Utility of magnetic resonance spectroscopic imaging for human epilepsy
Introduction
As well stated by many others, the impact of magnetic resonance (MR) imaging—starting in ~1980’s—for the clinical and scientific investigation of epilepsy has been profound. Since then, its importance has been maintained, with higher contrast and resolution in structural imaging being increasingly important towards the detection of ever smaller lesions. More recently however over the past 15 years+ has been wide spread appreciation of the sensitivity of the MR signal to key aspects of cerebral metabolism and physiology. This has been accompanied by continued technological development of many aspects of imaging, such as ultra-high field, signal-to-noise improvements and image acceleration.
MR spectroscopy MRS, which is unique in its ability to evaluate many cerebral compounds associated with brain metabolism, in particular has substantially benefited from such development. For epilepsy, given its commonly aberrant metabolism, it has long been thought that MRS could be informative. Interest in the MR spectroscopy of epilepsy thus arises from the interesting metabolic pathophysiology of hyperexcitable networks in addition to the very clinical need to better identify the seizure onset zone, which may be identified by such measurements. As is well known, the challenge of identifying this brain area can be high, as many “localization related epilepsy” patients can exhibit structural MRI that is either negative or is ambiguous. In this chapter we review what MRS means and some of its methodologic issues, including various approaches that MR spectroscopic studies have taken for epilepsy, and then findings in recent studies that suggest that there could be a clinical role for MRS in epilepsy.
Spectroscopy: what it means and how it is done
MR spectroscopy is one of the oldest types of magnetic resonance study, and is unique in its ability to evaluate the chemical composition of the brain. While there are a number of magnetically detectable nuclei including 31P, 23Na, 13C and others, 1H has the largest gyromagnetic ratio and being at 100% abundance, has the greatest sensitivity for both experimental and clinical use. We refer the reader to many excellent texts on MR science for more details on formation of images, etiology and implementation of contrast (1,2), detector and hardware requirements, focusing here on issues specific to 1H spectroscopy and spectroscopic imaging for epilepsy. Spectroscopy, by virtue of the need and interest to resolve between different chemical compounds—rather than acquiring data solely from the protons of water as is the case for all structural and functional MRI—introduces a significant fundamental complexity (hardware, software and analysis) to the MR imaging session. It is ironic that this oldest of physical methodologies has been amongst the more difficult to implement routinely. Nonetheless, with continued technological MR developments, the ability to routinely obtain high quality MR spectroscopic imaging studies is becoming much more feasible than even 5 years ago.
MR spectroscopy implies the presence of the chemical shift axis, that which separates different compounds. Just as the lipid signal needs to resolved from the water signal in certain types of MR imaging, spectroscopy places a higher requirement on the magnetic field homogeneity (B0) and detector performance (B1) so as to accurately and cleanly resolve compounds that could otherwise obscure each other. Interestingly in the early 1990’s, many MR systems were equipped to be able to perform at this level such that there were a number of early papers on MRS in epilepsy, finding tantalizing results [e.g., (3,4)]. However, vendors realized that MRI of water is simpler to manage, and so it has not been until again more recently with higher field (3T, 7T) has there been a return of equipment performance able to handle MR spectroscopy and spectroscopic imaging.
What is being measured?
While in theory many compounds are detectable by MR spectroscopy, the requirement for visibility means that only those 1H protons that are of relatively high concentration (~1 to 10 mm) and “tumble” adequately quickly on the NMR time scale in the intracellular milieu will be detected. As a result, these compounds typically include creatine, N-acetyl aspartate (NAA), choline and choline moieties, lactate, myo-inositol, glutamate, GABA, glutamine and others. By virtue of their interesting metabolic characteristics, robust detection and high concentrations, creatine, NAA and choline have found the greatest applicability for human use. NAA is synthesized only in neuronal mitochondria (5,6) and has been strongly correlated with oxidative metabolism (7-10). Many studies of brain disorders have found NAA to be an informative measure of neuronal function. Creatine, as a key component of phosphocreatine to be highly useful as a normalization factor for many bioenergetic parameters, being consistent over a cross section of species and within a given tissue type (11). While found in both neurons and astrocytes, the highest concentration of creatine is in the astrocytes and thus when considering an integrated unit of neuronal function, many groups have used the ratio of NAA/Cr as a normalized parameter. We characterize NAA/Cr as reflecting the bioenergetics of the neuronal/glial unit (bGNU), finding it to be very informative for identifying regions of energetic and neuronal dysfunction (10,12-14). Choline and choline containing compounds have been found to be elevated in conditions of increased cellular membrane concentrations and cellular turnover and thus NAA/Cho has been a key parameter of interest in tumors and multiple sclerosis.
Other compounds remain of great interest, e.g., myo-inositol commonly increased in settings of even mild edema, glutamate in its role as excitatory transmitter and bioenergetic metabolite, GABA as the major inhibitory neurotransmitter, and thus we anticipate continued work focusing on these. However, owing to these compounds more complex chemical structure, they are less robustly detected and thus it remains true that for many studies including epilepsy, creatine, NAA and choline are the workhorse compounds of interest.
The comparison between MRSI and 18fluoro-deoxyglucose-PET (FDG-PET) is inevitable, and while these are clearly complementary measurements, it is worthwhile considering some of the pertinent differences and similarities. FDG PET evaluates total glucose consumption, and has been long used to measure differences in glucose consumption in varying tissue types [FDG uptake in gray matter is approximately 3× that of white matter (15)] and with various states of activation. The amount of uptake reflects the amount of tissue present in the voxel of interest, and thus decreases in uptake can result from both decreased cerebral consumption and/or tissue atrophy (with potential for distortion of white and gray matter contributions). There is commonly variation in voxel size or point spread function with location in PET, but given improvements with human high resolution research tomograph (HRRT) cameras, the voxel resolution has improved to better than 2 to 3 mm in-plane resolution (16). In epilepsy, the success rate of FDG PET in identifying the region of seizure onset very much depends on the population studied with the best localization rates of ~80-90% in temporal lobe epilepsy, although it is probably less successful in the nonlesional neocortical epilepsy patients [for review (17-19)]. This lesser success rate reflects the challenge in neocortical epilepsy, with the region of seizure onset commonly much less well defined, with variations in propagation paths and volume of injury.
In MRSI, the use of the NAA/Cr ratio largely eliminates the sensitivity of the parameter to tissue volume due to minimal NAA and Cr in the cerebrospinal fluid (CSF); however there is some sensitivity to tissue type (gray, white matter), with the majority of workers finding that NAA/Cr is smaller in gray than in white matter [reflecting primarily a higher creatine concentration in gray (20-22)]. With most sampling strategies, the sampling volume of MRSI is regionally constant, and for practicable time limits of study, are typically 0.64 to 2 cc. Thus in epilepsy, where tissue atrophy can be variably gross or subtle, the ability of MRSI to detect dysfunction using the bGNU with tissue type correction is potentially excellent as it does not require use of asymmetry indices and is relatively independent of tissue atrophy.
Single voxel vs. spectroscopic imaging
A major challenge in the field of MRS has been the choice of single voxel versus spectroscopic imaging approaches for acquisition. For epilepsy, single voxel spectroscopy is primarily of use where the region of seizure onset is already known. With well positioned and adequately small voxel sizes to minimize dilutional effects from adjacent normal (gray and white) tissue, such studies can be informative. It is clear however that for many cases of epilepsy where the abnormalities may be both small and cryptic, spectroscopic imaging is a necessary and more informative approach. The challenges of spectroscopic imaging however are high, requiring excellent performance in both B0 field homogeneity and B1 amplitudes, these components not commonly a problem for many types of structural imaging. However for many advanced imaging systems, the performance of these components has been optimized through combinations of hardware and acquisition methods, and results from these are demonstrated in the studies below.
NAA/Cr abnormalities in MTLE
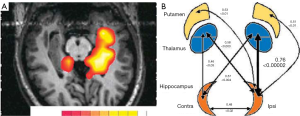
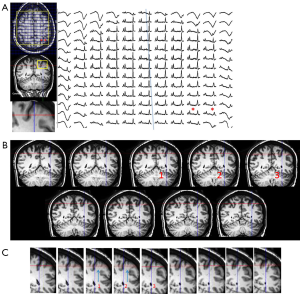
The first studies performed in the early 90’s established the ability of 1H-MRS to lateralize the seizure focus in temporal lobes and to test the validity of MRSI to already established methods of localization, using video-EEG as gold standard as well as MRI-volumetry (MRIV), FDG-PET and SPECT. Hugg 1993 (23) initially demonstrated a significant asymmetry of NAA left/right metabolite ratios and further studies have demonstrated comparable results. These studies demonstrate a decrease in NAA in the affected temporal lobe of TLE patients. Kuzniecky 1998 (24) investigated the validity of 1H-MRS in lateralizing the pathologic area in 30 consecutive preoperative TLE patients with mesial temporal lobe sclerosis on MRI. Volumetry correctly lateralized 93% of patients while 1H-MRS did so in 97%. Concordance between the described MRI modalities was 73%. Comparable results were described by Cendes 1997 (25) in 100 consecutive patients with intractable mesial TLE. In addition, 1H-MRS was also sensitive in detecting bilateral dysfunction. The degree of asymmetry in NAA/Cr ratios correlated with the degree of one sidedness of EEG abnormalities. These results support the fact that 1H-MRS is a valid method even in bilateral cases presenting a high concordance to the degree of bilateral EEG findings in patients with TLE. Figure 1 shows an example of such asymmetric bilaterality of metabolic dysfunction. It should also be noted that the temporal lobe abnormalities have also been identified with other imaging methods, including conventional and diffusion weighted imaging [e.g., (26-28)]. Recent data from Hetherington 2007 and Pan 2012 (29,30) has also demonstrated that the decrements in NAA are not just localized to the ipsilateral hippocampus, but consistent with existing PET finding a network of involved limbic and subcortical nuclei, as shown in Figure 1B.
While it is not surprising that metabolic dysfunction follows from uncontrolled seizure activity, it has also been known that mild to moderate oxidative stress can also cause major abnormalities in GABA (and glutamate). Saransaari and Oja 1997 (31) studied the mouse hippocampus with variable levels of peroxide stress to show increases in basal GABA release, ranging from 30 to more than 550%. These observations are of particular interest for epilepsy where GABA is thought to be a key component underlying the abnormal hyperexcitability. Given this and the known high energetic cost of neurotransmission and synaptic activity (32), we anticipated that GABA neurotransmission and metabolic function might be correlated, certainly in the seizure onset zone. This may be especially important given the recent in vitro work that has suggested GABA function may be either anti- or proconvulsant (33,34). This was evaluated in human epilepsy patients undergoing intracranial EEG and microdialysis analysis. Their microdialysis measurements of extracellular GABA (ecGABA) were compared to pre-operative measures of the bGNU, or NAA/Cr. All data from this study were acquired from the hippocampus, including patients with either hippocampal epilepsy or non-hippocampal (neocortical) epilepsy. Figure 2 shows the correlations from this study, finding very strong correlations between ecGABA and NAA/Cr. In the MTLE patients, ecGABA strongly negatively correlated with decreasing NAA/Cr, R=−0.94, P<0.001 and implies that ecGABA and mitochondrial function are largely representing parallel processes. There are numerous neurochemical and neurophysiological injury studies that specifically link metabolic function with GABA. For example, Nguyen and Picklo 2003 (36) suggested that injury-produced lipid peroxidation products inhibit the TCA cycle enzyme succinic semialdehyde dehydrogenase (SSAD, which metabolically degrades GABA). This would result in both decreased TCA cycle flow and decreased GABA clearance. Alternatively, electrophysiological data have suggested that in the context of injury and dysfunctional chloride transport, ecGABA can become excitatory (33) and can further propagate seizure-linked injury. While these data cannot specify which or several of these injurious processes may be ongoing in the seizure onset zone, it is evident that the relationship of ecGABA with NAA/Cr within the ipsilateral hippocampus is clearly distinctive in comparison to ecGABA correlations in the non-MTLE group.
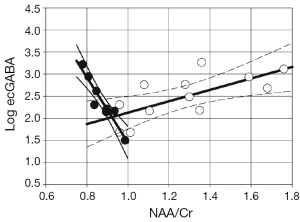
In the neocortical epilepsy patients and outside of the seizure onset zone, the relationship between ecGABA and NAA/Cr, is also significant but positive with R=+0.70, P<0.015. In these neocortical patients, the hippocampus being studied was ipsilateral to the cortical seizure onset region and thus may be a site of proximal propagation. Nonetheless, as it is not the seizure focus, these data remain conceptually similar to the results of Petroff 2001 (37), who found that well outside the seizure focus (studying the occipital lobe), patients with better seizure control have higher tissue GABA levels. Similar to the above, these correlational data do not permit the determination of the specific relationship between ecGABA and mitochondrial function. However as these data were made in relatively healthier tissue, the physiological relationships that link these parameters are likely to be different in comparison to that seen in the seizure focus. For example, a potentially common source for increased ecGABA even in healthy brain has been suggested from the readily reversible GABA transporter GAT-1 (38).
Thus comparing the in vivo human ecGABA and bGNU measure is consistent with rodent data showing relationships between metabolic injury with abnormal ecGABA concentrations, and probably neurotransmission as well. It is of note however that it is the hippocampal epilepsy patients (filled circles of Figure 2) that shows the increased ecGABA and is therefore consistent with the directionality of mitochondrial function causing elevated ecGABA as suggested from (31). The data from the non-hippocampal epilepsy patients may be more reflective of normal GABA and metabolic inter-dependence, although it is clear that more work will be needed to better understand the poise of these relationships.
MRS work in patients with structurally identified cortical malformations has revealed that NAA changes are specific to the epileptogenic zone, and are “normal” in patients whose seizures are well controlled. Furthermore, in patients with on-going seizures, the regions which are anatomically abnormal but nonepileptogenic also display normal NAA levels (39).
Neocortical epilepsy and prognostication of surgical outcome
While temporal lobe epilepsy, and in particular medial temporal lobe epilepsy is reasonably well defined from a perspective of anatomy and clinical seizure type, localization of extra-temporal and neocortical epilepsies is commonly much more challenging. This arises in part from the well-defined size, relative clarity and consistency of medial temporal lobe structures, enabling better identification of abnormalities. For neocortical epilepsy it is not surprising, given the large volume of neocortex that has potential for seizure onset (either MRI-negative, or MRI-ambiguous), the potential for variable propagation paths and variation between different subjects for volume of seizure onset, that this is a much more challenging problem. The promise is clearly there; for example, with structurally identified cortical malformations, Kuzniecky 1997 (39) showed that NAA is informative towards seizure onset, rather than structural abnormality alone. Other studies with non-lesional cases have been also suggestive but less clear; e.g., from earlier studies of MRS in patients with normal MRI anatomy, multiple studies have shown a reduction of NAA in the affected hemisphere [e.g., (40-42)]. The results of these studies and others show that 1H-MRS is able to contribute valuable information for assessing the affected hemisphere in MRI negative TLE patients.
Outcomes is another key test for such methods, and in the clearer clinical group of MTLE, several groups (43,44) have demonstrated that bilateral metabolic alterations in TLE patients with hippocampal sclerosis have a predictive value for postoperative outcome. Patients demonstrating severe bilateral or even metabolic changes contralateral to planned removal had a poorer postoperative seizure outcome than patients demonstrating unilateral metabolite alterations ipsilateral to the hemisphere in which surgery was performed. The results indicated that contralateral changes in 1H-MRS are a good predictor for poor seizure outcome. Thus far however, for neocortical epilepsy this type of outcomes prediction has been more difficult.
The challenges for MRSI for neocortical epilepsy are substantial, given the wide variety of pathologies and commonly limited clinical localization information. It may be argued that from the above cited studies in non-lesional epilepsy cases, the sensitivity of MRS has been moderate, although there is evidence for a clear tendency for reduced NAA in the involved hemisphere. However from the imaging perspective, there have also been uncertainties, e.g., the needed volume resolution pertinent for the large majority of cases or the expected severity of injury is not well defined. Thus with minimal a priori knowledge, there is need for excellent spectral quality and spatial resolution over large areas of brain volume. Ongoing development in MR performance however raises possibilities for progress in this. For example, substantive improvements in image quality can be obtained with ultra-high field (7T) imaging, where the higher signal-to-noise ratio can enable smaller voxel sizes. An initial test of 7T MRSI has been reported (45) (Table 1), where the concordance between the regional bGNU (NAA/Cr) abnormality with region of surgical resection (none, partial or complete) was compared with patient outcome (ILAE classification dichotomized to I-III and IV-VI).
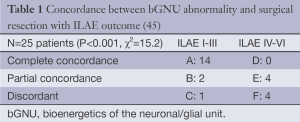
Full table
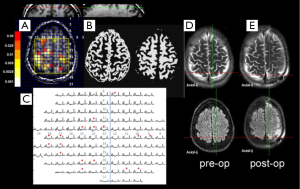
Figure 3 shows data from this report, showing a patient with neocortical epilepsy with a history of childhood meningitis who had unilateral (left) intracranial EEG coverage based on semiology. The resulting resection surgery included the L precuneus, which did overlap with the bGNU abnormality. Post-operatively this patient did well initially for 3 months, but has had an ILAE class IV outcome. In a group of 25 patients all of whom eventually underwent resective surgical treatment, there was concordance between the region of bGNU abnormality with resection region (i.e., did the region of resection overlap with metabolic abnormality or not) and eventual outcome. While it is clear that this is a small patient group, a Fisher’s exact 3×2 contingency statistical test found that the concordance between MRSI and surgical resection was significantly related to good outcome, P<0.001.
Where do we go from here? And why is it taking so long for implementation?
From an imaging perspective, it may be questioned as to how such studies may be optimally performed, in particular whether the 7T ultra-high field is requisite in comparison to the much more commonly available 3T platform. It should be noted however, that while the above work has been performed at 7T with developments in terms of field homogeneity and RF performance (46-48), such developments also have immediate impact for 3T or 4T. As Maudsley has published (49,50) and is relatively well known, even 3T suffers from similar (but somewhat milder) problems of field homogeneity especially in lower brain regions. Nonetheless, with optimized acquisitions, 3T can provide pertinent measurements; as shown in Figure 4 recently acquired at 3T, the NAA/Cr abnormality at the neocortical ribbon in the parietal lobe provoked a closer inspection of the MPRAGE T1 weight images to identify a subtle T1 hypointensity consistent with dysplasia.
So the dilemma for epilepsy is therefore reasonably clear. As a disorder that can vary substantially between different patients, commonly requires clinical and imaging scrutiny over large portions of the brain, requires high performance imaging equipment and expertise for acquisition and analysis, it is not surprising that epilepsy has thus far has received comparatively little effort from MR spectroscopic imaging community, and thus in turn, less focus from neurological experts. In comparison with other challenging neurological problems such as multiple sclerosis, dementia or neurodegenerative disorders, many of these conditions are not as demanding insofar as imaging for surgical management, e.g., the disorder is generally found in the same locus in all patients, does not require visualization in the cortical ribbon and/or caudal brain regions, and is not surgically quickly verifiable. Thus at this writing MRSI is performed for epilepsy only in a handful of interested academic imaging centers. The question that we have tried to address here is to what extent MRSI can be informative when executed with the best available effort. Clearly, while we have supported the pro- position—that MRSI can be highly informative—we also recognize that typical for any multi-component undertaking, MRSI is demanding in that each component needs to have a high rate of success. In all reality, whether or not MRSI can be implemented for broader use in epilepsy will depend on not just the above clinical and imaging requirements, but also economic factors that place a high premium on costs and patient throughput.
In this article, we have presented studies and data showing the added information that MRS has provided for understanding epilepsy and the challenge of seizure localization. It has been premised on metabolic interpretation, methodological improvements from field strength, acquisition and analysis. Most recently with approaches developed from 7T that can be adapted for 3T, we are suggesting that there is significant room for improvements for 3T performance for key brain regions important for epilepsy. With acceleration methods based on multi-banding or echo-planar sampling, the timeframe of acquisitions can be within acceptable from both patient and Radiology viewpoints. Overall, we anticipate that with focused effort on each component of this multi-component process, we anticipate that the bar for adequate performance of MRSI can be within reach, sufficient to establish its role in epilepsy localization. Given the sensitivity of the metabolic measurements to many aspects of seizure injury MRSI can provide an objective view of candidate regions of seizure injury, which may ultimately be useful for better understanding the network in the epileptic brain, surgery and outcomes prediction.
Acknowledgements
Funding: This work was supported by NIH R01 NS090417, EB011639 and FACES (Finding a Cure for Epilepsy and Seizures).
Disclosure: The authors declare no conflict of interest.
References
- Haacke EM, Brown RW, Thompson MR, Venkatesan R. Magnetic Resonance Imaging: Physical Principles and Sequence Design. New York: Wiley-Liss, 1999.
- Kuzniecky RI, Jackson GD. Magnetic Resonance in Epilepsy. Waltham: Academic Press, 2005.
- Cendes F, Andermann F, Preul MC, Arnold DL. Lateralization of temporal lobe epilepsy based on regional metabolic abnormalities in proton magnetic resonance spectroscopic images. Ann Neurol 1994;35:211-6. [PubMed]
- Bernasconi A, Tasch E, Cendes F, Li LM, Arnold DL. Proton magnetic resonance spectroscopic imaging suggests progressive neuronal damage in human temporal lobe epilepsy. Prog Brain Res 2002;135:297-304. [PubMed]
- Urenjak J, Williams SR, Gadian DG, Noble M. Specific expression of N-acetylaspartate in neurons, oligodendrocyte-type-2 astrocyte progenitors, and immature oligodendrocytes in vitro. J Neurochem 1992;59:55-61. [PubMed]
- Patel TB, Clark JB. Synthesis of N-acetyl-L-aspartate by rat brain mitochondria and its involvement in mitochondrial/cytosolic carbon transport. Biochem J 1979;184:539-46. [PubMed]
- Goldstein FB. The enzymatic synthesis of N-acetyl-L-aspartic acid by subcellular preparations of rat brain. J Biol Chem 1969;244:4257-60. [PubMed]
- Heales SJ, Davies SE, Bates TE, Clark JB. Depletion of brain glutathione is accompanied by impaired mitochondrial function and decreased N-acetyl aspartate concentration. Neurochem Res 1995;20:31-8. [PubMed]
- Bates TE, Strangward M, Keelan J, Davey GP, Munro PM, Clark JB. Inhibition of N-acetylaspartate production: implications for 1H MRS studies in vivo. Neuroreport 1996;7:1397-400. [PubMed]
- Pan JW, Takahashi K. Interdependence of N-acetyl aspartate and high-energy phosphates in healthy human brain. Ann Neurol 2005;57:92-7. [PubMed]
- Connett RJ. Analysis of metabolic control: new insights using scaled creatine kinase model. Am J Physiol 1988;254:R949-59. [PubMed]
- Muñoz Maniega S, Cvoro V, Chappell FM, Armitage PA, Marshall I, Bastin ME, Wardlaw JM. Changes in NAA and lactate following ischemic stroke: a serial MR spectroscopic imaging study. Neurology 2008;71:1993-9. [PubMed]
- Suhy J, Rooney WD, Goodkin DE, Capizzano AA, Soher BJ, Maudsley AA, Waubant E, Andersson PB, Weiner MW. 1H MRSI comparison of white matter and lesions in primary progressive and relapsing-remitting MS. Mult Scler 2000;6:148-55. [PubMed]
- Guevara CA, Blain CR, Stahl D, Lythgoe DJ, Leigh PN, Barker GJ. Quantitative magnetic resonance spectroscopic imaging in Parkinson's disease, progressive supranuclear palsy and multiple system atrophy. Eur J Neurol 2010;17:1193-202. [PubMed]
- Huisman MC, van Golen LW, Hoetjes NJ, Greuter HN, Schober P, Ijzerman RG, Diamant M, Lammertsma AA. Cerebral blood flow and glucose metabolism in healthy volunteers measured using a high-resolution PET scanner. EJNMMI Res 2012;2:63. [PubMed]
- Eggers C, Hilker R, Burghaus L, Schumacher B, Heiss WD. High resolution positron emission tomography demonstrates basal ganglia dysfunction in early Parkinson's disease. J Neurol Sci 2009;276:27-30. [PubMed]
- Spencer S, Huh L. Outcomes of epilepsy surgery in adults and children. Lancet Neurol 2008;7:525-37. [PubMed]
- Spencer SS. Long-term outcome after epilepsy surgery. Epilepsia 1996;37:807-13. [PubMed]
- Téllez-Zenteno JF, Dhar R, Wiebe S. Long-term seizure outcomes following epilepsy surgery: a systematic review and meta-analysis. Brain 2005;128:1188-98. [PubMed]
- Pouwels PJ, Frahm J. Regional metabolite concentrations in human brain as determined by quantitative localized proton MRS. Magn Reson Med 1998;39:53-60. [PubMed]
- Hetherington HP, Pan JW, Mason GF, Adams D, Vaughn MJ, Twieg DB, Pohost GM. Quantitative 1H spectroscopic imaging of human brain at 4.1 T using image segmentation. Magn Reson Med 1996;36:21-9. [PubMed]
- Schuff N, Ezekiel F, Gamst AC, Amend DL, Capizzano AA, Maudsley AA, Weiner MW. Region and tissue differences of metabolites in normally aged brain using multislice 1H magnetic resonance spectroscopic imaging. Magn Reson Med 2001;45:899-907. [PubMed]
- Hugg JW, Laxer KD, Matson GB, Maudsley AA, Weiner MW. Neuron loss localizes human temporal lobe epilepsy by in vivo proton magnetic resonance spectroscopic imaging. Ann Neurol 1993;34:788-94. [PubMed]
- Kuzniecky R, Hugg JW, Hetherington H, Butterworth E, Bilir E, Faught E, Gilliam F. Relative utility of 1H spectroscopic imaging and hippocampal volumetry in the lateralization of mesial temporal lobe epilepsy. Neurology 1998;51:66-71. [PubMed]
- Cendes F, Caramanos Z, Andermann F, Dubeau F, Arnold DL. Proton magnetic resonance spectroscopic imaging and magnetic resonance imaging volumetry in the lateralization of temporal lobe epilepsy: a series of 100 patients. Ann Neurol 1997;42:737-46. [PubMed]
- Concha L, Beaulieu C, Gross DW. Bilateral limbic diffusion abnormalities in unilateral temporal lobe epilepsy. Ann Neurol 2005;57:188-96. [PubMed]
- Seidenberg M, Kelly KG, Parrish J, Geary E, Dow C, Rutecki P, Hermann B. Ipsilateral and contralateral MRI volumetric abnormalities in chronic unilateral temporal lobe epilepsy and their clinical correlates. Epilepsia 2005;46:420-30. [PubMed]
- Araújo D, Santos AC, Velasco TR, Wichert-Ana L, Terra-Bustamante VC, Alexandre V Jr, Carlotti CG Jr, Assirati JA Jr, Machado HR, Walz R, Leite JP, Sakamoto AC. Volumetric evidence of bilateral damage in unilateral mesial temporal lobe epilepsy. Epilepsia 2006;47:1354-9. [PubMed]
- Hetherington HP, Kuzniecky RI, Vives K, Devinsky O, Pacia S, Luciano D, Vasquez B, Haut S, Spencer DD, Pan JW. A subcortical network of dysfunction in TLE measured by magnetic resonance spectroscopy. Neurology 2007;69:2256-65. [PubMed]
- Pan JW, Spencer DD, Kuzniecky R, Duckrow RB, Hetherington H, Spencer SS. Metabolic networks in epilepsy by MR spectroscopic imaging. Acta Neurol Scand 2012;126:411-20. [PubMed]
- Saransaari P, Oja SS. Enhanced GABA release in cell-damaging conditions in the adult and developing mouse hippocampus. Int J Dev Neurosci 1997;15:163-74. [PubMed]
- Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab 2001;21:1133-45. [PubMed]
- Woo NS, Lu J, England R, Dufour S, Mount DB, Deutch AY, Lovinger DM, Delpire E. Hyperexcitability and epilepsy associated with disruption of the mouse neuronal-specific K-Cl cotransporter gene. Hippocampus 2002;12:258-68. [PubMed]
- Palma E, Amici M, Sobrero F, Spinelli G, Di Angelantonio S, Ragozzino D, Mascia A, Scoppetta C, Esposito V, Miledi R, Eusebi F. Anomalous levels of Cl- transporters in the hippocampal subiculum from temporal lobe epilepsy patients make GABA excitatory. Proc Natl Acad Sci U S A 2006;103:8465-8. [PubMed]
- Cavus I, Pan JW, Hetherington HP, Abi-Saab W, Zaveri HP, Vives KP, Krystal JH, Spencer SS, Spencer DD. Decreased hippocampal volume on MRI is associated with increased extracellular glutamate in epilepsy patients. Epilepsia 2008;49:1358-66. [PubMed]
- Nguyen E, Picklo MJ Sr. Inhibition of succinic semialdehyde dehydrogenase activity by alkenal products of lipid peroxidation. Biochim Biophys Acta 2003;1637:107-12. [PubMed]
- Petroff OA, Hyder F, Rothman DL, Mattson RH. Homocarnosine and seizure control in juvenile myoclonic epilepsy and complex partial seizures. Neurology 2001;56:709-15. [PubMed]
- Richerson GB, Wu Y. Dynamic equilibrium of neurotransmitter transporters: not just for reuptake anymore. J Neurophysiol 2003;90:1363-74. [PubMed]
- Kuzniecky R, Hetherington H, Pan J, Hugg J, Palmer C, Gilliam F, Faught E, Morawetz R. Proton spectroscopic imaging at 4.1 tesla in patients with malformations of cortical development and epilepsy. Neurology 1997;48:1018-24. [PubMed]
- Woermann FG, McLean MA, Bartlett PA, Parker GJ, Barker GJ, Duncan JS. Short echo time single-voxel 1H magnetic resonance spectroscopy in magnetic resonance imaging-negative temporal lobe epilepsy: different biochemical profile compared with hippocampal sclerosis. Ann Neurol 1999;45:369-76. [PubMed]
- Connelly A, Van Paesschen W, Porter DA, Johnson CL, Duncan JS, Gadian DG. Proton magnetic resonance spectroscopy in MRI-negative temporal lobe epilepsy. Neurology 1998;51:61-6. [PubMed]
- Achten E, Boon P, Van De Kerckhove T, Caemaert J, De Reuck J, Kunnen M. Value of single-voxel proton MR spectroscopy in temporal lobe epilepsy. AJNR Am J Neuroradiol 1997;18:1131-9. [PubMed]
- Kuzniecky R, Hugg J, Hetherington H, Martin R, Faught E, Morawetz R, Gilliam F. Predictive value of 1H MRSI for outcome in temporal lobectomy. Neurology 1999;53:694-8. [PubMed]
- Eberhardt KE, Stefan H, Buchfelder M, Pauli E, Hopp P, Huk W, Tomandl BF. The significance of bilateral CSI changes for the postoperative outcome in temporal lobe epilepsy. J Comput Assist Tomogr 2000;24:919-26. [PubMed]
- Pan JW, Duckrow RB, Gerrard J, Ong C, Hirsch LJ, Resor SR Jr, Zhang Y, Petroff O, Spencer S, Hetherington HP, Spencer DD. 7T MR spectroscopic imaging in the localization of surgical epilepsy. Epilepsia 2013;54:1668-78. [PubMed]
- Avdievich NI, Pan JW, Baehring JM, Spencer DD, Hetherington HP. Short echo spectroscopic imaging of the human brain at 7T using transceiver arrays. Magn Reson Med 2009;62:17-25. [PubMed]
- Hetherington HP, Avdievich NI, Kuznetsov AM, Pan JW. RF shimming for spectroscopic localization in the human brain at 7 T. Magn Reson Med 2010;63:9-19. [PubMed]
- Pan JW, Lo KM, Hetherington HP. Role of very high order and degree B0 shimming for spectroscopic imaging of the human brain at 7 tesla. Magn Reson Med 2012;68:1007-17. [PubMed]
- Maudsley AA, Domenig C, Ramsay RE, Bowen BC. Application of volumetric MR spectroscopic imaging for localization of neocortical epilepsy. Epilepsy Res 2010;88:127-38. [PubMed]
- Maudsley AA, Domenig C, Sheriff S. Reproducibility of serial whole-brain MR spectroscopic imaging. NMR Biomed 2010;23:251-6. [PubMed]


