
Posterior reversible encephalopathy syndrome coexists with acute cerebral infarction: challenges of blood pressure management
Introduction
Posterior reversible encephalopathy syndrome (PRES) is a rare clinical neuroimaging disorder (1), which is characterized by vasogenic edema in the bilateral subcortical areas of the parietal and occipital lobes (2); there is frequent involvement of the frontal and temporal lobes, cerebellum, and brainstem (3). PRES can occur in any age group. The clinical manifestations of PRES are nonspecific and include acute headache, nausea, vomiting, and visual disorders (4), with seizure and coma occurring in severe cases (3). Sometimes PRES presents similarly to acute cerebral infarction with neurological dysfunction and thus can be easily misdiagnosed (5,6). With timely and appropriate treatment, the clinical symptoms and imaging changes of PRES can be rapidly improved, even completely recovered (7). Among the raft of causative factors of PRES, hypertension is one of the most common (8); hypertension is also the most common risk factor for acute cerebral infarction (9). However, the pathophysiological mechanisms between PRES and acute cerebral infarction are not the same. The lesion locations of PRES manifest as vasogenic edema (10,11), and as cytotoxic edema in cerebral infarction (3). The main principles of controlling BP in the early stage of these 2 diseases are different, even sometimes opposing (12). Clinically, the simultaneous appearance of PRES and acute cerebral infarction, caused by hypertension, is rare. And once the 2 diseases coexist, it is often challenging to decide on the best avenue for the regulation of hypertension. This article reports a case of PRES coexisting with acute lacunar cerebral infarction in an infrequent location of the hippocampus and pons and analyzes the antihypertensive therapy provided to the patient. Moreover, the early antihypertensive principles of PRES and acute cerebral infarction are summarized for our allied clinicians.
Case presentation
The patient was a 36-year-old male admitted to the emergency room for weakness in his left limbs. Without any apparent predisposition, the patient had experienced difficulty in raising his left upper limb and holding chopsticks, and his left leg was not flexible. Additional symptoms included dysphonia, bilateral temporo-occipital headache, confusion, and short-term memory loss. There was no reduction in the depth of the nasolabial groove, fever, or seizures. The patient denied having a medical history of hypertension, diabetes, coronary heart disease, renal artery stenosis, connective tissue disease, and alcohol or drug abuse. The presenting vital signs included a body temperature of 36.2 °C, heart rate 84 beats per minute (bpm), and blood pressure (BP) 214/145 mmHg. Upon neurological examination, he was conscious, having trouble speaking, comprehension and short-term memory were damaged, strength level of the left limbs was 5-, left Babinski sign (-), deep tendon reflexes of the 4 limbs were normal, and neck movement was unrestricted. Auxiliary examination revealed that routine complete blood count was normal, routine urine and feces were unremarkable; serum potassium 3.18 mmol/L; liver and kidney function were normal; fasting blood glucose 4.8 mmol/L; glycosylated hemoglobin 5.2%; the renin-aldosterone system was normal; coagulation and thyroid function were normal; both anti-nuclear Ab and anti-phospholipid Ab were negative, and screening for syphilis and AIDS returned negative results. The cerebrospinal fluid pressure was 140 mmH2O, with a protein level of 0.42 g/L; ultrasound of the renal parenchyma and adrenal gland did not find any tumor, and the abdominal aorta and renal vessel ultrasound revealed no severe stenosis; computed tomography (CT) of the head detected a suspicious low-density change in the pons (Figure 1A).
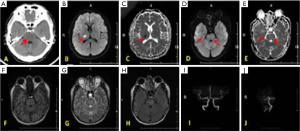
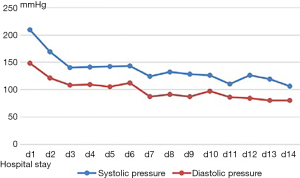
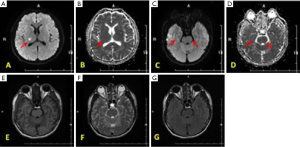
According to medical history and neurological examinations, the initial diagnosis was acute cerebral infarction. The antithrombotic agent aspirin and atorvastatin were taken orally, and oral nifedipine was administered to reduce BP. However, the head CT suggested that the pons lesions were inconsistent with the clinical manifestations. The patients’ BP decreased to 172/119 mmHg in the 24 h after admission. Magnetic resonance imaging (MRI) of the head showed acute lacunar infarction in the right thalamus and vasogenic edema in the pons and right hippocampus, without large vessel stenosis (Figure 1B,C,D,E,F,G,H,I,J). We added irbesartan to control the hypertension further, and the BP gradually decreased. On the 14th day, BP was controlled at 130/85 mmHg (Figure 2), a repeat head MRI revealed that the lesions in the pons and hippocampus had disappeared (Figure 3), and the patient had no remarkable sequelae. The final diagnosis was made in 3 parts: (I) acute lacunar cerebral infarction; (II) posterior reversible encephalopathy syndrome; (III) hypertension.
Discussion
There are several causes of PRES, including hypertension, eclampsia/preeclampsia (13), autoimmune disease (14), use of immunosuppressants (15), severe infection (16), renal insufficiency (17), and others. Hypertension is one of the most common causes of PRES (18). In our case, the BP was extremely high at the onset of disease, and there was no other relevant medical history, so we hypothesized that hypertension was the main reason for PRES.
Mechanism of vasogenic edema and cytotoxic edema induced by hypertension
Approximately 75–80% of PRES patients have significantly elevated BP (2). When the increased BP exceeds the upper limit of self-regulation in cerebrovascular injury, the endothelial cells become damaged and release vascular endothelial toxic substances, which increase vascular permeability and lead to brain tissue over perfusion (19), and subsequent vasogenic edema (20). For acute cerebral infarction caused by hypertension, the interruption of cerebral blood flow promotes ischemia and hypoxia in the brain tissue, which gives rise to metabolic disorders in nerve cells and changes the internal and external environment of neurons; then, cytotoxic edema ensues (4). In light of the above, we can deduce that the pathophysiological mechanisms caused by either vasogenic edema or cytotoxic edema are different. Therefore, we strongly believe that the principles of antihypertensive therapy in the 2 conditions require very distinct approaches.
Identification of vasogenic edema and cytotoxic edema
Diffusion-weighted imaging (DWI) combined with apparent diffusion coefficient (ADC) is the “gold standard” to identify the area of cerebral edema (21,22), and they also can distinguish PRES from acute cerebral infarction (4,23).
Vasogenic cerebral edema is caused by the breakdown of the blood-brain barrier and increased capillary permeability. Water molecules and some protein substances penetrate the extracellular fluid through the vascular wall and increase the osmotic pressure of the extracellular fluid. In areas of vasogenic edema, DWI usually presents as low or equal signals (23), but can sometimes present a slightly higher signal due to the “shine-through” effect of MRI T2 weighted image (T2WI) (24); however, ADC always presents a high signal for this pathology (21,22).
The reasons for cytotoxic edema are diverse, and it happens commonly in ischemia of the brain. Due to ischemia, the energy pump function of cell membranes is destroyed, and nerve cells develop metabolic disorders and edema. However, the blood-brain barrier function is reserved, and the perivascular and extracellular space is not significantly expanded. In cytotoxic edema, DWI shows a high signal, while ADC reveals a low signal (6). Both DWI and ADC can identify the above 2 types of brain tissue edema. When the head MRI of the patient reveals the coexistence of cytotoxic edema and vasogenic edema, it suggests that different pathogeneses are involved in the disease, which creates a challenge in determining the most appropriate treatment.
Early management of BP in PRES
There are no randomized controlled clinical trials (RCTs) to support optimal management of hypertension in PRES; however, the effective treatment of hypertension is essential. Antihypertensive medications should be used when the systolic blood pressure (SBP) exceeds 160 mmHg and/or diastolic blood pressure (DBP) exceeds 105–110 mmHg (6). It is usually recommended to reduce BP by about 25% within 24–48 h, depending on the level of BP at the time of the attack, or the basic BP situation. Moreover, intravenous antihypertensive treatment is feasible when necessary (25), although severe fluctuations of BP in a short period should be avoided. Fluctuating BP will further damage the blood-brain barrier and lead to the leakage of macromolecular substances from the vascular endothelial cells into the brain tissue (26). The above recommendations are based only on relevant case analyses or reviews, and clinical guidelines do not support them. The BP of the patient we currently report on accorded with the antihypertensive indications of PRES. Meanwhile, the drop level of BP reached 20% in 24 h, which was in line with the basic principles of antihypertensive treatment in PRES. Therefore, we speculate that the exemplary prognosis of the patient is attributed to the effective control of hypertension.
The differences between classical PRES and variants of PRES
The basic imaging pattern of PRES is limited to watershed areas of the brain, including the cortex, subcortex, and deep white matter. The parietal and occipital lobes are most frequently affected in PRES and are often distributed symmetrically (27). The frontal lobe, temporal lobe, cerebellum, basal ganglia, brainstem, and deep white matter are also commonly affected, are circumstantially called atypical parts, and account for about 4% of the affected areas (28). The most common pathogenesis of PRES is disordered cerebrovascular self-regulation, which is induced by hypertension. The vertebrobasilar arterial system is very sensitive to this process, so posterior circulation is most frequently involved (29). Some researchers have found that patients with supratentorial involvement tend to have slightly elevated BP, while patients with the brainstem, basal ganglia, and cerebellum involvement tend towards significantly elevated BP readings (27). We hypothesize that the affected sites of PRES relate to BP level, etiology, age, and other factors, which require further research.
Management of BP in acute cerebral infarction
The management of BP in acute cerebral infarction is a complicated issue, and it is necessary to increase the blood perfusion of ischemic brain tissue and avoid reperfusion injury. Fluctuating BP is typical in the acute phase (30), and high BP is most common. It has been reported that SBP is >140 mmHg in 77% of acute cerebral infarction patients (31). The pathophysiological mechanisms of hypertension are very complex, but the specific reasons are still not entirely clear. Contributing factors include a history of hypertension, psychological stress, the severity of the disease, thrombus load, location of infarction, and collateral circulation, and these factors comingle to raise the BP (30,32). Our patient denied having a history of hypertension, but he had never monitored his BP; thus, we cannot rule out the possibility of persistent hypertension. Current opinions on antihypertensive approaches in acute cerebral infarction vary greatly, mainly because most studies have been characterized by being single-center, small sample size, and lacking repeatability (33). Chamorro et al. (34) found that in cerebral infarction patients with a history of hypertension, high BP in the acute phase could increase brain perfusion, which was beneficial for clinical prognosis. Verschoof et al. (35) noted that hypotension in the acute phase of cerebral infarction increases the risk of death and complications during hospitalization, especially in patients with heart failure, shock, and gastrointestinal bleeding. Anderson et al. (36) proved that antihypertensive therapy can only reduce the risk of hemorrhage transformation, but cannot improve the clinical prognosis. There was a U-shaped relationship between BP and clinical prognosis, which indicated that maintaining SBP at <150 mmHg could achieve the lowest mortality, and death risk increased by 3.6% per 10 mmHg of elevation beyond that threshold (37). Therefore, maintaining BP at a high level may be beneficial for acute ischemic stroke patients, but this perspective is inconsistent with the therapeutic principles of PRES. Furthermore, higher BP is not always better for this purpose. A meta-analysis of 32 studies involving 10,892 patients suggested that hypertension in the acute stage was an independent risk factor for poor clinical prognosis (38). Leonardi-Bee et al. (31) pointed out that in the early phase when SBP exceeded 200 mmHg or was increased by more than 50% from the basal level, the risk of stroke relapse, bleeding transformation, and brain edema increased significantly. Different views exist on antihypertensive therapy for acute ischemic injury, and this presents a challenge to the management of BP in PRES. Meanwhile, in the acute phase of cerebral ischemic injury, severe fluctuations of BP should be avoided; Geng et al. (39) found that severe fluctuation of SBP within 24 h of onset was an independent risk factor for poor prognosis at 7 days. Manning et al. (40) also proved that fluctuations of SBP within 24 h of infarction was an independent risk factor for hemorrhagic transformation. Tanaka et al. (41) pointed out that the fluctuation of SBP in the acute phase was an independent predictor for death within a month. Avoiding severe fluctuations of BP in the acute phase is consistent with the treatment principles of PRES. We hypothesized that keeping BP within a moderate range would contribute to the protection of endothelial cells.
Regarding the timing for commencing antihypertensive therapy, Zhang et al. (42) suggested that aggressive antihypertensive treatment should be started within 48 h after the onset of symptoms, in order to reduce the risk of stroke recurrence. Saver et al. (43) found that antihypertensive treatment within 24 h would reduce cerebral perfusion in the ischemic area and increase the scope of infarction. In current clinical practice, the American Heart Association/American Stroke Association (AHA/ASA) guidelines of 2018 suggested that for the acute cerebral infarction, antihypertensive therapy should be initiated within 24–72 h following the attack, but if the BP exceeds 220/120 mmHg, or target-organs are damaged (acute renal insufficiency, aortic dissection, acute pulmonary edema, etc.), accompanied by BP over 200/100 mmHg. We should reduce BP aggressively earlier (32). Hence, we believe that early antihypertensive therapy in acute cerebral infarction is a general guiding principle, and similar to the administration of BP in PRES. However, the degree and speed of antihypertensive management should be considered carefully in combination with other factors.
BP management in patients who experience intravenous thrombolysis or intravascular therapy
Wang et al. (44) demonstrated that among patients who received intravenous thrombolytic therapy if SBP was controlled between 130–140 mmHg compared to 180 mmHg, it did not improve the 90-day clinical prognosis or reduce bleeding risk. Ahmed et al. (33) implied that the risk of symptomatic intracranial hemorrhage was 4 times higher in patients who would receive intravenous thrombolytic therapy with SBP greater than 170 mmHg compared to 141–150 mmHg. Aries et al. (45) showed that patients who received intravenous thrombolysis with a rapid reduction of BP experienced impaired perfusion of the collateral circulation into the ischemia penumbra and aggravation of the symptoms of clinical ischemia. Therefore, there are still controversial standpoints on whether aggressive antihypertensive therapy is appropriate for patients who receive intravenous thrombolytic treatment in acute cerebral infarction. The AHA/ASA recommends that for intravenous thrombolysis, the BP should be <185/110 mmHg before treatment, and no more than 180/105 mmHg within the first 24 h following treatment (32), which could increase reperfusion and reduce the risk of bleeding transformation (46).
Alcaraz et al. (47) proved that the reduction of BP by more than 10% during mechanical thrombectomy compared to pre-operation was not of benefit to the clinical prognosis. Ishitsuka et al. (48) suggested that the elevated BP in the acute phase was detrimental to the long-term prognosis in patients who received mechanical thrombectomy. Raychev et al. (49) found that if vascular recanalization was achieved, the risk of bleeding was lowest when the SBP was ≤170 mmHg, and excessive BP might lead to reperfusion injury. On the contrary, if patients did not achieve recanalization, hypotension would result in a state of hypoperfusion in the ischemic area. As a result, the antihypertensive treatment for patients who achieve mechanical thrombectomy is complex. We need to consider multiple factors, such as recanalization, collateral circulation, door to treatment time, risk factors, and thrombectomy devices. The American AHA/ASA suggests that BP should be controlled at <185/110 mmHg before endovascular therapy, and at the same time, lower BP should also be avoided (32). Independent of intravenous thrombolysis or endovascular treatment, early BP management is a challenging problem, with all kinds of potential outcomes. We need to consider a variety of factors and undertake antihypertensive therapy according to the current guidelines.
Management of BP in different subtypes of acute cerebral infarction
Methods of management of BP fluctuations in the acute phase are various because of different infarction subtypes, which may be related to diverse etiological factors (50). Patients with lacunar infarction have higher BP in the acute phase, which results in fibrinoid necrosis and hyaline degeneration in deep perforating branch arteries (51). Endothelial cell dysfunction appears earlier, accompanied by cerebral microvascular lesions, which lead to the cerebrovascular regulation curve shifting to the right (51). Meanwhile, it was found that active antihypertensive treatment in lacunar cerebral infarction would not adversely affect the clinical prognosis (52,53). In patients with cerebral infarction due to large arterial stenosis or occlusion, the collateral circulation may be opened simultaneously. Collateral circulation was opened because of elevated BP, which effectively enhanced cerebral perfusion. However, it can also boost the risk of hemorrhagic transformation, so it is necessary to take into account both under perfusion and over perfusion in the process of BP administration (54). For the patients with cardiogenic cerebral infarction, cardiac failure was often present, and the compensatory mechanism of elevated BP was subsequently destroyed. Therefore, in the acute phase, BP was always lower or normal (50). Marcheselli et al. (55) proposed that lower BP was found in cardiogenic stroke patients within 24 h of onset, which may be related to long-term oral antihypertensive drugs. Based on the above, different etiologies lead to various kinds of BP in the acute phase, which may be related to the pathophysiological mechanism. Hence, etiological factors should be fully considered in antihypertensive treatment. Currently, there is no uniform antihypertensive goal, and the individuation of antihypertensive should be routinely performed (56).
Possible causes of PRES coexisting with acute cerebral infarction
The coexistence of PRES and acute cerebral infarction is clinically rare, with an incidence of about 11–26%, and poor prognosis is often indicated (23). There are multiple possible reasons for this coexistence; firstly, vasogenic edema caused by PRES increases the pressure of brain tissue, compresses the intracranial micro arteries, and leads to local infarction (57); secondly, vascular endothelial toxic substances or hypertension promote cerebral artery contraction or spasm (58), this further facilitates the decline of local cerebral blood flow (59). However, the exact pathogenesis is still unclear. In this case, we speculate that BP was significantly increased at the initial stage of the disease, which destroyed the self-regulatory function of the cerebral vessels and led to vasogenic edema. Moreover, the vasoconstriction and spasm happened, and cytotoxic edema subsequently appeared.
Antihypertension in this case
According to the head CT, the cerebral hemorrhage was eliminated after 10 h. The initial diagnosis was acute cerebral infarction, and oral antihypertensive drugs were administered as BP was 214/145 mmHg, which exceeded the recommended guidelines for antihypertensive treatment. The MRI on the second day confirmed the presence of both vasogenic and cytotoxic edema, especially the results of DWI MRI and ADC MRI, which suggested that PRES (unusual parts of pons and right hippocampus) coexisted with acute lacunar infarction (right thalamus). The patients’ BP decreased to 172/119 mmHg at 24 h. According to the principles of early BP management of PRES, active antihypertensive treatment should be performed to avoid irreversible cytotoxic edema. For patients who had not received intravenous thrombolytic therapy, early antihypertensive treatment was generally not recommended. However, considering that the patient had only lacunar infarction, antihypertensive treatment would not affect the clinical prognosis. After careful consideration, we intensified the antihypertensive treatment. After 2 weeks, the edema of the patients’ pons and hippocampus and the prognosis were favorable.
Summary
Hypertension is the most common cause of PRES and acute cerebral infarction, but there are differences in the antihypertensive treatment approaches between them. There is no ideal treatment scheme for BP management when these 2 pathologies present concurrently. Generally speaking, in PRES patients with elevated BP, appropriate antihypertensive therapy should be initiated during the acute phase. However, for patients with acute cerebral infarction, early antihypertensive therapy is more complicated. It requires consideration of BP history level, BP at the onset, whether or not to administer intravenous thrombolysis or endovascular therapy, the severity of the disease, and the etiology of cerebral infarction. The coexistence of PRES and acute cerebral infarction are relatively rare in clinical practice. Antihypertensive therapy should be considered thoroughly, including the timing, speed, and targets. Individualized treatment should be carried out after a suitable therapeutic compromise has been decided upon.
Acknowledgments
Funding: The authors would like to thank the Key Project of Medical Science Research of Hebei Province, China (20190502) and Hebei Provincial Natural Science Foundation of China (No. H2019206406).
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/qims-20-392). The authors have no conflicts of interest to declare.
Ethical Statement: The patient provided written informed consent for publication of this study and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wu Q, Marescaux C, Wolff V, Jeung MY, Kessler R, Lauer V, Chen Y. Tacrolimus-associated posterior reversible encephalopathy syndrome after solid organ transplantation. Eur Neurol 2010;64:169-77. [Crossref] [PubMed]
- Bartynski WS. Posterior reversible encephalopathy syndrome, part 1: fundamental imaging and clinical features. AJNR Am J Neuroradiol 2008;29:1036-42. [Crossref] [PubMed]
- Yacong W, Qinying C, Lihong Z, Su'e Z, Liang S, Ou S. Acute cortical blindness caused by pre-eclampsia in the antepartum; posterior reversible encephalopathy syndrome (PRES). Afr Health Sci 2015;15:705-8. [Crossref] [PubMed]
- Chen TY, Lee HJ, Wu TC, Tsui YK. MR imaging findings of medulla oblongata involvement in posterior reversible encephalopathy syndrome secondary to hypertension. AJNR Am J Neuroradiol 2009;30:755-7. [Crossref] [PubMed]
- Kim HG, Lee KM, Lee JS. Unusual magnetic resonance imaging findings in a patient with posterior reversible encephalopathy syndrome. Quant Imaging Med Surg 2018;8:1066-8. [Crossref] [PubMed]
- Zhang L, Wang Y, Shi L, Cao J, Li Z, Wáng YX. Late postpartum eclampsia complicated with posterior reversible encephalopathy syndrome: a case report and a literature review. Quant Imaging Med Surg 2015;5:909-16. [PubMed]
- Grossbach AJ, Abel TJ, Hodis B, Wassef SN, Greenlee JD. Hypertensive posterior reversible encephalopathy syndrome causing posterior fossa edema and hydrocephalus. J Clin Neurosci 2014;21:207-11. [Crossref] [PubMed]
- Abalo-Lojo JM, Baleato-González S, Gonzalez F. Cortical blindness secondary to posterior reversible encephalopathy syndrome, recovered by successful blood pressure management. Arq Bras Oftalmol 2017;80:324-6. [Crossref] [PubMed]
- Feigin VL, Roth GA, Naghavi M, Parmar P, Krishnamurthi R, Chugh S, Mensah GA, Norrving B, Shiue I, Ng M, Estep K, Cercy K, Murray CJL, Forouzanfar MH. Global Burden of Diseases, Injuries and Risk Factors Study 2013 and Stroke Experts Writing Group. Global burden of stroke and risk factors in 188 countries, during 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet Neurol 2016;15:913-24. [Crossref] [PubMed]
- Fugate JE, Rabinstein AA. Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol 2015;14:914-25. [Crossref] [PubMed]
- Zuccoli G, Fitzgerald RT, Nardone R, Furtado AD, Abdel-Hamid H. The link between arterial blood pressure and vasogenic edema in pediatric PRES. Neuroradiology 2015;57:865-6. [Crossref] [PubMed]
- Servillo G, Bifulco F, De Robertis E, Piazza O, Striano P, Tortora F, Striano S, Tufano R. Posterior reversible encephalopathy syndrome in intensive care medicine. Intensive Care Med 2007;33:230-6. [Crossref] [PubMed]
- McKinney AM, Jagadeesan BD, Truwit CL. Central-variant posterior reversible encephalopathy syndrome: brainstem or basal ganglia involvement lacking cortical or subcortical cerebral edema. AJR Am J Roentgenol 2013;201:631-8. [Crossref] [PubMed]
- Kur JK, Esdaile JM. Posterior reversible encephalopathy syndrome--an underrecognized manifestation of systemic lupus erythematosus. J Rheumatol 2006;33:2178-83. [PubMed]
- Kishi Y, Miyakoshi S, Kami M, Ikeda M, Katayama Y, Murashige N, Kusumi E, Yuji K, Kobayashi K, Kato D, Hamaki T, Matsumura T, Kim SW, Morinaga S, Mori S, Kanemaru M, Hayashi T, Takaue Y, Taniguchi S. Tokyo Stem Cell Transplantation Consortium. Early central nervous system complications after reduced-intensity stem cell transplantation. Biol Blood Marrow Transplant 2004;10:561-8. [Crossref] [PubMed]
- Bartynski WS, Boardman JF, Zeigler ZR, Shadduck RK, Lister J. Posterior reversible encephalopathy syndrome in infection, sepsis, and shock. AJNR Am J Neuroradiol 2006;27:2179-90. [PubMed]
- Lamy C, Oppenheim C, Méder JF, Mas JL. Neuroimaging in posterior reversible encephalopathy syndrome. J Neuroimaging 2004;14:89-96. [Crossref] [PubMed]
- Merayo-Chalico J, Apodaca E, Barrera-Vargas A, Alcocer-Varela J, Colunga-Pedraza I, González-Patiño A, Arauz A, Abud-Mendoza C, Martínez-Martínez M, Gómez-Martín D. Clinical outcomes and risk factors for posterior reversible encephalopathy syndrome in systemic lupus erythematosus: a multicentric case-control study. J Neurol Neurosurg Psychiatry 2016;87:287-94. [Crossref] [PubMed]
- Fugate JE, Claassen DO, Cloft HJ, Kallmes DF, Kozak OS, Rabinstein AA. Posterior reversible encephalopathy syndrome: associated clinical and radiologic findings. Mayo Clin Proc 2010;85:427-32. [Crossref] [PubMed]
- Bartynski WS. Posterior reversible encephalopathy syndrome, part 2: controversies surrounding pathophysiology of vasogenic edema. AJNR Am J Neuroradiol 2008;29:1043-9. [Crossref] [PubMed]
- Furtado A, Hsu A, La Colla L, Zuccoli G. Arterial blood pressure but not serum albumin concentration correlates with ADC ratio values in pediatric posterior reversible encephalopathy syndrome. Neuroradiology 2015;57:721-8. [Crossref] [PubMed]
- Chen Z, Zhang G, Lerner A, Wang AH, Gao B, Liu J. Risk factors for poor outcome in posterior reversible encephalopathy syndrome: systematic review and meta-analysis. Quant Imaging Med Surg 2018;8:421-32. [Crossref] [PubMed]
- Covarrubias DJ, Luetmer PH, Campeau NG. Posterior reversible encephalopathy syndrome: prognostic utility of quantitative diffusion-weighted MR images. AJNR Am J Neuroradiol 2002;23:1038-48. [PubMed]
- Shaharir SS, Remli R, Marwan AA, Said MS, Kong NC. Posterior reversible encephalopathy syndrome in systemic lupus erythematosus: pooled analysis of the literature reviews and report of six new cases. Lupus 2013;22:492-6. [Crossref] [PubMed]
- Mansia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Struijker Boudier HA, Zanchetti A. European Society of Hypertension. European Society of Cardiology. 2007 ESH-ESC Guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Blood Press 2007;16:135-232. [Crossref] [PubMed]
- Liman TG, Bohner G, Endres M, Siebert E. Discharge status and in-hospital mortality in posterior reversible encephalopathy syndrome. Acta Neurol Scand 2014;130:34-9. [Crossref] [PubMed]
- Choh NA, Jehangir M, Rasheed M, Mira T, Ahmad I, Choh S. Involvement of the cervical cord and medulla in posterior reversible encephalopathy syndrome. Ann Saudi Med 2011;31:90-2. [Crossref] [PubMed]
- Samara A, Berry B, Ghannam M. Posterior reversible encephalopathy syndrome with isolated infratentorial involvement: A case report. Radiol Case Rep 2019;14:576-80. [Crossref] [PubMed]
- Fittro K, Dizon R. Understanding posterior reversible encephalopathy syndrome. JAAPA 2018;31:31-4. [Crossref] [PubMed]
- Carlberg B, Asplund K, Hägg E. Factors influencing admission blood pressure levels in patients with acute stroke. Stroke 1991;22:527-30. [Crossref] [PubMed]
- Leonardi-Bee J, Bath PM, Phillips SJ, Sandercock PA. IST Collaborative Group. Blood pressure and clinical outcomes in the International Stroke Trial. Stroke 2002;33:1315-20. [Crossref] [PubMed]
- Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2019;50:e344-e418. [Crossref] [PubMed]
- Ahmed N, Wahlgren N, Brainin M, Castillo J, Ford GA, Kaste M, Lees KR, Toni D. SITS Investigators. Relationship of blood pressure, antihypertensive therapy, and outcome in ischemic stroke treated with intravenous thrombolysis: retrospective analysis from Safe Implementation of Thrombolysis in Stroke-International Stroke Thrombolysis Register (SITS-ISTR). Stroke 2009;40:2442-9. [Crossref] [PubMed]
- Chamorro A, Vila N, Ascaso C, Elices E, Schonewille W, Blanc R. Blood pressure and functional recovery in acute ischemic stroke. Stroke 1998;29:1850-3. [Crossref] [PubMed]
- Verschoof MA, Groot AE, Vermeij JD, Westendorp WF, van den Berg SA, Nederkoorn PJ, van de Beek D, Coutinho JM. Association Between Low Blood Pressure and Clinical Outcomes in Patients With Acute Ischemic Stroke. Stroke 2020;51:338-41. [Crossref] [PubMed]
- Anderson CS, Huang Y, Lindley RI, Chen X, Arima H, Chen G, Li Q, Billot L, Delcourt C, Bath PM, Broderick JP, Demchuk AM, Donnan GA, Durham AC, Lavados PM, Lee TH, Levi C, Martins SO, Olavarria VV, Pandian JD, Parsons MW, Pontes-Neto OM, Ricci S, Sato S, Sharma VK, Silva F, Song L, Thang NH, Wardlaw JM, Wang JG, Wang X, Woodward M, Chalmers J, Robinson TG. ENCHANTED Investigators and Coordinators. Intensive blood pressure reduction with intravenous thrombolysis therapy for acute ischaemic stroke (ENCHANTED): an international, randomised, open-label, blinded-endpoint, phase 3 trial. Lancet 2019;393:877-88. [Crossref] [PubMed]
- Bangalore S, Schwamm L, Smith EE, Hellkamp AS, Suter RE, Xian Y, Schulte PJ, Fonarow GC, Bhatt DL. Get With the Guidelines-Stroke Steering Committee and Investigators. Blood pressure and in-hospital outcomes in patients presenting with ischaemic stroke. Eur Heart J 2017;38:2827-35. [Crossref] [PubMed]
- Willmot M, Leonardi-Bee J, Bath PM. High blood pressure in acute stroke and subsequent outcome: a systematic review. Hypertension 2004;43:18-24. [Crossref] [PubMed]
- Geng X, Liu X, Li F, Wang J, Sun H, Feng A, Sun Y, Sun H, Yang F, Zhao J, Tang Y. Blood pressure variability at different time periods within first 24 hours after admission and outcomes of acute ischemic stroke. J Clin Hypertens (Greenwich) 2020;22:194-204. [Crossref] [PubMed]
- Manning LS, Rothwell PM, Potter JF, Robinson TG. Prognostic Significance of Short-Term Blood Pressure Variability in Acute Stroke: Systematic Review. Stroke 2015;46:2482-90. [Crossref] [PubMed]
- Tanaka E, Koga M, Kobayashi J, Kario K, Kamiyama K, Furui E, Shiokawa Y, Hasegawa Y, Okuda S, Todo K, Kimura K, Okada Y, Okata T, Arihiro S, Sato S, Yamagami H, Nagatsuka K, Minematsu K, Toyoda K. Blood pressure variability on antihypertensive therapy in acute intracerebral hemorrhage: the Stroke Acute Management with Urgent Risk-factor Assessment and Improvement-intracerebral hemorrhage study. Stroke 2014;45:2275-9. [Crossref] [PubMed]
- Zhang R, Zhong C, Zhang Y, Xie X, Zhu Z, Wang A, Chen CS, Peng Y, Peng H, Li Q, Ju Z, Geng D, Chen J, Liu L, Wang Y, Xu T, He J. Immediate Antihypertensive Treatment for Patients With Acute Ischemic Stroke With or Without History of Hypertension: A Secondary Analysis of the CATIS Randomized Clinical Trial. JAMA Netw Open 2019;2:e198103. [Crossref] [PubMed]
- Saver JL. Blood pressure management in early ischemic stroke. JAMA 2014;311:469-70. [Crossref] [PubMed]
- Wang X, Song L, Yang J, Sun L, Moullaali TJ, Sandset EC, Delcourt C, Lindley RI, Robinson TG, Minhas JS, Arima H, Chalmers J, Kim JS, Sharma V, Wang JG, Pontes-Neto O, Lavados PM, Olavarría VV, Lee TH, Levi C, Martins SO, Thang NH, Anderson CS. ENCHANTED Investigators. Interaction of Blood Pressure Lowering and Alteplase Dose in Acute Ischemic Stroke: Results of the Enhanced Control of Hypertension and Thrombolysis Stroke Study. Cerebrovasc Dis 2019;48:207-16. [Crossref] [PubMed]
- Aries MJ, Elting JW, De Keyser J, Kremer BP, Vroomen PC. Cerebral autoregulation in stroke: a review of transcranial Doppler studies. Stroke 2010;41:2697-704. [Crossref] [PubMed]
- Maïer B, Fahed R, Khoury N, Guenego A, Labreuche J, Taylor G, Blacher J, Zuber M, Lapergue B, Blanc R, Piotin M, Mazighi M. Association of Blood Pressure During Thrombectomy for Acute Ischemic Stroke With Functional Outcome: A Systematic Review. Stroke 2019;50:2805-12. [Crossref] [PubMed]
- Alcaraz G, Chui J, Schaafsma J, Manninen P, Porta-Sánchez A, Pereira VM, Venkatraghavan L. Hemodynamic Management of Patients During Endovascular Treatment of Acute Ischemic Stroke Under Conscious Sedation: A Retrospective Cohort Study. J Neurosurg Anesthesiol 2019;31:299-305. [Crossref] [PubMed]
- Ishitsuka K, Kamouchi M, Hata J, Fukuda K, Matsuo R, Kuroda J, Ago T, Kuwashiro T, Sugimori H, Nakane H, Kitazono T, Investigators FSR. High blood pressure after acute ischemic stroke is associated with poor clinical outcomes: Fukuoka Stroke Registry. Hypertension 2014;63:54-60. [Crossref] [PubMed]
- Raychev R, Liebeskind DS, Yoo AJ, Rasmussen M, Arnaudov D, Brown S, Saver J, Simonsen CZ. Physiologic predictors of collateral circulation and infarct growth during anesthesia - Detailed analyses of the GOLIATH trial. J Cereb Blood Flow Metab 2020;40:1203-12. [Crossref] [PubMed]
- Moores M, Yogendrakumar V, Bereznyakova O, Alesefir W, Pettem H, Stotts G, Dowlatshahi D, Shamy M. Normal Systolic Blood Pressure at Presentation With Acute Ischemic Stroke Predicts Cardioembolic Etiology. J Am Heart Assoc 2020;9:e014399. [Crossref] [PubMed]
- Altmann M, Thommessen B, Rønning OM, Reichenbach AS, Fure B. Blood pressure differences between patients with lacunar and nonlacunar infarcts. Brain Behav 2015;5:e00353. [Crossref] [PubMed]
- Gómez-Choco M, García-Sánchez SM, Font MÀ, Mengual JJ, Blanch P, Castellanos P, Cortés-Fernández MS, Martín-Castillejos C, Lleixa M, Martín-Baranera M, Armario P. Biomarkers levels and brachial and central blood pressure during the subacute phase of lacunar stroke and other ischemic stroke subtypes. J Hum Hypertens 2020;34:404-10. [Crossref] [PubMed]
- Yamamoto Y, Nagakane Y, Ohara T, Tanaka E, Morii F, Koizumi T, Muranishi M, Takezawa H. Intensive blood pressure-lowering treatment in patients with acute lacunar infarction. J Stroke Cerebrovasc Dis 2013;22:1273-8. [Crossref] [PubMed]
- Harper G, Castleden CM, Potter JF. Factors affecting changes in blood pressure after acute stroke. Stroke 1994;25:1726-9. [Crossref] [PubMed]
- Marcheselli S, Cavallini A, Tosi P, Quaglini S, Micieli G. Impaired blood pressure increase in acute cardioembolic stroke. J Hypertens 2006;24:1849-56. [Crossref] [PubMed]
- Vemmos KN, Spengos K, Tsivgoulis G, Zakopoulos N, Manios E, Kotsis V, Daffertshofer M, Vassilopoulos D. Factors influencing acute blood pressure values in stroke subtypes. J Hum Hypertens 2004;18:253-9. [Crossref] [PubMed]
- Granata G, Greco A, Iannella G, Granata M, Manno A, Savastano E, Magliulo G. Posterior reversible encephalopathy syndrome--Insight into pathogenesis, clinical variants and treatment approaches. Autoimmun Rev 2015;14:830-6. [Crossref] [PubMed]
- Bartynski WS, Boardman JF. Catheter angiography, MR angiography, and MR perfusion in posterior reversible encephalopathy syndrome. AJNR Am J Neuroradiol 2008;29:447-55. [Crossref] [PubMed]
- Gao B, Yu BX, Li RS, Zhang G, Xie HZ, Liu FL, Lv C. Cytotoxic Edema in Posterior Reversible Encephalopathy Syndrome: Correlation of MRI Features with Serum Albumin Levels. AJNR Am J Neuroradiol 2015;36:1884-9. [Crossref] [PubMed]


