classified 82.6% (19 of 23) patients when using the IAP language index as the gold standard. When FA of the ARC was combined with fMRI language activation patterns and handedness, 95.6% (22 of 23) of patients were correctly classified. Overall, results of these studies indicate that increased FA and decreased MD of left frontotemporal fiber tracts are associated with better performances on measures of language functioning in patients with TLE. However, there appears to be a decreased asymmetry of language networks in many patients with LTLE that is reflected in both functional and microstructural measures and may indicate a partial or complete language reorganization associated with chronic TLE.
Pre-post surgical changes in language networks
Clinically, the greatest value of understanding language networks in patients with TLE lies in the ability to predict the likelihood and nature of language decline following epilepsy surgery. However, few studies have addressed this in a systematic way. Yogarajah et al. (25) measured white matter networks and language performances before and after (average of 4.5 months) anterior temporal lobectomy (ATL) in patients with TLE and found widespread decreases in FA surrounding the resection zone, in addition to increases in FA after left ATL in a cluster of regions that included the ipsilateral external capsule, posterior limb of the internal capsule, and corona radiata (see Figure 2). Mean pre- and post-operative FA and parallel diffusivity (i.e., diffusion parallel to primary fiber orientation) in this cluster were significantly correlated with postoperative verbal fluency and naming scores in patients with LTLE. Specifically, greater increases in parallel diffusivity following left ATL were associated with smaller declines in language performances. The authors suggest that this cluster of altered diffusion following left ATL corresponds to a ventromedial language network located medial to the dorsolateral language network, which may be less susceptible to injury following ATL and have a greater propensity for structural reorganization. In a more recent study, Pustina et al. (19) used DTI to measure white matter changes before and up to several years after ATL, examining the relationship between changes in FA and verbal fluency. In patients with LTLE, better preoperative phonemic fluency was related to higher FA in the left superior corona radiata, right SLF, and right UNC. However, better post-operative phonemic and semantic fluency were associated with higher FA of the right SLF only. Given the relationship of all three clusters with language performance before surgery but only the right SLF after surgery, the authors suggest that an adaptive inter-hemispheric shift occurs following left ATL. Thus, although left ATLs increase risk for postoperative language decline, there is a high likelihood of functional and structural reorganization to the contralateral side that may help to preserve language in many patients.
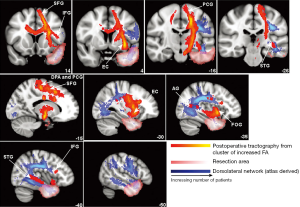
Taken together, studies using DTI to examine language networks in patients with TLE have supported the idea that multiple frontotemporal fiber tracts play a role in naming, fluency and reading comprehension, whether using neuropsychological measures or fMRI language tasks. However, the left ARC remains the fiber tract most associated with language performances in healthy controls and patients with TLE. Although there does not appear to be a one-to-one correspondence between functional and microstructural asymmetries, patients with LTLE are more likely to have less left-lateralized DTI and fMRI patterns, consistent with the concept of inter-hemispheric language reorganization in TLE. In addition to new insights into pre-operative language reorganization, DTI has provided evidence that post-operative reorganization and adaptive changes may occur within parallel language networks following left ATL, and this reorganization may occur within intra-hemispheric (3,25) or inter-hemispheric (12,19) networks.
What can DTI tell us about memory dysfunction in TLE?
Between 29-47% of patients with TLE present with verbal and/or nonverbal impairments in episodic memory (26,27), making memory dysfunction the most common cognitive co-morbidity in TLE. Thus, imaging of networks that underlie memory functioning has received considerable attention in the epilepsy literature.
Microstructural damage to the hippocampus itself, as well as to fiber tracts that originate in or course through the TL, have been implicated in memory dysfunction in TLE. Lui et al., (28) examined the relationship between medial temporal and whole brain diffusion abnormalities in TLE patients and found that the apparent diffusion coefficient (ADC) of the ipsilateral hippocampus and parahippocampus was significantly increased in patients. ADC within the hippocampus, particularly the left hippocampus, was related to poorer verbal and visuospatial memory in patients. There was also a strong trend between visuospatial memory and ADC of the right hippocampus that did not reach significance (P=0.08). Work from our lab has supported the importance of hippocampal MD in verbal memory, showing that hippocampal volume and MD of the left hippocampus contribute unique variance to memory scores (29).
The UNC has received considerable attention in studies of memory functioning due to its connections between prefrontal and medial temporal regions associated with memory (30). Diehl et al. (31) explored the relationship of the UNC to auditory and verbal scores of both immediate and delayed memory abilities in patients with TLE, finding that reduced FA of the left UNC was associated with poorer auditory memory in both LTLE and RTLE, while increased ADC and radial diffusivity were associated with poorer auditory memory in LTLEs only. For the right UNC, reduced FA was associated with poorer performance on visual delayed memory in LTLE, but no associations were found in patients with RTLE. This study provides evidence that damage to fiber tracts that emanate from the medial TL, in addition to the hippocampus, contribute to memory impairment in patients with TLE and that there is some material-specificity in these associations (i.e., left UNC damage is associated with verbal memory impairment, whereas right UNC damage is associated with visual memory impairment).
More recent studies have examined multiple fiber tracts and found broader associations with memory performances in TLE (18). Work from our lab has shown that increases in MD of the left UNC, parahippocampal cingulum (PHC), IFOF, and bilateral ARC, as well as decreases in FA of the right ARC are associated with poorer delayed memory for prose passages in patients with TLE. Similarly, in a cohort of 12 individuals primarily composed of LTLE patients (10 LTLE, 2 RTLE), Riley et al. (32) found a strong trend (P=0.053) for reduced FA in the ipsilateral anterior TL, which includes portions of these tracts, to be associated with poorer delayed verbal memory scores.
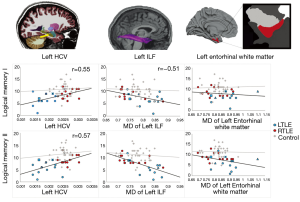
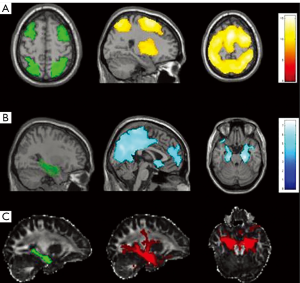
One question of clinical importance is how well DTI measures predict memory performances relative to other imaging measures described in the literature. Our lab sought to examine the contribution of DTI relative to structural-MRI for explaining verbal memory performance (33). We found that MD of the left ILF was a stronger predictor of verbal memory scores compared to morphometric measures including medial temporal cortical thickness, gray-white contrast and left hippocampal volume. The best model for predicting verbal memory performance included left hippocampal volume, MD of left ILF, and MD within left entorhinal white matter (see Figure 3). Together, these structures explained 60% of the variance in delayed memory performances. These results suggest that DTI and structural MRI may provide complementary information, as both the size of the hippocampus and microstructural integrity of surrounding white matter appear to be important to verbal memory in TLE.
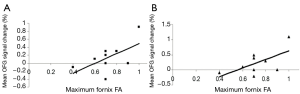
Voets et al. (34) combined DTI with fMRI to examine reorganization within memory networks in LTLE and to determine the coherence between functional and diffusion measures. Using a tensor independent component analysis on fMRI activations from a complex scene encoding task, they were able to conduct a voxel-based analysis of FA along the UNC and fornix. Results showed that maximum FA along the fornix was associated with altered functional connectivity between nilateral medial TL and the ipsilateral orbitofrontal cortex (see Figure 4). No relationship was found between UNC FA and functional activations, or between any of the DTI measures and neuropsychological scores. However, increased medial temporal-prefrontal activations ipsilateral to the seizure focus were associated with better performances on immediate and delayed story recall. The authors concluded that (I) LTLE is associated with adaptive changes within medial temporal-prefrontal memory networks ipsilateral to the seizure focus rather than inter-hemispheric shifts in memory; and that (II) white matter integrity of a primary hippocampal efferent pathway (i.e., fornix) may underlie these functional changes. Although no direct relationship was demonstrated between fornix FA and memory measures, the authors acknowledge that their small sample size (N=9) may have attenuated any potential effects.
Pre-post surgical changes in memory networks
Although many studies have examined changes in memory networks following ATL using functional imaging [(4,35-39) for a review see (40)], no studies have systematically used DTI to examine the relationship between pre- to postoperative changes in memory networks and changes in memory performances in TLE. In a recent post-hoc analysis of five patients with LTLE (33), we show that changes in left hippocampal volume, MD of left ILF, and MD of left entorhinal white matter all appear to correlate with change in memory performances one year following left ATL. However, our sample size was too small to draw reliable conclusions. Furthermore, this analysis was restricted to the subset of regions that were significant pre-surgical predictors of memory performance. Therefore, whether or not change in memory was associated with an increase in contralateral or adjacent ipsilateral regions (suggestive of inter- or intra-hemispheric reorganization) was not evaluated. Nevertheless, these preliminary data indicate that diffusion of left TL white matter and hippocampal volume may both be important to the prediction of verbal memory outcomes, at least following left ATL.
In summary, hippocampal volume and diffusivity seem to be sensitive predictors of memory performance in patients with TLE. Furthermore, efferent and afferent tracts of the hippocampus, in addition to those that course through the lateral TL, appear to contribute uniquely to the memory performance in patients with TLE. Finally, microstructural integrity within medial temporal fibers (i.e., fornix) may underlie functional plasticity in memory networks, suggesting some coherence between structural and functional measures.
What can DTI tell us about working memory/executive function networks in TLE?
Executive functioning refers to a broad category of higher-level cognitive abilities that includes fluency, set-shifting, decision-making, reasoning, and response inhibition, as well as working memory (41). The use of DTI to investigate executive functions in patients with TLE is an area of emerging interest since impairment in any of these complex cognitive processes can be associated with lower educational attainment, employment and social functioning in epilepsy (42). It is now recognized that 25-30% of patients with TLE show evidence of executive dysfunction (27,43), and a similar proportion demonstrate white matter changes in frontal networks (44). However, to date, the majority of studies have focused on the extent of white matter diffusivity or executive dysfunction in TLE. Only recently have some groups started to examine the complex executive function-DTI associations in TLE in a systematic manner.
Unlike memory and language networks, there is less consensus as to which fiber networks underlie executive dysfunction in TLE. Wang et al. (45) found that increased MD in multiple deep nuclei (i.e., caudate, putamen, thalamus) and decreased FA in the left frontal and occipital lobe were associated with poorer phonemic fluency in patients with TLE. They also found that impaired working memory was associated with increased MD in the splenium of corpus callosum (CC) and left putamen, and increased MD in the posterior limb of internal capsule bilaterally was associated with slower inhibition/switching performance. However, in a follow-up study, the same group (20) did not find any associations between white matter microstructure and similar measures of executive dysfunction in TLE. Dulay et al. [2009] examined associations between cingulum FA and working memory, problem solving, mental flexibility, phonemic fluency and learning performances in 8 patients with TLE. They found that the patients with executive dysfunction (defined as an average z-score of −1.64 or lower) had significantly lower mean FA values in their right and left cingulum. They also found that increased FA in the left cingulum was associated with poorer working memory, problem solving, and phonemic fluency. Similarly, Widjaja et al. (46) analyzed lobar diffusion in a mixed sample of children (N=40) with predominantly frontal, temporal and frontotemporal epilepsy in conjunction with an extensive neuropsychological battery. In this study, FA decreases in right temporal FA regions were associated with both language and executive function impairment (r=0.535, P<0.001; r=0.617, P<0.001). In addition, there were correlations between FA and executive function in bilateral frontal, left temporal, right parietal, and the body of the CC (r=0.500-0.542, all P values between 0.001 and 0.005). In nine patients with TLE, Riley et al. (47) analyzed rule learning, switching, and inhibition/switching performance together with less conventional DTI metrics (e.g., mean proportion of fibers connecting the caudate to a number of prefrontal regions). In this small sample, they found that reduced caudate volume was associated with higher perseveration on rule learning and switching, while reduced microstructural connections between left caudate and the dorsal prefrontal cortex were associated with slower inhibition/switching performance.
Combining fMRI and DTI, Winston et al. (48) studied working memory impairment in 54 patients with TLE and unilateral hippocampal sclerosis using a combination of a visuospatial n-back fMRI paradigm and a composite measure of working memory from the Weschler Adult Intelligence Scale-III (WAIS-III; see Table 1) to create a single working memory index. In all patients with TLE, white matter integrity (FA and MD) within a frontoparietal network, in addition to the integrity of the contralateral TL, was associated with working memory performance. Similar to Widjaja et al.’s (46) findings in the TL, Winston et al. interpreted these results as supporting the importance of both a frontoparietal network and TL structures beyond the hippocampus in working memory performance. Taking a connectivity approach, Stretton et al. (49) examined both functional connectivity and structural connectivity of the underlying white matter tracts emanating from the hippocampus (see Figure 4). They found that in controls, lower FA within both left and right hippocampal networks were associated with greater ipsilateral frontoparietal functional connectivity. Interestingly, LTLE patients demonstrated the opposite effect, where greater FA within right hippocampal networks was related to increased right frontoparietal functional connectivity, suggesting potential structural compensation of the contralateral hippocampus.
Full table
Despite some evidence that white matter microstructure within frontal networks (i.e., frontoparietal and/or frontotemporal) contributes to executive functions, numerous studies have not found reliable associations between reductions in prefrontal FA and executive dysfunction in TLE (46,50). As described above, Widjaja et al. found executive dysfunction (i.e., impaired divided attention, response inhibition, problem solving, and mental flexibility) to be primarily associated with reductions in right TL, but not frontal lobe FA. Kucukboyaci et al. (50) examined canonical correlations between frontotemporal fiber tracts (e.g., IFOF, UNC, ARC, and PHC) and measures of executive functioning and found that worse visuomotor set-shifting performance was associated with decreased FA of the left IFOF, whereas worse verbal set-shifting performance was associated with decreased FA in the left ARC and left UNC, but only in controls. Conversely, this relationship appeared to be absent in patients with TLE, suggesting a breakdown in typical microstructural-function associations that could reflect aberrant developmental or degenerative processes associated with TLE.
Pre-post surgical changes in executive function networks
Despite evidence suggesting changes in executive functioning in many patients following ATL (51), as well as separate studies finding changes in fiber tracts thought to underlie various aspects of executive functioning [see (42) for a review; (44)] none of these studies have examined how changes in executive function relate to changes in diffusion measures following ATL.
What can DTI tell us about global functioning in epilepsy?
In addition to studies examining the relationship between DTI measures and performance within specific cognitive domains, a few studies have explored how white matter microstructure relates to more global measures of cognitive functioning. Vaessen et al. (52) applied graph theoretical analysis to examine how widespread white matter connectivity relates to full scale IQ (FSIQ; WAIS-III) and overall level of cognitive impairment in a cohort of patients that included both TLE and frontal lobe epilepsy. Patients classified with severe cognitive impairment (N=7) showed lower clustering (a measure of brain network segregation) and higher path length (a measure of brain network integration) compared to controls and patients with little to no cognitive impairment. They found that higher FSIQ was associated with higher clustering and lower path lengths, but there was no significant relationship between whole brain white matter volumes and IQ (see Figure 5). They suggest that microstructural measures of network topology may provide a more sensitive measure of impairment than whole brain white matter volume alone. Using a broad range of neuropsychological measures, Alexander et al. (53) found that increased FA of the left fornix was related to higher processing speed, yet when analyzing memory tasks they did not find any significant correlations. In further analysis of their results, the authors found that the association between the left fornix and processing speed was only present in patients without hippocampal sclerosis.
The relationship between DTI measures and global intelligence has also been examined in children with epilepsy. In a study described previously, Widjaja et al. (46) took a broader look at cognitive function in children with localization related epilepsy (LRE) and found that FA of the CC was associated with both verbal IQ and performance IQ. In general, the authors note that LRE of childhood onset is associated with more global cognitive deficits compared to LRE of adult onset, and that aberrant development of critical white matter tracts may underlie these broader impairments. Evidence of atypical diffusion and its relationship to poorer cognition in children with epilepsy suggests that seizures or other precipitating events may disrupt the typical developmental trajectories of many white matter tracts early on, and this may underlie an atypical course of cognitive development for children with epilepsy. Longitudinal studies of DTI and cognition in pediatric populations can shed light on these issues, while also providing greater insight into the nature of structural and functional reorganization in children with specific epilepsy syndromes. Ultimately, this information will facilitate our understanding of the nature of structure-function relationships in epilepsy across the lifespan.
Current limitations of DTI and the promise of advanced diffusion imaging for better understanding network dysfunction in TLE
Since its inception, DTI has generated considerable interest among researchers and clinicians for its unique ability to probe the underlying structure of white matter and to quantify the nature and location of network dysfunction in patients with epilepsy. Despite its promise, there are several limitations of the tensor model that may lessen its clinical application and the degree to which DTI measures relate to clinical variables.
First, standard DTI has a low spatial resolution relative to the underlying white matter structure. That is, the voxel size is typically 2-3 mm and the tensor model assumes that all fibers within a voxel are well-described by a single orientation estimate. In reality, a single voxel is likely to include multiple fiber tracts with unique orientations, and therefore, DTI is not well equipped to resolve crossing fibers. Second, partial voluming of gray matter, white matter, and cerebrospinal fluid is often problematic, complicating the measurement of tissue structure, particularly in peripheral white matter and gray matter (54). Third, the standard tensor models diffusion as a Gaussian distribution (55), which does not appear to reflect biological reality. A number of additional limitations of DTI include distortions resulting from motion, eddy currents, and inhomogeneous static magnetic fields. For excellent reviews of both technical and model-based limitations of DTI, see Winston (56) and Abhinav et al. (57).
These known limitations of DTI have led to the emergence of a number of advanced diffusion models with greater promise for resolving crossing fibers and quantifying more specific intravoxel tissue properties. Among these advanced diffusion measures, the ball-and-stick model, diffusion kurtosis imaging (DKI) and restriction spectrum imaging (RSI) have gained recent popularity for measuring network pathology associated with neurosurgical populations and neurodegenerative disease (57). The ball-and-stick method is a specific form of multiple tensors that models free diffusion (ball) and axons (sticks) and is implemented within the FSL software package. DKI is another extension of conventional DTI, which utilizes multiple diffusion weightings (b-values) to quantify the non-monoexponential decay in diffusion data and is sensitive to changes in non-Gaussian components of water diffusion (58). RSI is a model-based, multiple b-value approach that can separate cylindrically-restricted diffusion within axons from other tissue properties (e.g., vasogenic edema), allowing for improved visualization and quantification of fiber tracts in regions of edema or associated pathology (59). These are just a few of the emerging methods that may provide additional insight into the complex mechanisms underlying network damage in epilepsy.
These advanced diffusion techniques have only begun to emerge within the epilepsy literature and be used to examine network dysfunction in TLE. Lee et al. (60) used DKI to examine the microstructural integrity of early myelinating versus late myelinating tracts in TLE and found greater reductions in mean kurtosis (MK), parallel kurtosis, and axial kurtosis in late vs early myelinating tracts, including the ILF and SLF. Furthermore, kurtosis metrics were more sensitive to group differences in late myelinating tracts relative to FA, which may reflect their greater sensitivity to complex changes in heterogeneous tissue compartments (i.e., axonal loss and permeability changes between intra- and extra-axonal tissue compartments) relative to standard diffusion measures. Thus, advanced diffusion measures show great promise for improving the quantification of fiber bundles with complex anatomy as well as providing more specific measures of pathology in TLE, and it is possible that they will also provide greater insight into how network changes lead to cognitive dysfunction in TLE.
Summary and future directions
Over the past two decades, DTI has provided considerable insight into the relationship between network changes and cognitive dysfunction in TLE, particularly with respect to language and memory. In concert with fMRI, DTI has increased our understanding of pre- and postoperative reorganization of language and memory networks and indicated the possibility that these adaptive network changes may occur both inter- or intrahemispherically. In particular, language studies have shown that, in addition to the ARC, a number of other frontotemporal tracts including the UNC, ILF and IFOF are important for naming and fluency performances. Furthermore, DTI studies have demonstrated that in addition to the hippocampus, the UNC, ILF, ARC, PHC, IFOF and fornix appear to be part of a complex memory network. The literature has been far less conclusive as to which white matter networks are associated with executive dysfunction and global cognitive impairment in TLE, or how changes in white matter networks following ATL influence these domains. These studies have been complicated by relatively small sample sizes, the diverse aspects of executive functioning surveyed in the literature (i.e., inhibition, task switching, working memory, etc.) and heterogeneous patient cohorts. Thus, future studies that address intricate associations between white matter integrity and these more complex cognitive functions in TLE are greatly needed.
Finally, there are a myriad of unanswered questions of theoretical and clinical importance to the field of epilepsy that DTI studies are uniquely poised to address, alone or in combination with other imaging modalities. Among the most provocative are the following: What are the network changes that occur in response to initial precipitating injuries (IPIs) in epilepsy, and how do these changes set the course for functional reorganization? Can specific preoperative markers of network integrity in TLE help to quantify individual risk of cognitive decline following different surgical approaches (e.g., ATL versus laser ablation)? Does cognitive rehabilitation of memory or other cognitive impairments in TLE result in adaptive network changes that map onto measurable cognitive gains? Answering these questions could have a pivotal impact on the field by providing insight into the development of cognitive dysfunction in TLE, as well as how cognitive networks are altered by surgical, pharmacological, and neuropsychological interventions. In doing so, multi-site projects with larger patient samples and funding structures that encourage both reproducibility and translational applications will be essential for obtaining results that are both statistically and scientifically meaningful.
Conclusions
Improving structural-cognitive correlations in TLE will likely depend on a variety of factors that include further development of imaging hardware, diffusion models, and post-processing algorithms/analytic models. As noted, advanced diffusion models show great promise for providing better quantitative data with regards to crossing fibers and intravoxel tissue microstructure, and they may strengthen microstructural associations with cognition in TLE. However, most cognitive processes are complex and likely recruit multiple cortical and subcortical networks that may vary across individuals (61). Furthermore, partial or atypical reorganization of function may occur in many patients, further complicating even the more reliable structure-function associations (i.e., language). Given that no single neuroimaging measure is without limitations, multimodal imaging studies that combine information from functional, structural, and diffusion imaging may provide the greatest insight into network dysfunction in patients with TLE.
Acknowledgements
The work was supported by the National Institute of Neurological Disorders and Stroke (R01NS065838 to CR McDonald) and the Epilepsy Foundation Pre-Doctoral Research Training Fellowship (#299443, NE Kucukboyaci).
Disclosure: The authors declare no conflict of interest.
References
- Powell HW, Duncan JS. Functional magnetic resonance imaging for assessment of language and memory in clinical practice. Curr Opin Neurol 2005;18:161-6. [PubMed]
- Powell HW, Parker GJ, Alexander DC, Symms MR, Boulby PA, Barker GJ, Thompson PJ, Koepp MJ, Duncan JS. Imaging language pathways predicts postoperative naming deficits. J Neurol Neurosurg Psychiatry 2008;79:327-30. [PubMed]
- Powell HW, Parker GJ, Alexander DC, Symms MR, Boulby PA, Wheeler-Kingshott CA, Barker GJ, Koepp MJ, Duncan JS. Abnormalities of language networks in temporal lobe epilepsy. Neuroimage 2007;36:209-21. [PubMed]
- Powell HW, Richardson MP, Symms MR, Boulby PA, Thompson PJ, Duncan JS, Koepp MJ. Preoperative fMRI predicts memory decline following anterior temporal lobe resection. J Neurol Neurosurg Psychiatry 2008;79:686-93. [PubMed]
- Duffau H. Toward the application of the hodotopical concept to epilepsy surgery. World Neurosurg 2011;75:431-3. [PubMed]
- Piper RJ, Yoong MM, Kandasamy J, Chin RF. Application of diffusion tensor imaging and tractography of the optic radiation in anterior temporal lobe resection for epilepsy: a systematic review. Clin Neurol Neurosurg 2014;124:59-65. [PubMed]
- Winston GP, Yogarajah M, Symms MR, McEvoy AW, Micallef C, Duncan JS. Diffusion tensor imaging tractography to visualize the relationship of the optic radiation to epileptogenic lesions prior to neurosurgery. Epilepsia 2011;52:1430-8. [PubMed]
- Ahmadi ME, Hagler DJ Jr, McDonald CR, Tecoma ES, Iragui VJ, Dale AM, Halgren E. Side matters: diffusion tensor imaging tractography in left and right temporal lobe epilepsy. AJNR Am J Neuroradiol 2009;30:1740-7. [PubMed]
- Concha L, Beaulieu C, Collins DL, Gross DW. White-matter diffusion abnormalities in temporal-lobe epilepsy with and without mesial temporal sclerosis. J Neurol Neurosurg Psychiatry 2009;80:312-9. [PubMed]
- Gross DW, Concha L, Beaulieu C. Extratemporal white matter abnormalities in mesial temporal lobe epilepsy demonstrated with diffusion tensor imaging. Epilepsia 2006;47:1360-3. [PubMed]
- Kemmotsu N, Girard HM, Bernhardt BC, Bonilha L, Lin JJ, Tecoma ES, Iragui VJ, Hagler DJ Jr, Halgren E, McDonald CR. MRI analysis in temporal lobe epilepsy: cortical thinning and white matter disruptions are related to side of seizure onset. Epilepsia 2011;52:2257-66. [PubMed]
- Rodrigo S, Oppenheim C, Chassoux F, Hodel J, de Vanssay A, Baudoin-Chial S, Devaux B, Meder JF. Language lateralization in temporal lobe epilepsy using functional MRI and probabilistic tractography. Epilepsia 2008;49:1367-76. [PubMed]
- Lin JJ, Mula M, Hermann BP. Uncovering the neurobehavioural comorbidities of epilepsy over the lifespan. Lancet 2012;380:1180-92. [PubMed]
- Upadhyay J, Hallock K, Ducros M, Kim DS, Ronen I. Diffusion tensor spectroscopy and imaging of the arcuate fasciculus. Neuroimage 2008;39:1-9. [PubMed]
- Hasan KM, Iftikhar A, Kamali A, Kramer LA, Ashtari M, Cirino PT, Papanicolaou AC, Fletcher JM, Ewing-Cobbs L. Development and aging of the healthy human brain uncinate fasciculus across the lifespan using diffusion tensor tractography. Brain Res 2009;1276:67-76. [PubMed]
- Ashtari M. Anatomy and functional role of the inferior longitudinal fasciculus: a search that has just begun. Dev Med Child Neurol 2012;54:6-7. [PubMed]
- Caverzasi E, Papinutto N, Amirbekian B, Berger MS, Henry RG. Q-ball of inferior fronto-occipital fasciculus and beyond. PLoS One 2014;9:e100274. [PubMed]
- McDonald CR, Ahmadi ME, Hagler DJ, Tecoma ES, Iragui VJ, Gharapetian L, Dale AM, Halgren E. Diffusion tensor imaging correlates of memory and language impairments in temporal lobe epilepsy. Neurology 2008;71:1869-76. [PubMed]
- Pustina D, Doucet G, Evans J, Sharan A, Sperling M, Skidmore C, Tracy J. Distinct types of white matter changes are observed after anterior temporal lobectomy in epilepsy. PLoS One 2014;9:e104211. [PubMed]
- Wang XQ, Lang SY, Hong LU, Lin MA, Yan-ling MA, Yang F. Changes in extratemporal integrity and cognition in temporal lobe epilepsy: a diffusion tensor imaging study. Neurol India 2010;58:891-9. [PubMed]
- Kucukboyaci NE, Kemmotsu N, Leyden KM, Girard HM, Tecoma ES, Iragui VJ, McDonald CR. Integration of multimodal MRI data via PCA to explain language performance. Neuroimage Clin 2014;5:197-207. [PubMed]
- Powell HW, Parker GJ, Alexander DC, Symms MR, Boulby PA, Wheeler-Kingshott CA, Barker GJ, Noppeney U, Koepp MJ, Duncan JS. Hemispheric asymmetries in language-related pathways: a combined functional MRI and tractography study. Neuroimage 2006;32:388-99. [PubMed]
- Vernooij MW, Smits M, Wielopolski PA, Houston GC, Krestin GP, van der Lugt A. Fiber density asymmetry of the arcuate fasciculus in relation to functional hemispheric language lateralization in both right- and left-handed healthy subjects: a combined fMRI and DTI study. Neuroimage 2007;35:1064-76. [PubMed]
- Ellmore TM, Beauchamp MS, Breier JI, Slater JD, Kalamangalam GP, O'Neill TJ, Disano MA, Tandon N. Temporal lobe white matter asymmetry and language laterality in epilepsy patients. Neuroimage 2010;49:2033-44. [PubMed]
- Yogarajah M, Focke NK, Bonelli SB, Thompson P, Vollmar C, McEvoy AW, Alexander DC, Symms MR, Koepp MJ, Duncan JS. The structural plasticity of white matter networks following anterior temporal lobe resection. Brain 2010;133:2348-64. [PubMed]
- Hermann B, Seidenberg M, Lee EJ, Chan F, Rutecki P. Cognitive phenotypes in temporal lobe epilepsy. J Int Neuropsychol Soc 2007;13:12-20. [PubMed]
- Hermann B, Seidenberg M. Epilepsy and cognition. Epilepsy Curr 2007;7:1-6. [PubMed]
- Lui YW, Nusbaum AO, Barr WB, Johnson G, Babb JS, Orbach D, Kim A, Laliotis G, Devinsky O. Correlation of apparent diffusion coefficient with neuropsychological testing in temporal lobe epilepsy. AJNR Am J Neuroradiol 2005;26:1832-9. [PubMed]
- Girard HM, Kemmotsu N, Kucukboyaci NE, Tecoma ES, Iragui VJ, McDonald CR. Diffusion of subcortical structures enhances the prediction of verbal memory performance and seizure lateralization in mesial temporal lobe epilepsy. American Epilepsy Society Annual Meeting. 2011:abstr 1.241.
- Von Der Heide RJ, Skipper LM, Klobusicky E, Olson IR. Dissecting the uncinate fasciculus: disorders, controversies and a hypothesis. Brain 2013;136:1692-707. [PubMed]
- Diehl B, Busch RM, Duncan JS, Piao Z, Tkach J, Lüders HO. Abnormalities in diffusion tensor imaging of the uncinate fasciculus relate to reduced memory in temporal lobe epilepsy. Epilepsia 2008;49:1409-18. [PubMed]
- Riley JD, Franklin DL, Choi V, Kim RC, Binder DK, Cramer SC, Lin JJ. Altered white matter integrity in temporal lobe epilepsy: association with cognitive and clinical profiles. Epilepsia 2010;51:536-45. [PubMed]
- McDonald CR, Leyden KM, Hagler DJ, Kucukboyaci NE, Kemmotsu N, Tecoma ES, Iragui VJ. White matter microstructure complements morphometry for predicting verbal memory in epilepsy. Cortex 2014;58:139-50. [PubMed]
- Voets NL, Adcock JE, Stacey R, Hart Y, Carpenter K, Matthews PM, Beckmann CF. Functional and structural changes in the memory network associated with left temporal lobe epilepsy. Hum Brain Mapp 2009;30:4070-81. [PubMed]
- Voets NL, Zamboni G, Stokes MG, Carpenter K, Stacey R, Adcock JE. Aberrant functional connectivity in dissociable hippocampal networks is associated with deficits in memory. J Neurosci 2014;34:4920-8. [PubMed]
- Bonelli SB, Powell RH, Yogarajah M, Samson RS, Symms MR, Thompson PJ, Koepp MJ, Duncan JS. Imaging memory in temporal lobe epilepsy: predicting the effects of temporal lobe resection. Brain 2010;133:1186-99. [PubMed]
- Cheung MC, Chan AS, Lam JM, Chan YL. Pre- and postoperative fMRI and clinical memory performance in temporal lobe epilepsy. J Neurol Neurosurg Psychiatry 2009;80:1099-106. [PubMed]
- Rabin ML, Narayan VM, Kimberg DY, Casasanto DJ, Glosser G, Tracy JI, French JA, Sperling MR, Detre JA. Functional MRI predicts post-surgical memory following temporal lobectomy. Brain 2004;127:2286-98. [PubMed]
- Richardson MP, Strange BA, Thompson PJ, Baxendale SA, Duncan JS, Dolan RJ. Pre-operative verbal memory fMRI predicts post-operative memory decline after left temporal lobe resection. Brain 2004;127:2419-26. [PubMed]
- Binder JR, Sabsevitz DS, Swanson SJ, Hammeke TA, Raghavan M, Mueller WM. Use of preoperative functional MRI to predict verbal memory decline after temporal lobe epilepsy surgery. Epilepsia 2008;49:1377-94. [PubMed]
- Lezak MD, Lezak MD. eds. Neuropsychological assessment. 4th Edition. New York: Oxford University Press, 2004.
- Stretton J, Thompson PJ. Frontal lobe function in temporal lobe epilepsy. Epilepsy Res 2012;98:1-13. [PubMed]
- Dabbs K, Jones J, Seidenberg M, Hermann B. Neuroanatomical correlates of cognitive phenotypes in temporal lobe epilepsy. Epilepsy Behav 2009;15:445-51. [PubMed]
- Winston GP, Stretton J, Sidhu MK, Symms MR, Duncan JS. Progressive white matter changes following anterior temporal lobe resection for epilepsy. Neuroimage Clin 2013;4:190-200. [PubMed]
- Wang XQ, Iang SY, Lu H, Ma L, Mao YL, Yang F. Executive function impairment in patients with temporal lobe epilepsy: neuropsychological and diffusion-tensor imaging study. Zhonghua Yi Xue Za Zhi 2007;87:3183-7. [PubMed]
- Widjaja E, Skocic J, Go C, Snead OC, Mabbott D, Smith ML. Abnormal white matter correlates with neuropsychological impairment in children with localization-related epilepsy. Epilepsia 2013;54:1065-73. [PubMed]
- Riley JD, Moore S, Cramer SC, Lin JJ. Caudate atrophy and impaired frontostriatal connections are linked to executive dysfunction in temporal lobe epilepsy. Epilepsy Behav 2011;21:80-7. [PubMed]
- Winston GP, Stretton J, Sidhu MK, Symms MR, Thompson PJ, Duncan JS. Structural correlates of impaired working memory in hippocampal sclerosis. Epilepsia 2013;54:1143-53. [PubMed]
- Stretton J, Winston GP, Sidhu M, Bonelli S, Centeno M, Vollmar C, Cleary RA, Williams E, Symms MR, Koepp MJ, Thompson PJ, Duncan JS. Disrupted segregation of working memory networks in temporal lobe epilepsy. Neuroimage Clin 2013;2:273-81. [PubMed]
- Kucukboyaci NE, Girard HM, Hagler DJ Jr, Kuperman J, Tecoma ES, Iragui VJ, Halgren E, McDonald CR. Role of frontotemporal fiber tract integrity in task-switching performance of healthy controls and patients with temporal lobe epilepsy. J Int Neuropsychol Soc 2012;18:57-67. [PubMed]
- Martin RC, Sawrie SM, Edwards R, Roth DL, Faught E, Kuzniecky RI, Morawetz RB, Gilliam FG. Investigation of executive function change following anterior temporal lobectomy: selective normalization of verbal fluency. Neuropsychology 2000;14:501-8. [PubMed]
- Vaessen MJ, Jansen JF, Vlooswijk MC, Hofman PA, Majoie HJ, Aldenkamp AP, Backes WH. White matter network abnormalities are associated with cognitive decline in chronic epilepsy. Cereb Cortex 2012;22:2139-47. [PubMed]
- Alexander RP, Concha L, Snyder TJ, Beaulieu C, Gross DW. Correlations between Limbic White Matter and Cognitive Function in Temporal-Lobe Epilepsy, Preliminary Findings. Front Aging Neurosci 2014;6:142. [PubMed]
- Kang X, Herron TJ, Turken AU, Woods DL. Diffusion properties of cortical and pericortical tissue: regional variations, reliability and methodological issues. Magn Reson Imaging 2012;30:1111-22. [PubMed]
- Basser PJ. Relationships between diffusion tensor and q-space MRI. Magn Reson Med 2002;47:392-7. [PubMed]
- Winston GP. The physical and biological basis of quantitative parameters derived from diffusion MRI. Quant Imaging Med Surg 2012;2:254-65. [PubMed]
- Abhinav K, Yeh FC, Pathak S, Suski V, Lacomis D, Friedlander RM, Fernandez-Miranda JC. Advanced diffusion MRI fiber tracking in neurosurgical and neurodegenerative disorders and neuroanatomical studies: A review. Biochim Biophys Acta 2014;1842:2286-97.
- Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K. Diffusional kurtosis imaging: the quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med 2005;53:1432-40. [PubMed]
- McDonald CR, White NS, Farid N, Lai G, Kuperman JM, Bartsch H, Hagler DJ, Kesari S, Carter BS, Chen CC, Dale AM. Recovery of white matter tracts in regions of peritumoral FLAIR hyperintensity with use of restriction spectrum imaging. AJNR Am J Neuroradiol 2013;34:1157-63. [PubMed]
- Lee CY, Tabesh A, Spampinato MV, Helpern JA, Jensen JH, Bonilha L. Diffusional kurtosis imaging reveals a distinctive pattern of microstructural alternations in idiopathic generalized epilepsy. Acta Neurol Scand 2014;130:148-55. [PubMed]
- Hagmann P, Jonasson L, Maeder P, Thiran JP, Wedeen VJ, Meuli R. Understanding diffusion MR imaging techniques: from scalar diffusion-weighted imaging to diffusion tensor imaging and beyond. Radiographics 2006;26 Suppl 1:S205-23. [PubMed]


