
Common infectious diseases of the central nervous system—clinical features and imaging characteristics
Introduction
The central nervous system (CNS) primarily consists of the brain and spinal cord, which are enclosed within the surrounding meninges and bone structures such as the calvarium and spine. Infectious diseases of the CNS include a wide spectrum of infections caused by various pathogens affecting one or more of these components as well as the spaces between these components. Different components or compartments of the CNS are affected circumstantially; each of the disease entities will have different imaging pattern and characteristics according to their different transmission routes, locations and appearance, i.e. meningitis, brain abscess, septic emboli, spinal epidural empyema and osteomyelitis (OM). Despite the advances of modern medicine, accurate diagnoses of a specific disease entity of the CNS infections have proven to be very challenging as their clinical presentations are very general and nonspecific, such as fever, headaches, weakness, numbness or back pain.
The infectious diseases of the CNS are most commonly caused by various types of bacteria or viruses. Other less common organisms include fungi or protozoans, etc. These pathogens enter the CNS through two main routes of transmission: hematogenous spread from distant infections such as endocarditis or urinary tract infection, or direct extension from infections of the adjacent structures such as sinusitis or mastoiditis. Hematogenous transmission tends to produce diffuse or multifocal lesions in different vascular distributions, such as diffuse meningitis or septic emboli. Direct extension of the infections usually gives rise to focal lesions, such as subdural or epidural empyema. Whenever local infection is identified, the adjacent anatomic structures (e.g., paranasal sinuses, mastoid air cells, middle ears) should be examined diligently to pinpoint the initial source. Extended involvement of other CNS compartments and vascular systems is not uncommon, as it can coexist or become complications of primary meningitis or encephalitis, such as ventriculitis or thrombophlebitis.
Neuroimaging techniques are essential for diagnosing various CNS infections and help differentiate different disease entities. The two main utilized imaging modalities are computed tomography (CT) and magnetic resonance imaging (MRI), each with their own advantages and limitations. CT scanners are widely available in hospitals and clinics, being operated 24 hours a day. Nonenhanced CT (NECT) images can be acquired quickly and with little patient preparations. CT scan is highly sensitive to acute intracranial hemorrhages (parenchymal or extra-axial), calcified lesions or bony deformities. NECT of head often serves as a gateway for more advanced imaging or more complexed patients’ management, such as screening for lumbar puncture (LP) or used for CT-guide biopsy or drainage. Administration of intravenous contrast in CT usually increases the sensitivity of detecting an underlying abnormality and further delineating its characters. Vascular complications of CNS infections can be accessed by CT angiogram of head, either in arterial or venous structures. The main drawback of CT is its radiation to patients. Other disadvantages include relatively low soft tissue resolution, limited imaging display planes and less sensitive contrast enhancement. Small extra-axial fluid collections can be missed. Meningitis or other subtle structural changes may not be detected at early stages. MR imaging is much more sensitive for detecting early changes of CNS infections and depicting various imaging findings during each disease process, due to its high anatomy resolution, superb soft tissue differentiation, multiplanar acquisition, and versatile sequences of delineating different characters of pathologic processes of CNS infections. Vasogenic edema caused by infection appears hyperintense on T2-weighted (T2WI) or FLAIR images; A small hemorrhagic focus within the infection could may manifest as an exaggerated blooming artifact on gradient echo sequence (GRE); Hyperintense signals on diffusion weighted images (DWI) are highly characteristic of many types of infectious lesions and separating them apart from other etiologies. Administration of intravenous contrast in MRI increase the sensitivity and specificity of the MRI technique, helping further delineate the pathology, exclude most noninfectious etiologies, narrow down the differential diagnoses. The disadvantages of MR include lesser available MR scanners in hospitals, longer examination time and certain limitations of the patients. Claustrophobia could be a problem for some patients. Patients with pacemakers or other battery-operated devices as well as metallic foreign bodies in critical organs are also contraindications for MR, although MR-compatible pacemaker started to appear on the market in recent years.
Additionally, vascular complications of CNS infections, either in arterial or venous compositions can be accessed by CT or MR angiogram/venogram, although DSA remains the gold standard for evaluating these conditions.
Side effects of contrast such as allergic reactions can be seen using either iodinated contrast for CT or DSA and Gadolinium containing contrast for MR. Both contrasts should be used with caution for patients with renal failure. Nephrogenic systemic fibrosis is a special disease entity related to the Gadolinium composition of MR contrast.
In this article, we have reviewed various infectious diseases of the CNS, described their clinical presentations, laboratory findings, imaging characteristics and patterns, differential diagnosis and treatment options. Most importantly, a comprehensive review of the patient’s clinical picture prior to reviewing imaging can help direct the search and identify subtle but expected abnormalities that might be otherwise overlooked.
Acute meningitis/encephalitis
Acute meningitis is an infection of the membranes (meninges) covering the brain and spinal cord. It is the most common infectious disease of the CNS. Either leptomeninges (pia and arachnoid matter) or pachymeninges (dura matter) can be affected. The leptomeningeal meningitis is more common than the pachymeningeal meningitis and is more conspicuous on neuroimaging.
The most common etiology of acute meningitis is bacteria. Other common pathogens include viruses and fungi. Protozoans or parasites are less commonly seen in Western countries. The bacterial cases are usually more fulminating than viral ones and often life-threatening but not without exceptions. In newborns, group B Streptococcus, gram-negative bacteria such as E. coli, Klebsiella-Entero and pseudomonas are the main causes of meningitis. Other pathogens include staphylococci and listeria monocytogenes. Beyond the newborn period, the most common causes of bacterial meningitis are Neisseria meningitidis, Streptococcus pneumoniae, and Hemophilus influenza which account for 80% of cases (1-6). All three of these organisms are respiratory pathogens. They first colonized within the mucosa of nasopharynx, oropharynx or larynx, then entered the bloodstream. The most common route of transmission is through hematogenous spread. Others gain access to the CNS via direct spread from local infectious foci, after head trauma or neurosurgery, or during vaginal delivery from untreated GBS-colonized mothers.
The classic triad of clinical presentations of bacterial meningitis consists of fever, headache and nuchal rigidity, although most patients rarely present with all three of the textbook definitions. They often have at least two of the four symptoms of headache, fever, neck stiffness, and altered mental status. Other common presentations include seizures, and focal neurologic deficits. CNS symptoms depend on areas of involvement, cerebral, cranial nerve or spinal nerves. In infants and young children, decreased oral intake, temperature instability, lethargy, and seizures are vague clinical clues calling for a higher index of suspicion for more serious diseases (4-6).
Diagnosis of meningitis is mainly based on clinical findings in suspected patients combining typical symptoms and abnormal lab tests, such as positive CSF gram stain or culture. LP remains the gold standard for diagnosing meningitis. A head CT should be performed before LP to exclude increased intracranial pressure due a mass or other contraindication. Positive cerebral spinal fluid (CSF) cytology is found on the initial LP in 50–70% and in nearly all cases after 3 attempts. Characteristic CSF findings include increased WBC count >1,000/mL; elevated protein level >200 mg/dL and decreased glucose <40 mg/dL. The reported sensitivity of Gram stain for bacterial meningitis has varied from 50% to 90%; however, the specificity approaches 100% when present. CSF culture yield is low and can be negative when blood culture is positive. But blood culture does not point to the source and location of the infection. The yield of Gram stain and culture are significantly reduced if patients have received antibiotics before LP. Other CSF tests such as polymerase chain reaction (PCR) or Latex agglutination test (LAT) are not routinely recommended. However, LP is a labor-intensive interventional procedure, requiring trained medical personnel. Other major drawbacks include the laboratory time required for CSF culture (7,8).
Neuroimaging is extremely valuable in evaluation for meningitis. Nonenhanced CT (NECT) of brain may be normal in early state of meningitis, although negative result cannot exclude the presence of meningitis. Mild sulci effacement, mild ventriculomegaly or acute obstructive hydrocephalus could be an early indication of meningitis. Once the exudate from the inflamed meninges replaces CSF, the previously clear CSF may become almost isodense to brain parenchyma on NECT. Following IV contrast administration, the inflamed meninges and sulci become intensely enhanced. Gadolinium-enhanced MRI is the preferred imaging modality over CT because of its sensitivity and specificity. On MRI, the purulent exudates are T1 isointense with brain parenchyma and T2 isointense with CSF, but hyperintense in subarachnoid spaces on fluid-attenuation inversion recovery (FLAIR) MRI (9). The meninges and subarachnoid spaces often show restricted diffusion on diffusion weighted imaging (DWI). Enhanced MRI shows either diffuse “sugar coating” or thicker, lumpy, multinodular enhancement along the affected letpo- or pachy-meninges. Up to 50% of cases exhibit subarachnoid enhancement with curvilinear patterns that highlight the gyri and sulci. Dura-arachnoid space enhancement is less frequently seen. Post-contrast FLAIR increases the sensitivity of detecting subtle cases (10-12) (Figure 1).
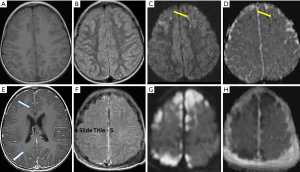
In addition to meningitis, the underlying brain parenchyma often shows coexisting encephalitis or can become affected as a complication of meningitis. The encephalitis is in close proximity of the meningitis with the involved brain parenchyma show similar hypointense T1 and hyperintense T2/FLAIR signals as those of meningitis. It also shows significant restricted diffusion which does not follow the vascular distribution. This feature helps its differentiation from a common infarct as a result of vessel occlusion due to thrombus or atherosclerosis. Its enhancement pattern is variable and ill-defined, unlike the mass-like appearance of a neoplasm. Other complications of meningitis include brain abscess, subdural/epidural empyema, ventriculitis, venous thrombosis, obstructive hydrocephalus, etc. They often demonstrate markedly different imaging findings, some of which will be discussed later in this article.
Differential diagnoses of meningeal enhancement include meningeal carcinomatosis, intracranial hypotension, reactive inflammation after LP, etc. Patients’ clinical history and presentations often provide diagnostic directions. The sulcal-cisternal FLAIR hyperintensity is not specific and can present in other conditions like subarachnoid hemorrhage, CSF metastases, susceptibility or flow artifact, or simply due to a patient being on 100% oxygen support (12).
Prompt and high-dose antibiotics are the mainstay of treatment, with the best being organism-specific. Broad spectrum antibiotics are used in case there is no known causative agent. The use of dexamethasone to reduce intracranial inflammation is advocated after numerous studies proving its association with decreased mortality in bacterial meningitis. Survivors of meningitis/encephalitis often suffer long-term neurological deficits, such as hearing loss, cognitive or motor deficit, visual impairment.
Herpes simplex encephalitis
There are two types of Herpes simplex viruses (HSV), Herpes simplex virus type I (HSV-1) and Herpes simplex virus type II (HSV-2). HSV infections in human are extremely common, with almost half of the US population aged 14–49 seropositive for HSV-1 and 11.9% seropositive for HSV-2 (13).
HSV-1 is oral herpes, transmitted by oral to oral contact. It is often acquired silently earlier in life, staying dormant within the body and becoming reactivated during adulthood.
HSV-1 encephalitis is the most common cause of sporadic fatal encephalitis worldwide, accounting for 10–20% of the roughly 20,000 annual viral encephalitis cases. All age groups are affected, with a third of all cases occurring in children and adolescents. HSV-1 encephalitis includes nearly all cases of HSV encephalitis after early infancy. It is caused by reactivation of the dormant HSV-1 virus. HSV-1 can be harbored in the human body in a dormant form indefinitely, usually in the Gasserian ganglia of the trigeminal nerves. The reactivation of the virus enters the brain through the trigeminal nerve pathway to preferentially affect the ipsilateral temporal lobe. Bilateral temporal lobe involvement is less common but usually asymmetrical (14,15).
HSV-1 encephalitis is fulminant necrotizing encephalitis. The patients’ clinical presentations are usually abrupt but nonspecific, consisting of sudden onset of fever, headache, seizures, and impaired consciousness, etc. The symptoms often progress with rapid deterioration, with a mortality rate of 70% (13,14). CSF analysis is nonspecific and commonly shows a lymphocytic pleocytosis, increase erythrocytes (RBC) and elevated protein. Definitive diagnosis is made by detecting HSV-1 DNA in CSF by PCR testing which has a very high sensitivity and specificity, but the result often takes days to reveal (14). Due to its invasive nature, brain biopsy is not usually required unless in ambivalent or difficult cases. Early intervention by intravenous acyclovir could be lifesaving when HSV encephalitis is suspected but not confirmed.
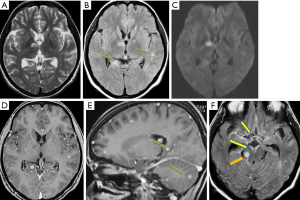
Temporal lobe abnormalities on neuroimaging are highly suspicious of HSV-1 encephalitis, although in early stages, CT of brain with and without contrast could be normal. Suspicion for herpes encephalitis should never be excluded based on a normal CT. Half of the patients usually demonstrate abnormalities on NECT, with low density noted in the temporal lobe and insular cortex, along with mild mass effect. In advanced stages, the affected areas show significant swelling, focal hemorrhages, necrosis and heterogeneous enhancement. On MRI, FLAIR images show asymmetric hyperintensity of the temporal lobes and insula with some restricted diffusion on DWI, correlating histologically with cytotoxic edema. In one study, DWI was found to be slightly superior in diagnosing acute and subacute herpes encephalitis (15,16). Susceptibility weighted imaging (SWI) are more sensitive to show petechial hemorrhages in early disease course which can be easily overlooked on NECT. Contrast enhanced images may show leptomeningeal enhancement and variable gyriform or parenchymal enhancement. Symmetrical involvement of both temporal lobes and the lateral putamen is atypical and may be suggestive of alternate disease processes (Figure 2).
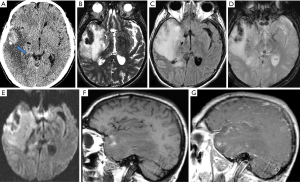
Differential diagnosis include other types of bacterial or viral encephalitis as their symptoms and imaging findings can sometimes be the same and definitive diagnosis can only be made by brain biopsy. Other differential diagnoses include limbic encephalitis, glioma, infarct, etc. Clinical presentations of these conditions are significantly different from HSV-1 encephalitis and much less fulminant. Limbic encephalitis is a group of autoimmune diseases involving the limbic system, including medial temporal lobe, hippocampus, amygdala, etc. It often has a subacute onset of symptoms, such as short-term memory loss, seizures and psychiatric symptoms, and can be associated with cancer within 5 years of diagnosis. Key imaging features which help to differentiate herpes encephalitis from limbic encephalitis is that herpes encephalitis will almost always spare the basal ganglia and is usually unilateral involvement, whereas limbic encephalitis usually involves the basal ganglia and often is bilateral. On MR, limbic encephalitis commonly shows hyperintense T2 signals without hemorrhage or enhancement. A glioma also has an insidious onset. A low grade one shows an infiltrative lesion with hyperintense T2 signals and mild mass effect, but no associated hemorrhage and does not enhance. A higher-grade glioma becomes more heterogenous with significant vasogenic edema and mass effect. It shows areas of necrosis and hemorrhages as well as heterogenous enhancement. Restricted diffusion is usually absent. An infarct of the temporal lobe follows the vascular territories, i.e., an MCA infarct involves the anterior and superior temporal lobe by MCA and a PCA infarct involves the inferior temporal lobe. Its signal intensities on DWI are more intense and more uniform than that of the encephalitis.
HSV-2 is genital herpes, characteristically affecting neonates through direct transplacental transmission or by perinatal transmission via the birth canal. The incidence is about 3–30 per 100,000 live births. In the United States, there are an estimate 1,500 cases of neonatal HSV-2 infection annually (17). Neonate HSV-2 infections can manifest as three forms: localized type with skin, eye and mouth involvement (SEM), CNS infection (encephalitis) without or with SEM, disseminated type. In this article, we concentrate on neonatal HSV-2 encephalitis.
HSV-2 encephalitis involved in approximately 30% of HSV-2 infected neonates. The infected neonates tend to present at 2–4 weeks after birth with fever, seizures, irritability, lethargy and poor feeding. The symptoms can deteriorate rapidly to coma and death. CSF analysis usually shows a mononuclear cell pleocytosis, normal or moderately low glucose and mildly elevated protein. Elevated RBC count is not usually present. Like HSV1, the standard diagnostic test for HSV-2 is also the CSF PCR to detect HSV-2 DNA in CSF (75–100% positive) (16,17).
Prenatal ultrasound of head or fetal MRI may show brain damages or congenital anomalies but results are nonspecific, and the yield is low. Neonatal ultrasound often underestimates the extent of brain damages and should not be used as the sole imaging study in suspected cases (16). NECT of head could be normal at the beginning but quickly show multiple areas of hypo-attenuation, loss of gray-white matter differentiation and sulci effacement in the brain, consistent with brain destructions and vasogenic edema. MRI is more sensitive and may show an early loss of the gray-white distinction with heterogeneously T2 hyperintensity and variable restricted diffusion in the cortex and white matters. Post contrast images show various meningeal or parenchymal enhancements of the diseased areas. Unlike HSV-1, HSV-2 is nonspecific in its regional involvement and may be diffuse or localized but does not show preference for any particular area of the brain parenchyma. In late stages, the affected brain shows multiple cystic encephalomalacia, volume loss and dystrophic calcifications (13,18) (Figure 3).
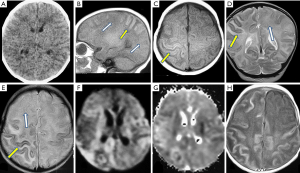
Differential diagnoses include acute meningoencephalitis of either bacterial or viral types, or acute disseminated encephalomyelitis (ADEM). Findings of acute meningoencephalitis have been discussed in the above section above. ADEM usually occurs in children between 3–7 years old and often has a prodrome of viral infection (75%). Neuroimaging studies typically demonstrate diffuse, poorly demarcated, large lesions predominately in cerebral white matters. Bilateral basal ganglia are often involved as well.
Despite treatment, prognosis of HSV-2 encephalitis remains poor and the mortality rate is high. Survivors often have long-lasting neurological damages including seizure disorders, mental or motor retardation, microcephaly, etc.
Eastern equine encephalitis (EEE)
Acute encephalitis caused by arboviruses in the United State is very rare but nonetheless it often captures the headlines of the national or regional news when it happens due to the devastating nature of the disease. Five types of arboviruses found in the US can cause encephalitis: Eastern equine virus, Western equine virus, West Nile virus, St. Louis virus and La Crosse virus. They account for 10% of cases of encephalitis. EEE is the most severe and fulminating type of encephalitis among those. It has a mortality rate of at least 30%, the highest among all arbovirus-caused encephalitis. From 2003 to 2016, a total of 121 human cases were reported, with a median of 8 cases reported annually. The national average annual incidence was 0.03 cases/million people (19). The annual cases rose dramatically in 2019 during which, 38 new cases were reported including 15 deaths.
Eastern equine virus is a mosquito-borne virus, found in the northeast regions of the US, the Great Lakes and along the Gulf Coast. Originally this virus was identified from the horses in Massachusetts in 1831. It was later also identified in other animals such birds and reptiles. Humans acquire EEE through the bite of an infected mosquito releasing the viruses into the bloodstream. The infected mosquito in turn acquires the virus through biting the infected animals and sucking their blood. The first identified human case was in 1938 when there was an outbreak in Massachusetts during which there were 25 deaths from 34 cases (20).
In the United States, although EEE can be sporadic occurring throughout the year, small outbreaks tend to occur each summer. Most people bitten by infected mosquitos are asymptomatic or show mild symptoms such as low-grade fever, malaise, and joint or muscle cramps. Less than 5% of infected people develop acute encephalitis. Patients over 50 years old or younger than 15 years old are at higher risk. Patients on immunosuppressant therapy are also more vulnerable.
Patients usually present with nonspecific but rapidly progressing symptoms at about 4–10 days after the infected mosquito bite: sudden onset of high fever, chills, headaches, vomiting and seizures. Any travel history or outdoor activities should be carefully examined. The symptoms deteriorate quickly to disorientation, coma, and death. Survivors generally have severe sequelae including convulsions, paralysis, and mental retardation.
Diagnosis of EEE is difficult as the clinical picture is often vague. Diagnosis is usually made by demonstrations of immunoglobulin M (IgM) antibody to EEE in CSF or a 4 time increase in serum antibody titers against EEE virus. Neutrophilic pleocytosis may be seen in CSF which is unusual in viral encephalitis (21). In some cases, virus, viral antigen or viral genomic sequences can be isolated from tissue, blood, or CSF. Serum IgM antibodies alone should be confirmed by demonstration of virus-specific neutralizing antibodies. The specimens must be sent to special laboratories in the country and the results are not available until a few days to a couple of weeks after specimen receipt.
Neuroimaging findings are very helpful as they often become abnormal in early stage. On NECT, diffuse heterogeneous hypointense areas are seen involving bilateral basal ganglia and thalami, associated with significant swelling and mass effect. The lateral ventricles and the third ventricle are effaced. Usually there is no evidence of frank hemorrhages. On MRI, heterogeneously hypointense T1 and hyperintense T2/FLAIR signals within bilateral basal ganglia and thalami appear to be striking features and persistent in multiple reports. This is thought to be due to combination of edema, inflammation and ischemia (21,22). Particularly linear areas of hyperintense T2/FLAIR signals within the external and internal capsules with relative sparing of the lentiform nuclei are seen in considerable amount of cases for which a “parentheses sign” has been proposed which may represent a distinguishable feature of EEE (23). The validity of this “parentheses sign” is still debatable and further confirmation is needed when more imaged cases are available. In some cases, abnormal signals extend to the midbrain and pons. DWI shows iso-intense signals without obvious restricted diffusion. In most cases, no enhancement is noted. There are also some reports exhibiting focal areas of hyperintense T2/FLAIR signals affecting the cortex and subcortical white matters of cerebral hemispheres (Figure 4).
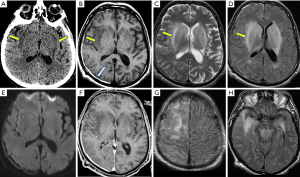
Other types of arthropod-borne viral encephalitis such as West Nile encephalitis, St. Louis encephalitis, show similar imaging findings and should be included in the differential diagnoses. Other diseases involving bilateral basal ganglia and thalami include Creutzfeldt-Jacob disease and hypoxic-anoxic encephalopathy, which have distinct clinical histories, presentations and lab tests. Persistent hyperintense diffusion signals of the diseased areas are most sensitive signs and differentiate them from EEE.
There is no effective anti-viral drug for EEE. Treatment is mostly supportive, including intravenous fluids, intubation, steroids and anticonvulsant. One thirds of patients die despite treatment. Fifty percent of survivors suffer permanent brain damage. At present, no human EEE vaccine is available.
CNS tuberculosis
Tuberculosis infection remains a leading cause of morbidity and mortality in developing countries, which is caused by Mycobacterium tuberculosis (TB), a contagious acid-fast bacillus. According to World Health Organization (WHO), the global incidence of TB is estimated at 10 million, with approximately 1.5 million patients died of TB in 2018 (24). In the United States, the incidence of TB infection is lower; most cases (70%) occur in immigrants from countries with high rates of endemic TB, according to the Center of Disease Control and Prevention (CDC) (25,26). Patients with immunosuppressed status, such as HIV-infected, post-chemotherapy cancer patients are particularly vulnerable to TB. According to CDC, 87.3% of reported TB cases in 2019 have known HIV status; among these 4.9% cases have coexisting HIV infection (25,26).
As a cause of CNS infection, intracranial TB is usually acquired hematogenous from the extracranial sources, most frequently in the GI tract or pulmonary system. It is estimated that about 10% of TB cases present as CNS infections with 60–70% of those occurring in children and young adults under 20 years of age. Tuberculosis infection in the brain manifests in two major ways: TB meningitis and one or multiple caseating granulomas (tuberculomas). Meningitis accounts about 70–80% of CNS presentations and tuberculomas account for nearly all of the remaining 20–30%. Rarely, CNS tuberculosis can present as an abscess (18,26). CNS TB infection has a mortality of 25–30%.
The typically symptoms of TB meningitis are like those observed in bacterial meningitis: nuchal rigidity, fever, headaches, vomiting, but in a subacute form and progressing gradually. Other findings include altered mental status, personality changes, cranial nerve palsies, …, depending on the location or forms of infection (focal tuberculoma vs. meningeal involvement), with its severity ranging from headaches and mild meningeal symptoms to seizures and coma.
Diagnosis of CNS tuberculosis is challenging due to suboptimal sensitivity and specificity of diagnostic tests. A definitive TB diagnosis in extra-neural site can be a strong indicator for CNS TB. LP should be avoided if there is concern for elevated intracranial pressure and risk of brainstem herniation. When CSF could be obtained, it typically shows lymphocytic pleocytosis, elevated protein, and decreased glucose. Microscopic proof of acid-fast bacilli (AFB) in CSF can give immediate result but usually require a large volume; Positive CSF culture for M. tuberculosis can be seen in less than 50% cases; the nucleic acid amplification test (NAAT) of CSF must be used in combination with AFB smear and culture. It has a rapid turnaround time with high sensitivity and specificity. But it has not been approved by the US Food and Drug Administration. A definitive diagnosis of tuberculoma is established by brain biopsy for histopathology and AFB stain and culture. Many times, a presumptive diagnosis is made for initiation of treatment in the setting of relevant clinical and epidemiologic factors and typical CSF findings (27,28).
In early stages of TB meningitis, NECT is normal in approximately 30% of cases. Typical manifestations of TB meningitis include hydrocephalus, basilar exudates, periventricular infarcts, and multiple tuberculomas. Hydrocephalus can be seen in up to 75% cases. Basilar exudates commonly appear iso- or hyperdense on NECT which cause effacement of the related sulci and basal cisterns. On MR, these thick proteinaceous exudates demonstrate hypo or iso-intense T1 signals and hyperintense FLAIR signals. Contrast enhanced CT or MR may show uniform and intense enhancement along basilar meninges. Acute cerebral infarcts may occur in up to 50% patients with advanced disease and mostly involving basal ganglia (18,27,28).
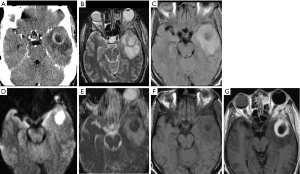
On CT, tuberculomas appear as as one or more round or lobulated hypo- or iso-dense lesions with edema out of proportion to mass effect but little enhancement in early stages and encapsulated hyperdense lesions with ring enhancement on late stages. These vary widely in size and most are less than 2.5 cm in diameter. They may demonstrate central calcification in chronic lesions. On MR, tuberculomas appear as discrete, single or multiple hypointense T1 and hyperintense T2 lesions with ring or homogeneous disc-shaped enhancement. Larger tuberculomas with necrotic centers may show restricted diffusion within the fluid cavities on DWI. They usually exert considerable surrounding edema and mass effect (18). Sometimes, innumerable small tuberculomas of a few millimeters in sizes can be observed in the brain of patients with a military TB (Figure 5).
Differential diagnosis of TB meningitis includes other pyogenic meningitis, especially those with predilection to basal cisterns such as Mycobacterium avium infection (MAI), syphilis or other granulomatous infections. Meningeal carcinomatosis is another common differential diagnosis if patient has a known neoplasm elsewhere. The differential diagnoses of tuberculoma include neurocysticercosis, a pyogenic abscess and a primary or metastatic neoplasm differentials.
The classic RIPE (rifampin, isoniazid, pyrazinamide, ethambutol) therapy is the first-line treatment for tuberculosis. Generally, 12 months of antibiotic therapy is considered adequate to eliminate even intracranial tuberculomas. The CDC also recommends dexamethasone for 6–8 weeks for management of tuberculous meningitis. In cases of hydrocephalus that were noncommunicating or resistant to diuretic therapy, the placement of a ventriculoperitoneal shunt is effective in relieving intracranial pressure and improving outcomes even in severe cases.
Extra-axial empyema
Intracranial empyemas have two types: epidural empyema and subdural empyema. Epidural empyema is also called epidural abscess, a collection of pus between the dura matter and the calvarium. Subdural empyema is a collection of pus between the arachnoid matter and the dura matter. Epidural empyemas have biconvex shapes that are confined by sutures but can cross midline by dissecting across dural sinuses. Subdural empyemas have crescent shapes that are not confined by sutures but limited by falx cerebri, tentorium cerebelli, and foreman magnum. It otherwise can spread freely to adopt the convexity of the cerebral hemispheres or line the interhemispheric fissure. Both types of empyemas can occur at any age though more common in adults, with no gender predilection and are responsible for 20–33% of all intracranial infection (5).
In older children and adults, the empyemas are predominantly acquired through direct spread from an adjacent infectious focus, such as paranasal sinusitis, otitis media, mastoiditis, or skull OM, while in infants, the leading cause of empyemas are pyogenic meningitis. Alternatively, distal infections can seed into these potential extra axial spaces through hematogenous transmission or can track up the CSF flow from spinal infection. Subdural empyema was also reported as a complication following invasive procedures such as evacuation of chronic subdural hematomas, and as such can be confused with a recurrent subdural hematoma without advanced neuroimaging (29-31). Causative organisms are dependent on the initial source of infection; in infants, the group B streptococci, E. coli and listeria monocytogenes are the most common causes of pyogenic meningitis; while in older population the isolated culprits include aerobic and anaerobic streptococci, staphylococci, aerobic gram-negative bacilli, anaerobes, and fungi (5,18).
Diagnosing extra-axial empyema is difficult based on clinical findings alone due to nonspecific presenting symptoms and laboratory tests. Fever, headache, lethargy, vomiting, and seizures, in combination with sinusitis, otitis media, mastoiditis, recent neurosurgery or head trauma should raise clinical suspicion. LP is not indicated as it provides little useful information and may precipitate brain herniation. Early diagnosis of extra axial empyema is crucial as patient may progress rapidly to coma or death.
Neuroimaging findings often provide more definite diagnosis. NECT is the gateway imaging modality that may show abnormal epidural or subdural fluid collections though subtle cases can be missed. The purulent materials may be hypodense to isodense to brain on NECT. Subtle effacement of the underlying cortical sulci or mild compression of the ventricle hints at mass effect in less obvious cases. On CECT, there is diffuse enhancement along the periphery of the fluid collections. MRI is the imaging modality of choice for evaluating empyemas. On none-enhanced MRI, empyema shows an equal inconspicuousness on T1WI—hypointense to isointense to the brain parenchyma, and mildly hyperintense to CSF. On T2WI and FLAIR, empyema gives off heterogeneous high intensity signals. Markedly restricted diffusion of the stagnant pus in extra axial spaces are the characteristic and crucial findings of empyemas, differentiating them from other nonrestricted extra-axial collections like subdural effusion, hygroma, or hematoma. Many times, the adjacent brain parenchyma shows similar hypointense T1 and hyperintense T2/FLAIR signals with restricted diffusion indicating associated encephalitis or ischemia. After intravenous contrast administration, the granulomatous or fibrous capsules surrounding the empyemas demonstrate diffuse meningeal enhancement in both CT and MR (32-34). Differentiation of subdural and epidural empyemas is based on its locations and morphological appearance, as to whether the collection is bounded by calvarial sutures or falx (Figures 6,7).
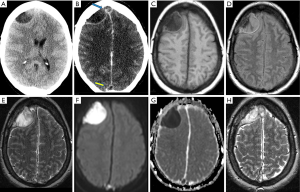
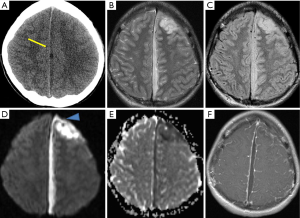
The epidural or subdural hematomas are the main differential diagnoses for epidural or subdural empyema respectively. On NECT, acute hemorrhages appear hyperdense. This hyperdensity gradually decreases over time. MRI signal characteristics of hemorrhages change over time as well. From acute to subacute stages, the hematoma changes from iso/hypo- intense to hyperintense on T1WI and iso/hyper- intense to hyperintense on T2 WI. From the subacute to chronic stages, it appears hyperintense on both T1WI and T2WI. Another differential diagnosis is subdural effusion which is usually a complication of meningitis. Its appearance and location are similar to the subdural empyema, but the fluid remains hypodense on CT and hypointense on T1WI of MR without enhancement. It shows no restricted diffusion on DWI.
The major complications of empyema include cerebritis, abscess formation, infarct, and cortical vein or dural sinus thrombosis, which have been related to significantly worse prognoses. Mortality can be as high as 10–20% despite early diagnosis and appropriate treatment with surgical evacuation and aggressive antibiotic therapy (5,32,33). Wide craniotomy is often preferred over Burr hole drainage, which has better clearance of empyema and improved clinical outcome.
Brain abscess
Brain abscess is a collection of pus surrounded by a well-vascularized capsule, enclosed in the brain tissues, usually as a result of a bacterial or fungal infection. Its development has 4 stages: at the first, the related brain tissues develop an acute infection, i.e., early cerebritis (1–4 days); second, the cerebritis progresses to “later cerebritis” (4–10 days), with the brain tissues become necrotic, starting to form a cavity containing purulent materials, i.e., a pyogenic abscess. The third stage (11–14 days) and the fourth stage (>14 days) delineate the formation of a vascularized capsule surrounding the abscess (35). Brain abscess is a serious and potentially life-threatening condition. Without treatment, it is invariably fatal.
Brain abscess is an uncommon disease and its incidence varies among different countries. It accounts for 8% of intracranial masses in developing countries and 1–2% in western countries. In the United States, approximately 1,500–2,500 cases are diagnosed annually. Over the past three decades, mortality from brain abscess has decreased from 30–40% to 5–20%, with the higher rates reported in developing nations. The highest incidence group is adult men below 30 years of age while children between ages 4–7 are the second highest group who often are associated with cyanotic heart diseases. About 25% of patients are less than 15 years of age. The affected male to female ratio is 2:1 to 3:1 for undetermined reasons (35,36).
Like many other CNS infections, brain abscess can be acquired via two routes: hematogenous dissemination through septicemia from a distant extracranial source such as pulmonary infectious processes and urinary tract infection; or direct spread from nearby infectious focus such as paranasal sinusitis, mastoiditis, or otitis media. Other possibilities include complications of local head trauma or recent neurosurgical interventions. Brain abscesses associated with septicemia commonly cause multiple abscesses, mostly in the distribution of the middle cerebral artery (MCA) and usually at the gray-white matter junction. Direct spread of primary infection intracranially usually causes a single abscess in the contiguous brain tissues.
The most common etiology is bacterial, while fungal or parasitic etiologies are less common. Up to 30% of brain abscesses are reported with unknown origin. In immunocompetent patients, the most common causative microorganisms are Streptococcus species, Staphylococcus aureus and pneumococci. Klebsiella commonly affects diabetics while Aspergillus and Nocardia are common in transplant patients. Bacterial abscesses are less common in immunocompromised patients (37). Toxoplasmosis and Mycobacterium tuberculosis are most common in HIV/AIDS patients (see later sections).
The patients’ clinical symptoms depend on the location of the abscess, degree of mass effect, and associated complications. Unfortunately, the most frequent presenting symptoms are nonspecific, including headache, fever, focal neurologic deficit, nausea, vomiting, and seizure.
Diagnosis is based on combination of clinical findings and neuroimaging results. Serology tests are nonspecific. LP is usually not helpful for diagnosis and often contraindicated if patients show papilledema or other focal symptoms or signs because of possible increased intracranial pressure.
Neuroimaging findings of cerebral abscesses vary depending on the stages of the abscesses and time of imaging. Early cerebritis appears as an irregular area of hypodensity that does not enhance following contrast injection. As cerebritis evolves, the hypodense lesion enlarges with thick and diffuse ring enhancement following contrast injection. The ring enhancement represents breakdown of the blood-brain barrier and the development of an inflammatory capsule. In the capsular stage, NECT shows a hypodense cavitating lesion with some dependent debris as well as surrounding vasogenic edema and mass effect. Post contrast CT shows a thick ring or nodular rim enhancement. The rim enhancement may not be uniform in thickness and is often less prominent at the medial or ventricular surface of the abscess (36-38). On MR, the typical findings of an abscess, i.e., in the capsular stage, are a cystic lesion with central hypointense T1 and hyperintense T2/FLAIR signals. The capsule may be visible as a hypointense or intermediate rim on T2/FLAIR images. On the susceptibility images, there may be a hypointense rim suggesting hemorrhages. The surrounding vasogenic edema shows irregular hypointense T1 and hyperintense T2/FLAIR signals. The marked hyperintensity of pus in the necrotic center on DWI with corresponding hypointense signals on ADC map is the characteristic finding of a pyogenic brain abscess (38,39). On postcontrast MRI, a smooth thin continuous ring is most suggestive of an abscess (Figure 8).
Major differential considerations include cystic neoplasms and tumefactive multiple sclerosis (MS). Most cystic neoplasms of the brain will not demonstrate restricted diffusion aside from dermoid tumors and CNS lymphoma. Additionally, clinical history will be highly important with symptoms of fever, headache, and leukocytosis raising much higher concern for abscess versus tumor. Tumefactive MS is a rare form of MS in which the MS plaque shows a “tumor-like” appearance with its size greater than 2 cm, associated with surrounding edema and mass effect. It usually has a hypointense T2 rim and incomplete ring enhancement. Multiple smaller but classical MS plaques are often seen in the rest of the white matters. Patient’s clinical history also provide additional clue for differential diagnosis.
Complications of brain abscesses are various, including local mass effect with intracranial herniation, hydrocephalus, meningitis, extra-axial empyema and venous/dural sinus thrombosis. Successful treatment depends on a combination of intravenous antibiotics and neurological intervention for abscess drainage. A target-specific antibiotic is applied when the culprit is known. Otherwise, a broad-spectrum antibiotic is employed. Radiology imaging is paramount for the early detection of a brain abscess and evaluation for its complications as well as monitoring its treatment.
Ventriculitis/ependymitis
Ventriculitis refers to infection and inflammation of the ventricles including and its contents including ventriculitis, ependymitis, ventricular empyema, and pyocephalus. Specifically, ependymitis refers to the diseased ependymal lining. Ventriculitis is an uncommon complication of severe intracranial infections, such as meningitis, encephalitis or brain abscesses. It is the result of the spread of pyogenic infections into the ventricles or along the ependyma. The routes of transmission include extension of pre-existing meningitis/encephalitis to the ventricles via the subependymal or choroid plexus, rupture of brain abscess, or contiguous extension from trauma or neurosurgical interventions such as an external ventricular drainage. Ventriculitis is estimated to occur in approximately 30% of patients with meningitis. It is more often seen in neonatal meningitis which occurs in 20% of cases but comprises 80% to 90% of all patients with ventriculitis. Staphylococcus and Enterobacter are the two most common causative microorganisms (40).
Besides the clinical manifestations of the primary infections, such as meningoencephalitis or pyogenic abscess, there are no reliable clinical signs of ventriculitis. Nonresponsive to antibiotics may raise a high index of suspicion. Blood tests are usually nonspecific. Gram stain or culture of CSF can be done and may be positive if drainage is required for obstructive hydrocephalus. Other CSF findings are nonspecific for infection: leukocytosis, decreased glucose and elevated protein levels. Increased intracranial pressure is usually present.
The diagnosis of ventriculitis is challenging as the clinical pictures are nonspecific but devastating. NECT may be normal or show periventricular hypodensities which could be due to periventricular inflammatory changes or transependymal fluids. Suppurative debris layering in the dependent areas of ventricles appears hyperdense and is the most characteristic finding in one series. Ependymal enhancement may possibly be seen on contrast enhanced CT. Characteristic MRI imaging includes restricted diffusivity on DWI evident within the dependent debris or along the ependymal surface. The layering debris in the occipital horns is T1 hyperintense relative to CSF and T2 hypointense. A periventricular halo of FLAIR/T2 as well as focal or diffuse enhancement along the ventricles is highly suggestive of ependymitis. Restricted diffusion on DWI along the ependymal surface or in the dependent areas of the ventricles are strong evidence of ventriculitis/ependymitis and accumulated pus. Sometimes, a poorly marginated swollen and enhancing choroid plexus is noted, consistent with choroid plexitis. This could be another characteristic MRI finding of ventriculitis (40,41) (Figure 9).
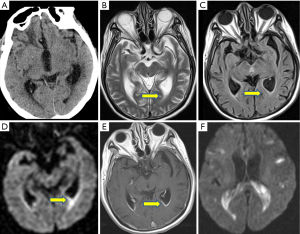
Differential diagnoses of ventriculitis on imaging include subarachnoid or ependymal tumor seeding and intraventricular hemorrhages. Although subarachnoid or ependymal tumor seeding may show ependymal enhancement but hyperintense diffusion signals along the ventricles generally separate them from ventriculitis/ependymitis. The clots of intraventricular hemorrhages also do not show restricted diffusion on DWI.
Ventriculitis/ependymitis is associated with high morbidity and mortality. Early detection and treatment offer the best hope for survival. The mainstay of treatment is intravenous antimicrobial therapy which can reach effective concentrations in CSF. Specific antibiotics are chosen based on CSF culture and penetration into CSF when meningeal inflammation is present. For catheter-related ventriculitis, removal of all components of the infected shunt or EVD, in combination with antimicrobial therapy is recommended.
Septic-embolic encephalitis (SEE)
SEE is caused by infected thromboemboli occluding the blood vessels of the brain. The affected brain manifests with two attributes of the disease: an ischemic insult due to vascular occlusion and an infectious insult from a deep-seated infection nidus. The ischemia may lead to brain infarcts; The infection may develop into brain abscesses. Mycotic aneurysms can also develop from the locations of septic emboli, which account for less than 10% neurological complications of infective endocarditis (IE). Intracranial hemorrhages could be complications of ischemic stroke, brain abscesses or rupture of a mycotic aneurysm (42,43).
SEE is usually a part of a systemic infection with septic emboli in multiple organs of the body, and the brain being the most common location. The most common origin of septic emboli is IE. Staphylococcus aureus is the leading causative microorganism, followed by Streptococcus viridian, enterococci and coagulase-negative staphylococci. IE patients with vegetation size >3 cm, Staphylococcus aureus infection, mitral valve involvement, and anticoagulation therapy are at higher risks of septic emboli. Other commonly reported sources of septic emboli include sepsis from intravenous drug use, infection of instrumental devices such as indwelling catheter or pacer wires, pulmonary abscess, etc. (44-46).
Symptoms of SEE vary from asymptomatic to severe with a high mortality. Ischemic stroke is the most common presenting symptom of SEE; 15% of patients present with transient ischemia. Neurological deficits depend on the location and extend of the disease. Other symptoms include altered mental status, psychosis, headaches, etc.
Serology tests show signs of systemic infection such as leukocytosis, elevated ESR, etc. The inciting pathogen may be identified by gram stain. Blood culture is almost always positive. LP is not usually performed as it provides little useful information. Onset of neurologic deficits in patients with a systemic infection should raise a high index of suspicion for SEE. Positive blood culture and neuroimaging findings point to a more definitive diagnosis.
Neuroimaging findings of SEE are commonly composed of ischemic infarct and brain abscesses. The ischemic infarct mostly occurs in the distribution of MCA and rarely in posterior circulation. Among IE patients, 20–55% present symptomatically with ischemic or hemorrhagic stroke; 60–80% are asymptomatic but show positive findings on brain MRI. Abscesses caused by septic emboli are usually multiple, small and of various ages, different than the brain abscesses described in the earlier section of this article. They could be seen in multiple vascular distributions, but mostly concentrated along the peripheral watershed territory or gray-white interface. If manifesting as cerebral microbleeds, the lesions are preferentially found in cortical areas (42-44). NECT scans show multiple low-attenuation foci, with or without microhemorrhage. Punctate or ring enhancement is noted after contrast administration. MR scans show multiple abscesses of various sizes in the brain—multiple cavitating lesions with rim-enhancement. The central lumen is associated with restricted diffusion. Hemorrhagic lesions can be correlated with “blooming” artifacts on susceptibility sequences. Similar to CT, contrast enhanced T1 images often demonstrate punctate or ring enhancement, similar to micro abscesses (Figure 10).
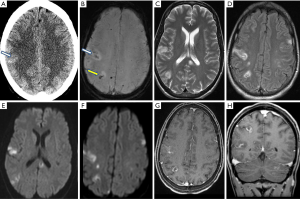
The main differential diagnoses include metastasis and MS. The images of metastasis show multiple round well-circumscribed ring-enhancing lesions which are often similar in sizes and typically locate in or near gray-white matter junctions. They are usually associated with significant vasogenic edema but lack of central restricted diffusion. MS usually has a long relapsing and remitting course, commonly present with typical imaging findings such as periventricular Dawson’s fingers, corpus callosum lesions, etc. The enhancement of MS plaques varies from solid to incomplete ring enhancement which only occurs in active demyelinating phase.
Management of SEE requires a multidisciplinary approach. Early organism-specific antibiotics are always recommended unless cryptogenic in which case a broad-spectrum antibiotic is recruited. Anticoagulation and thrombolysis are not therapeutic and should be avoided due to complications. Endovascular intervention can be helpful in case of acute septic emboli or mycotic aneurysm. Early cardiac surgery for endocarditis is proven safe in the setting of recent cerebral ischemic events (42). Current guidelines recommend surgery to prevent recurrent embolism in high-risk patients or prophylactic surgical intervention for the patients with vegetation >10 mm with severe valvular dysfunction.
Human immunodeficiency virus (HIV)-associated opportunistic infections of the CNS
HIV damages the immune system of the human body, making it vulnerable to infections and neoplasms. Most of these opportunistic infections were rare in the pre-HIV epidemic era and manifested due to the immunocompromised status of HIV patients. Acquired immunodeficiency syndrome (AIDS) is a more advanced stage of HIV infection. Well-known CNS infections in this population include toxoplasmosis, cryptococcosis, progressive multifocal leukoencephalopathy (PML), cytomegalovirus encephalitis, neurosyphilis, and tuberculosis. In the late era of highly active or combined antiretroviral therapy (HAART or cART), opportunistic CNS infections in HIV-positive patients have declined drastically. In a retrospective study, cerebral lesions were detected in 21.8% of patients in the pre-HAART era versus 7.6% in the early HAART era. (47). Lack of access to HAART or cART, with persistent low T-lymphcyte (CD4) cell count, has been associated with higher risk of CNS infections. A small percentage of these patients may experience immune reconstitution inflammatory syndrome (IRIS)—paradoxical worsening of clinical condition following initiation of HAART, in setting of active infection. HAART should be held for at least two weeks to reduce the risk of IRIS.
Toxoplasma encephalitis (TE)
TE or cerebral toxoplasmosis caused by toxoplasma gondii—an obligate intracellular protozoan—is the most common CNS infection in AIDS patients and the leading cause of death within this population. Its incidence is 15–50%, increased to about 30% when their CD4 <100. Studies have emphasized that the importance of continued HAART and prophylactic treatments could reduce the risk of TE by 50% when patients are on antiretroviral therapy and 53% when patients are mainly treated with prophylaxis (47,48).
Clinical symptoms TE is vague and indolent. The most common presenting symptom is headache, often accompanied by altered mental status and fever. Movement disorder and ataxia should raise suspicion for toxoplasmosis, correlating to radiologic or postmortem findings of brain abscesses most commonly involving the basal ganglia and the corticomedullary junction. Other nonspecific symptoms include but are not limited to dementia, ataxia, lethargy, and seizures. A presumptive diagnosis can be made if the patient has a CD4 count <100 cells/mL, in combination of compatible clinical symptoms, positive serological anti-toxopalsma IgG antibodies and typical neuroimaging findings. Definite diagnosis is made with detection of DNA of toxoplasma by PCR in CSF (50–98% sensitivity; 96–100% specificity), with brain biopsy required in certain cases (49).
Neuroimaging findings are not pathognomonic, but some features are more commonly present. On NECT, TE typically manifests as multiple hypodense lesions associated with significant vasogenic edema, often with a predilection for the basal ganglia and thalami. Corticomedullary junction is another common location. Calcifications are not present except in congenital TE. Thin smooth rim enhancement or sometimes eccentric nodular enhancement can be seen after intravenous contrast administration, which becomes more conspicuous with higher white count, and usually absent with CD4 <50 cells/cc (50). On MRI, TE demonstrates multiple focal lesions with hypointense T1 and hyperintense or mixed T2 signals, in the similar locations within the brain. These lesions may have hyperintense T1 signals along the rim, presumably representing coagulative necrosis or hemorrhage. Typical ring enhancement is seen in 90% cases. Nodular enhancement is sometimes noted. At times, a small eccentric nodule abutting an enhancing ring (i.e., inflamed vascular zone surrounding the necrotic abscess) was called a “target sign” and was thought to be highly suggestive of toxoplasmosis. Marked perilesional edema appear hypointense on T1 and hyperintense on T2/FLAIR images. On DWI, the core tissues of ring-enhancing toxoplasma abscesses usually show no restricted diffusion, unlike pyogenic abscesses. The peripheral hyperintensity on DWI may represent the presence of hemorrhage within their walls or due to T2 shine-through phenomenon of associated edema (51,52) (Figure 11).
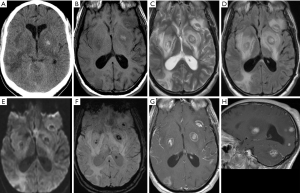
The differential diagnosis of multiple ring enhancing lesions in the brain is extensive. The most common ones are metastatic disease and septic emboli among the non-immunocompromised patients and lymphoma among the immunodeficient patient. Metastatic disease often has a known history of tumor and presents as multiple round well-circumscribed ring-enhancing lesions which are often similar in sizes and typically locate in or near gray-white matter junctions. They are usually associated with significant vasogenic edema but lack of central restricted diffusion. Ring enhancing lesions of SEE tend to locate at corticomedullary junction, more commonly in MCA distribution without predilection to basal ganglia. Emboli also cause acute infarcts in the associated vessel distribution/territory which are not seen in toxoplasmosis or metastatic disease. The infection can cause multiple abscesses of various sizes in the brain with central lumen associated with restricted diffusion. Lymphoma may sometimes manifest as ring enhancing lesions. But their sizes are usually larger than those in TE, and more infiltrative in appearance and more heterogeneous in enhancement. They typically show restricted diffusion. The corpus callosum and periventricular areas are more commonly involved (49,51).
The treatment of TE comprise antimicrobial therapy directed against T. gondii and antiretroviral therapy for immune recovery. A combination of pyrimethamine with sulfadiazine and folinic acid is the most common regime, typically for 6 weeks. Initial therapy is followed by secondary prophylaxis or maintenance therapy which is continued until CD4 counts are over 200 cells/microliters for more than three months.
Cryptococcal meningoencephalitis (CME)
Cryptococcus neoformans is a yeast-like fungus that may result in opportunistic infections in immunocompromised patients. It is the most common fungal infection in HIV-positive patients and the AIDS-defining illness for 60–70% of HIV-positive patients. CNS cryptococcal infection is the third most common infections of the brain besides HIV encephalopathy and TE. It likely occurs in patients with recently diagnosed HIV infection and with CD4 counts <100 cells/µ. This infection is responsible for 600,000 deaths worldwide each year, predominantly in populations with less access to HAART therapy. Its incidence has significantly declined in recent years with the advent of HAART (53,54).
The most common manifestation of CNS cryptococcal infection is meningoencephalitis (CME) as brain parenchyma is almost always involved, though sometimes only detected by histologic examination (55). The inflammatory response in the brain is generally milder than that seen in bacterial meningoencephalitis. Cryptococci tend to proliferate in the subarachnoid spaces and spread into the perivascular spaces. Growth of the microorganism leads to formation of mucoid materials in the perivascular spaces, commonly referred as gelatinous pseudocysts. They are usually seen in the basal ganglia, corticomedullary junction and dentate nuclei. In general, involvement of the brain is diffuse, but localized infections-cryptococcomas, may also be seen in about 10% of patients.
Symptoms of CME usually develop indolently over a period of weeks. They are nonspecific including headache, fever, malaise. Classical meningeal symptoms and signs, such as neck stiffness, photophobia and vomiting are present in 1/4 to 1/3 of patients. Isolated fever and headaches in advanced AIDS patients with CD4 <100 cells/mL should raise a high index of suspicion for CME.
LP shows a decreased WBC count with a mononuclear predominance, slightly elevated protein and low glucose concentration in CSF; elevated opening pressure has been observed in 70% of AIDS patients but less frequent in non-HIV-infected patients. Diagnosis of CME is established by identification of cryptococcal yeast on Indian ink stain smear of CSF (75%) or CSF culture; other highly sensitive and highly specific diagnostic tests in AIDS patients (greater than 90% sensitivity and specificity) are finding cryptococcal antigen (CrAg) and positive cryptococcal PCR in CSF (53,54,56).
The challenge in diagnosing CNS cryptococcal infections lies in its wide spectrum of nonspecific neuroimaging findings, from normal to nonspecific patterns of meningitis, meningoencephalitis, and multiple abscesses. Typical findings of a subacute cryptococcal meningitis or meningoencephalitis include meningeal enhancement without or with abnormal signals of the adjacent brain. The meningeal enhancement is not commonly present on both CT and MR but tend to build along the brain base when present. A communicating hydrocephalus may raise clinical suspicion. Gelatinous pseudocysts on NECT show multiple small hypodense cysts in or around the basal ganglia and the corticomedullary junctions. On MRI, these cysts appear hypointense on T1 and hyperintense on T2/FLAIR but are slightly different than CSF signals. They may show no or mild peripheral enhancement. Perilesional edema is generally not evident. Restricted diffusion is usually not seen in most lesions, but its presence in the absence of meningeal enhancement or parenchymal enhancement should be a strong indicator of this disease. Cryptococcomas, either as a solid enhancing mass or as disseminated enhancing nodules in the midbrain and basal ganglia, are found in 4–11% cases. Multiple military enhancing parenchymal and leptomeningeal nodules with choroid plexus involvement are demonstrated in rare cases (18,52,54) (Figure 12).
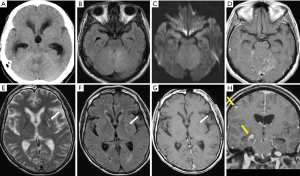
The standard antifungal therapy, Amphotericin B in combination with flucytosine, often performs poorly. Steroids do not improve survival and may contribute to adverse effects in cryptococcal meningitis, thus are only recommended in a subgroup of patients who have cryptococcomas with mass effect or IRIS (53,54).
Progressive multifocal leukoencephalopathy (PML)
PML is a rare and fatal opportunistic infection, caused by the John Cunningham (JC) virus. It occurs in up to 5% of AIDS patients, especially when patient’s CD4 counts is <200 cells/mL. People on chronic immunosuppressive medications are also at increased risk. Although the infection rate has gone down in the late-HAART era, poor prognosis still makes PML a daunting diagnosis. If untreated, the mortality rate is 30–50% within the first three months of diagnosis (57-59).
The JC virus is ubiquitously present in human bodies in dormant forms. Reactivation of the latent virus affects oligodendrocytes of the brain in immunocompromised patients leading to massive demyelination of white matter.
Clinical signs and symptoms are diverse, depending on affected locations of the brain and the degree of damage, typically including clumsiness, progressive weakness, visual defects (cortical blindness), language disturbance, behavioral changes, etc. Symptoms may evolve over the course of weeks to months, progressing rapidly in the last few months which usually leads to severe disability or frequently death (57-59).
Diagnosis of PML can be made on combination of positive CSF viral PCR of JC virus and progressive white matter disease on neuroimaging. Routine blood studies should include HIV serology and viral load if HIV status is unknown. PCR is the preferred way of diagnosis, but brain biopsy remains the gold standard when another disease entity such as CNS lymphoma is part of the differential diagnosis. Histopathologic triad of lytic demyelination of oligodendrocytes, bizarre astrocytes, and enlarged oligodendroglial nuclei are characteristic on brain biopsy or autopsy (58-60).
Typical white matter disease caused by PML consists of unilateral or bilateral but asymmetrical areas of demyelination that do not conform to any vascular territories in the brain. Mass effect is seen in acute phase, while encephalopathic changes with atrophy and volume loss are observed in chronic PML. Contrast enhancement is rare and, if it occurs, is patchy. The supratentorial lobar white matter is the most common location. PML lesions usually begin in the subcortical white matter of the parieto-occipital or frontal lobes but may also involve the corpus callosum, brainstem, pyramidal tracts, and cerebellum. The second most common location is cerebellar peduncle. At the early stage, patients may show small, scattered subcortical white matter foci, which later develop bilateral asymmetric, large confluent lesions extending to the subcortical U fibers. The so-called “scalloping out” of the gray-white border refers to sparing the cortical ribbon. The cortex is generally spared even in advanced disease (59,61,62).
CT of the head may reveal focal ill-defined hypodensities in subcortical and periventricular white matters without mass effect and little contrast enhancement. These lesions could be unilateral and small at the beginning but invariably progress to a more confluent appearance with bilateral but asymmetrical distribution in the white matters. On MR, these lesions appear hypointense on T1 and hyperintense on T2/FLAIR images. “Spongy” lesions with marked hyperintense foci within the background of relatively less hyperintense confluence are found in later stages of PML. On DWI, PML lesions may show a hyperintense rim along the periphery or advancing edge of the lesion but no or little hypointense signals on corresponding ADC images (61,62). Enhancement is not typically seen in HIV-related PML without IRIS (18,63,64). IRIS is a syndrome of paradoxical worsening of neuroimaging findings when patients’ clinical symptoms improve while receiving HAART therapy. Interestingly, lesion enhancement on CT or MRI is found to be a predictor of long-term survival in AIDS patients with PML in one study (62,63) (Figure 13).
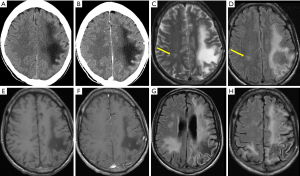
Major differential diagnoses of PML include posterior reversible encephalopathy syndrome (PRES) in non-immunocompromised patients and lymphoma in immunodeficient patients. PRES can be caused by malignant hypertension, eclampsia and some medical treatments. Typical finding is relative symmetrical edema of subcortical white matters, especially on both sides of the parietal and occipital lobes. CT images show ill-defined hypodensities of the affected areas without mass effect. On MRI, these lesions show hypointense T1 and hyperintense T2 signals without enhancement. Because these lesions represent vasogenic cerebral edema, DWI shows iso- or hypo-intense signals with hyperintense signals on ADC map (65). CNS lymphoma may mimic PML lesions on non-enhancement images. But it often shows cortical involvement, moderate edema and diffuse contrast enhancement as well as restricted diffusion. CSF analysis is also helpful for its differentiation.
There is no effective treatment PML, besides restoring the immune system with HAART. Paradoxical brain damage resulting in fulminant IRIS following HAART further complicates the PML course, subsequently requiring early and long-term steroid therapy. Prognosis is poor even with treatment. Studies have shown a 6-month mortality of 45.5% in late-presenting HIV-positive patients with PML, and a 1-year survival of 46.4% in patients on HAART.
Spinal osteomyelitis (OM)/discitis and spinal epidural abscess (SEA)
OM and discitis of the spine are bacterial infections of the vertebral bodies and the intervertebral discs. SEA is an infectious fluid collection (pus) within the epidural spaces of the spinal canal. SEA is usually concomitant with spinal OM and discitis, although at early stages, each of them can exist independently.
Native vertebral OM/discitis cases are uncommon, with an estimated incidence of 4.8 cases per population of 100,000. The incidences have doubled from 1998 to 2013 (66). It is a serious condition with a reported 1-year mortality rate of 11%. OM primarily occurs in adults, with half of patients are more than 59 years old. Male (51%) and female are almost equally affected. However younger people have become more frequently affected in the United States due to the IV drug abuse epidemic (67).
Staphylococcus aureus is the most common isolated organism in native OM or OM after previous instrumentation (indwelling catheter, spinal surgery) as well as SEA, accounting for 1/2 to 2/3 of cases in developed countries. Coagulate-negative Staphylococcus and Streptococcus are second most common in native OM and OM after instrumentation respectively (68). Other etiologies include Gram-negative or anaerobic organisms. OM in IV drug abusers had a higher prevalence of polymicrobial infection (69). Identification of the inciting bacteria could be done by CT guided biopsy and open surgery biopsy which, when combined, showed more yield than blood cultures (77% vs. 58%).
The most common route of transmission of OM/discitis is hematogenous spread from other part of body, such as urinary tract infection or endocarditis. Contiguous spread from the infected adjacent structures such as aorta, esophagus can be seen, and many times accompanied by paravertebral phlegmon or abscesses. Previous trauma or instrumentation (i.e., spinal surgery) increases the risks of the infection. IV drug abuser and previous instrumentation have higher incidence of methicillin-resistant Staphylococcus aureus (MRSA) infection. The rate of recurrent infection is also higher among MRSA infections. Higher mortality rate is associated with increased age, male gender, and other related clinical conditions such as congestive heart failure, cerebrovascular diseases, liver or renal diseases (70-72).
The lumber spine is most commonly affected, followed by thoracic spine and cervical spine. Since segmental arteries supplying the vertebrae usually bifurcate to supply two adjacent end plates of contiguous vertebrae, thus hematogenous vertebral OM often causes bone destruction in two adjacent vertebral bodies and usually destroys their intervertebral disc. Posterior extension of OM/discitis causes epidural phlegmon or abscess. The facet joints and the posterior elements may be involved. When the facet joint is involved, it is called septic arthritis of the facet joint, which nowadays becomes facetitis, a newly invented medical term.
The most common presentation of OM/discitis is back pain, localized at the affected area of spine. Back pain usually begins insidiously and progressively worsens over several weeks to months. At the beginning, the pain may be worse at night and relieved by bed rest. Fever may be seen in the affected individual but not a consistent presentation. Neurologic deficits are more common with accompanied epidural abscess. Definitive diagnosis of OM/discitis or epidural SEA is made by positive culture from the biopsy specimen of the infected sites. Back pain, fever and classic radiographic finings of OM/discitis are sufficient to start antimicrobial therapy for suspected cases. Positive blood culture significantly increased the index of suspicion. LP is usually not helpful and may increase the risk of the pathogen entering the thecal sac. Laboratory studies of the blood are nonspecific and often show leukocytosis, elevated erythrocyte sedimentation rate (ESR) and positive C-reactive protein (CRP) (69-72).
Plain radiography of the spine is insensitive for detecting OM/discitis until late stages of the disease. Permeated osteolytic area within the vertebral body, cortical irregularities and intervertebral disc destruction can be seen in the advanced stage, but earlier and more easily identified by CT. Contrast enhancement is of limited value for bony or disc destructions but may delineate the associated epidural empyema, phlegmon or abscesses within the adjacent paravertebral spaces and/or paraspinal muscles. On MR, the bone marrow or disc edema are early findings of OM/discitis, which demonstrate hypointense T1 and hyperintense T2/STIR signals as well as diffuse enhancement after intravenous contrast. Frank or advanced OM and discitis demonstrate bony destructions or disc irregularities of the infected bony structures or discs. The corresponding hyperintense T2 and STIR signals as well as enhancement become more heterogeneous (Figure 14). Accompanied phlegmon or abscess may be identified in the related epidural space as well as the adjacent perivertebral and paraspinal spaces or muscles. In later stage of the disease, the vertebral body may show a pathological fracture (70-72).
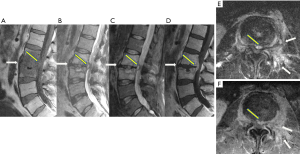
SEA is one of the most frequent causes for urgent MRI in the Emergency Department, especially with the clinical triad of back pain, fever, and neurological deficits involving the extremities. It is considered a neurological emergency as its complication of cord compression or infarct may cause irreversible neurological deficits, paralysis or even death. It has similar epidemiology as OM/discitis. In addition to the hematogenous seeding from septicemia into the epidural space, the spread of bacterial infection can also arise from the direct invasion from adjacent infected structures such as joint or disc spaces.
Although CT may reveal evidence of discitis or OM if present, SEA commonly escapes detection. MRI with contrast is the only true modality that can clearly diagnose SEA. Identification of an epidural fluid collection with rim-enhancement is generally sufficient for a presumptive diagnosis of SEA in the right clinical setting. The disease could present as either a phlegmonous or liquid form. Phlegmonous forms will demonstrate prominent heterogeneously enhancing soft tissues while a liquid form will show a single or multiple fluid collections with peripheral rim-like enhancement (73). Longitudinal extension is common with average extent ranging from 3 to 5 spinal cord segments (74). Characteristically, restricted diffusion of the epidural fluid collection is the hallmark of epidural abscess and separate if from other types of epidural fluid collections, such as a hematoma or an arachnoid cyst. But DWI images of spine are not usually acquired in most institutions (75). Additionally, SEA is often associated with inflammatory reaction or infection of the adjacent paraspinal muscles, psoas muscles and facet joints (Figure 15).
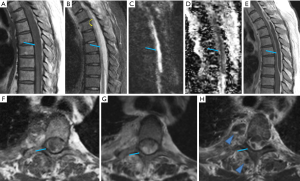
Differential diagnosis of OM/discitis include degenerative disease and osteolytic metastasis. Although back pain is also common in degenerative disease of spine, but the patients’ clinical history is usually different and the features of infection such as fever, is not typically present. Bony or disc destructions, and pathological fractures do not exist. Spinal metastasis, beside distinct history of a primary tumor elsewhere, often demonstrates vertebral body destructions and can be associated with a pathological fracture. But the intervertebral disc is typically spared as the avascular disc is resistant to tumor invasion (76). The associated epidural metastasis appears a solid enhancing mass focused on the level of bony involvement rather than an elongated epidural fluid collection with peripheral enhancement of SEA. Another major differential diagnosis of SEA is an acute epidural hematoma of the spine. Beyond different clinical history, key features to distinguish SEA from epidural hematoma are mostly based on signal characteristics on MR. The signals of the epidural hematoma appear similar to acute hematoma intracranially. They change with time due to evolution of the blood products. The acute epidural hematoma should not demonstrate peripheral enhancement (77). Spinal epidural metastases can also mimic SEA, though their clinical courses are usually more chronic and not associated with clinical history of fever, leukocytosis, or elevated ESR.
Treatment of OM/discitis usually begins with intravenous therapy by broad-spectrum empiric antibiotics for patients with sepsis or neurological comprises. When the causative microorganism is known, antimicrobial therapy should be tailored to the identified pathogen. Vancomycin is reserved for MRSA infection, commonly seen in IV drug abusers.
Surgical decompression is the most common treatment method for SEA, followed by IV antimicrobial therapy.
Conclusions
Infectious diseases of the CNS usually have very high morbidity and mortality. Despite advances in medicine, accurate and prompt diagnosis of these infections are still very challenging due to the poor specificity of the patients’ presenting signs and symptoms, especially in pediatric, elderly, and immunocompromised populations. Objective findings on neuroimaging studies play a critical role in pinpointing the exact diagnoses so that treatments can be initiated in a timely fashion. Since prompt treatment is paramount for the patients’ condition and may even be lifesaving, it is pivotal for clinicians to become familiar with the features of a wide spectrum of the CNS infections, their imaging patterns and characteristics as well as differential diagnoses. A pattern approach method of these diseases can also be developed. As a result, the patients can have a significantly improved prognosis with reduced morbidity and mortality.
Tables 1,2 list the major clinical features and imaging features of the common infectious diseases of the CNS mentioned in this article.
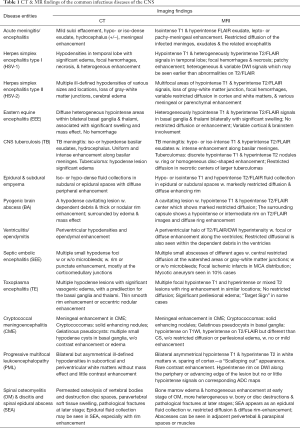
Full table
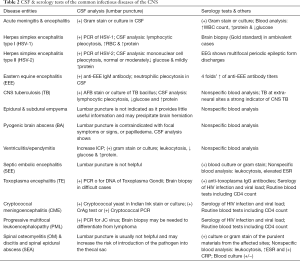
Full table
Acknowledgments
I would like to express special thanks to Christopher M. Lin who provided valuable tips of formatting various document, figures, and tables using Microsoft Office Suite, and proofread this manuscript for every revision. I would also like to thank my colleague Dr. Tara M. Catanzano for her continuous encouragement and support for my academic endeavors.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/qims-20-886). The authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Thigpen MC, Whitney CG, Messonnier NE, Zell ER, Lynfield R, Hadler JL, Harrison LH, Farley MM, Reingold A, Bennett NM, Craig AS, Schaffner W, Thomas A, Lewis MM, Scallan E, Schuchat A. Emerging Infections Programs Network. Bacterial meningitis in the United States, 1998-2007. N Engl J Med 2011;364:2016-25. [Crossref] [PubMed]
- Oordt-Speets AM, Bolijn R, van Hoorn RC, Bhavsar A, Kyaw MH. Global etiology of bacterial meningitis: A systematic review and meta-analysis. PLoS One 2018;13:e0198772. [Crossref] [PubMed]
- Prober CG, Srinivas NS, Mathew R. Nelson Textbook of Pediatrics. Chapter 603, 2936-2948.e2.
- Sáez-Llorens X, McCracken GH Jr. Bacterial meningitis in children. Lancet 2003;361:2139-48. [Crossref] [PubMed]
- Mandell GL, Bennett JE, Dolin R. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. 7th edition. Philadelphia, PA: Churchill Livingstone/Elsevier, 2010.
- van de Beek D, de Gans J, Spanjaard L, Weisfelt M, Reitsma JB, Vermeulen M. Clinical features and prognostic factors in adults with bacterial meningitis. N Engl J Med 2004;351:1849-59. [Crossref] [PubMed]
- Troendle M, Pettigrew A. A systematic review of cases of meningitis in the absence of cerebrospinal fluid pleocytosis on lumbar puncture. BMC Infect Dis 2019;19:692. [Crossref] [PubMed]
- Dorsett M, Liang SY. Diagnosis and Treatment of Central Nervous System Infections in the Emergency Department. Emerg Med Clin North Am 2016;34:917-42. [Crossref] [PubMed]
- Hazany S, Go JL, Law M. Magnetic resonance imaging of infectious meningitis and ventriculitis in adults. Top Magn Reson Imaging 2014;23:315-25. [Crossref] [PubMed]
- Mohan S, Jain KK, Arabi M, Shah GV. Imaging of meningitis and ventriculitis. Neuroimaging Clin N Am 2012;22:557-83. [Crossref] [PubMed]
- Smirniotopoulos JG, Murphy FM, Rushing EJ, Rees JH, Schroeder JW. Patterns of contrast enhancement in the brain and meninges. Radiographics 2007;27:525-51. [Crossref] [PubMed]
- Tosaka M, Sato N, Fujimaki H, Tanaka Y, Kagoshima K, Takahashi A, Saito N, Yoshimoto Y. Diffuse pachymeningeal hyperintensity and subdural effusion/hematoma detected by fluid-attenuated inversion recovery MR imaging in patients with spontaneous intracranial hypotension. AJNR Am J Neuroradiol 2008;29:1164-70. [Crossref] [PubMed]
- James SH, Kimberlin DW. Neonatal Herpes Simplex Virus Infection. Infect Dis Clin North Am 2015;29:391-400. [Crossref] [PubMed]
- Whitley RJ. Herpes Simplex Virus Infections of the Central Nervous System. Continuum (Minneap Minn) 2015;21:1704-13. [Crossref] [PubMed]
- Renard D, Nerrant E, Lechiche C. DWI and FLAIR imaging in herpes simplex encephalitis: a comparative and topographical analysis. J Neurol 2015;262:2101-5. [Crossref] [PubMed]
- Fa F, Laup L, Mandelbrot L, Sibiude J, Picone O. Fetal and neonatal abnormalities due to congenital herpes simplex virus infection: a literature review. Prenat Diagn 2020;40:408-14. [Crossref] [PubMed]
- Corey L, Whitley RJ, Stone EF, Mohan K. Difference between herpes simplex virus type 1 and type 2 neonatal encephalitis in neurological outcome. Lancet 1988;1:1-4. [Crossref] [PubMed]
- Osborn AG, Hedlund GL, Salzman KL. Osborn's brain: imaging, pathology, and anatomy. Salt Lake City: Amirsys, 2013.
- Lindsey NP, Staples JE, Fischer M. Eastern Equine Encephalitis Virus in the United States, 2003-2016. Am J Trop Med Hyg 2018;98:1472-7. [Crossref] [PubMed]
- Armstrong PM, Andreadis TG. Eastern equine encephalitis virus--old enemy, new threat. N Engl J Med 2013;368:1670-3. [Crossref] [PubMed]
- Deresiewicz RL, Thaler SJ, Hsu L, Zamani AA. Clinical and neuroradiographic manifestations of eastern equine encephalitis. N Engl J Med 1997;336:1867-74. [Crossref] [PubMed]
- Babi MA, Raleigh T, Shapiro RE, McSherry J, Applebee A. MRI and encephalography in fatal eastern equine encephalitis. Neurology 2014;83:1483. [Crossref] [PubMed]
- Nickerson JP, Kannabiran S, Burbank HN. MRI findings in eastern equine encephalitis: the "parenthesis" sign. Clin Imaging 2016;40:222-3. [Crossref] [PubMed]
- World Health Organization. Global tuberculosis report 2019. Available online: https://www.who.int/tb/publications/global_report/en/. (Accessed on October 28, 2019).
- CDC – National Center for Health Statistics – Tuberculosis Data and Statistics. Available online: https://www.cdc.gov/tb/statistics/default.htm. August 10, 2019.
- Langer AJ, Navin TR, Winston CA, LoBue P. Epidemiology of Tuberculosis in the United States. Clin Chest Med 2019;40:693-702. [Crossref] [PubMed]
- Méchaï F, Bouchaud O. Tuberculous meningitis: Challenges in diagnosis and management. Rev Neurol (Paris) 2019;175:451-7. [Crossref] [PubMed]
- Chin JH. Tuberculous meningitis: Diagnostic and therapeutic challenges. Neurol Clin Pract 2014;4:199-205. [Crossref] [PubMed]
- Weingarten K, Zimmerman RD, Becker RD, Heier LA, Haimes AB, Deck MD. Subdural and epidural empyemas: MR imaging. AJR Am J Roentgenol 1989;152:615-21. [Crossref] [PubMed]
- McIntyre PB, Lavercombe PS, Kemp RJ, McCormack JG. Subdural and epidural empyema: diagnostic and therapeutic problems. Med J Australia 1991;154:653-7. [Crossref] [PubMed]
- Lundy P, Kaufman C, Garcia D, Partington MD, Grabb PA. Intracranial subdural empyemas and epidural abscesses in children. J Neurosurg Pediatr 2019;24:14-21. [Crossref] [PubMed]
- Mattogno PP, La Rocca G, Signorelli F, Visocchi M. Intracranial subdural empyema: diagnosis and treatment update. J Neurosurg Sci 2019;63:101-2. [PubMed]
- Bartt RE. Cranial epidural abscess and subdural empyema. Handb Clin Neurol 2010;96:75-89. [Crossref] [PubMed]
- Tsuchiya K, Osawa A, Katase S, Fujikawa A, Hachiya J, Aoki S. Diffusion-weighted MRI of subdural and epidural empyemas. Neuroradiology 2003;45:220-3. [Crossref] [PubMed]
- Haimes AB, Zimmerman RD, Morgello S, Weingarten K, Becker RD, Jennis R, Deck MD. MR imaging of brain abscesses. AJR Am J Roentgenol 1989;152:1073-85. [Crossref] [PubMed]
- Shih RY, Koeller KK. Bacterial, Fungal, and Parasitic Infections of the Central Nervous System: Radiologic-Pathologic Correlation and Historical Perspectives. Radiographics 2015;35:1141-69. [Crossref] [PubMed]
- Rath TJ, Hughes M, Arabi M, Shah GV. Imaging of cerebritis, encephalitis, and brain abscess. Neuroimaging Clin N Am 2012;22:585-607. [Crossref] [PubMed]
- Ganau M, Mankad K, Srirambhatla UR, Tahir Z, D'Arco F. Ring-enhancing lesions in neonatal meningitis: an analysis of neuroradiology pitfalls through exemplificative cases and a review of the literature. Quant Imaging Med Surg 2018;8:333-41. [Crossref] [PubMed]
- Lai PH, Chung HW, Chang HC, Fu JH, Wang PC, Hsu SH, Hsu SS, Lin HS, Chuang TC. Susceptibility-weighted imaging provides complementary value to diffusion-weighted imaging in the differentiation between pyogenic brain abscesses, necrotic glioblastomas, and necrotic metastatic brain tumors. Eur J Radiol 2019;117:56-61. [Crossref] [PubMed]
- Fukui MB, Williams RL, Mudigonda S. CT. AJNR Am J Neuroradiol 2001;22:1510-6. [PubMed]
- Pezzullo JA, Tung GA, Mudigonda S, Rogg JM. Diffusion-weighted MR imaging of pyogenic ventriculitis. AJR Am J Roentgenol 2003;180:71-5. [Crossref] [PubMed]
- García-Cabrera E, Fernández-Hidalgo N, Almirante B, Ivanova-Georgieva R, Noureddine M, Plata A, Lomas JM, Gálvez-Acebal J, Hidalgo-Tenorio C, Ruíz-Morales J, Martínez-Marcos FJ, Reguera JM, de la Torre-Lima J, de Alarcón González A. Group for the Study of Cardiovascular Infections of the Andalusian Society of Infectious Diseases. Spanish Network for Research in Infectious Diseases. Neurological complications of infective endocarditis: risk factors, outcome, and impact of cardiac surgery: a multicenter observational study. Circulation 2013;127:2272-84. [Crossref] [PubMed]
- Okazaki S, Yoshioka D, Sakaguchi M, Sawa Y, Mochizuki H, Kitagawa K. Acute ischemic brain lesions in infective endocarditis: incidence, related factors, and postoperative outcome. Cerebrovasc Dis 2013;35:155-62. [Crossref] [PubMed]
- Hess A, Klein I, Iung B, Lavallée P, Ilic-Habensus E, Dornic Q, Arnoult F, Mimoun L, Wolff M, Duval X, Laissy JP. Brain MRI findings in neurologically asymptomatic patients with infective endocarditis. AJNR Am J Neuroradiol 2013;34:1579-84. [Crossref] [PubMed]
- Shen G, Shen X, Pu W, Zhang G, Lerner A, Gao B. Imaging of cerebrovascular complications of infection. Quant Imaging Med Surg 2018;8:1039-51. [Crossref] [PubMed]
- Faisal W, Burnton G, Imlay-Gillespie L, Robilliard J. Cerebral abscesses and septic pulmonary emboli due to Serratia marcescens infection arising from a Portacath. J Microbiol Immunol Infect 2010;43:538-41. [Crossref] [PubMed]
- Antinori A, Larussa D, Cingolani A, Lorenzini P, Bossolasco S, Finazzi MG, Bongiovanni M, Guaraldi G, Grisetti S, Vigo B, Gigli B, Mariano A, Dalle Nogare ER, De Marco M, Moretti F, Corsi P, Abrescia N, Rellecati P, Castagna A, Mussini C, Ammassari A, Cinque P, d'Arminio Monforte A. Italian Registry Investigative NeuroAIDS. Prevalence, associated factors, and prognostic determinants of AIDS-related toxoplasmic encephalitis in the era of advanced highly active antiretroviral therapy. Clin Infect Dis 2004;39:1681-91. [Crossref] [PubMed]
- Matinella A, Lanzafame M, Bonometti MA, Gajofatto A, Concia E, Vento S, Monaco S, Ferrari S. Neurological complications of HIV infection in pre-HAART and HAART era: a retrospective study. J Neurol 2015;262:1317-27. [Crossref] [PubMed]
- Bowen LN, Smith B, Reich D, Quezado M, Nath A. HIV-associated opportunistic CNS infections: pathophysiology, diagnosis and treatment. Nat Rev Neurol 2016;12:662-74. [Crossref] [PubMed]
- Lee GT, Antelo F, Mlikotic AA. Cerebral Toxoplasmosis. RadioGraphics 2009;29:1200-5. [Crossref] [PubMed]
- Garg RK, Sinha MK. Multiple Ring-Enhancing Lesions of the Brain. J Postgrad Med 2010;56:307-16. [Crossref] [PubMed]
- Chong-Han CH, Cortez SC, Tung GA. Diffusion-Weighted MRI of Cerebral Toxoplasmosis Abscess. AJR 2003;181:1711-4. [Crossref] [PubMed]
- Williamson PR, Jarvis JN, Panackal AA, Fisher MC, Molloy SF, Loyse A, Harrison TS. Cryptococcal meningitis: epidemiology, immunology, diagnosis and therapy. Nat Rev Neurol 2017;13:13-24. [Crossref] [PubMed]
- Spec A, Powderly WG. Cryptococcal meningitis in AIDS. Handb Clin Neurol 2018;152:139-50. [Crossref] [PubMed]
- Lee SC, Dickson DW, Casadevall A. Pathology of cryptococcal meningoencephalitis: analysis of 27 patients with pathogenetic implications. Hum Pathol 1996;27:839-47. [Crossref] [PubMed]
- Kashef Hamadani BH, Franco-Paredes C, McCollister B, Shapiro L, Beckham JD, Henao-Martínez AF. Cryptococcosis and cryptococcal meningitis: New predictors and clinical outcomes at a United States academic medical centre. Mycoses 2018;61:314-20. [Crossref] [PubMed]
- Zhai S, Brew BJ. Progressive multifocal leukoencephalopathy. Handb Clin Neurol 2018;152:123-37. [Crossref] [PubMed]
- Kartau M, Sipilä JO, Auvinen E, Palomäki M, Verkkoniemi-Ahola A. Progressive Multifocal Leukoencephalopathy: Current Insights. Degener Neurol Neuromuscul Dis 2019;9:109-21. [Crossref] [PubMed]
- Berger JR, Aksamit AJ, Clifford DB, Davis L, Koralnik IJ, Sejvar JJ, Bartt R, Major EO, Nath A. PML diagnostic criteria: consensus statement from the AAN Neuroinfectious Disease Section. Neurology 2013;80:1430-8. [Crossref] [PubMed]
- Mornese Pinna S, Scarvaglieri E, Milia MG, Imperiale D, Ghisetti V, Audagnotto S, Prochet A, Lipani F, Bonora S, Di Perri G, Calcagno A. Detectable cerebrospinal fluid JCV DNA in late-presenting HIV-positive patients: beyond progressive multifocal leukoencephalopathy? J Neurovirol 2017;23:763-7. [Crossref] [PubMed]
- Küker W, Mader I, Nägele T, Uhl M, Adolph C, Klose U, Herrlinger U. Progressive multifocal leukoencephalopathy: value of diffusion-weighted and contrast-enhanced magnetic resonance imaging for diagnosis and treatment control. Eur J Neurol 2006;13:819-26. [Crossref] [PubMed]
- Sahraian MA, Radue EW, Eshaghi A, Besliu S, Minagar A. Progressive multifocal leukoencephalopathy: a review of the neuroimaging features and differential diagnosis. Eur J Neurol 2012;19:1060-9. [Crossref] [PubMed]
- Berger JR, Levy RM, Flomenhoft D, Dobbs M. Predictive factors for prolonged survival in acquired immunodeficiency syndrome-associated progressive multifocal leukoencephalopathy. Ann Neurol 1998;44:341-9. [Crossref] [PubMed]
- Krey L, Raab P, Sherzay R, Berding G, Stoll M, Stangel M, Wegner F. Severe Progressive Multifocal Leukoencephalopathy (PML) and Spontaneous Immune Reconstitution Inflammatory Syndrome (IRIS) in an Immunocompetent Patient. Front Immunol 2019;10:1188. [Crossref] [PubMed]
- Schweitzer AD, Parikh NS, Askin G, Nemade A, Lyo J, Karimi S, Knobel A, Navi BB, Young RJ, Gupta A. Imaging characteristics associated with clinical outcomes in posterior reversible encephalopathy syndrome. Neuroradiology 2017;59:379-86. [Crossref] [PubMed]
- Issa K, Diebo BG, Faloon M, Naziri Q, Pourtaheri S, Paulino CB, Emami A. The Epidemiology of Vertebral Osteomyelitis in the United States From 1998 to 2013. Clin Spine Surg 2018;31:E102-8. [Crossref] [PubMed]
- Allison DC, Holtom PD, Patzakis MJ, Zalavras CG. Microbiology of bone and joint infections in injecting drug abusers. Clin Orthop Relat Res 2010;468:2107-12. [Crossref] [PubMed]
- Kim UJ, Bae JY, Kim SE, Kim CJ, Kang SJ, Jang HC, Jung SI, Song KH, Kim ES, Kim HB, Park WB, Kim NJ, Park KH. Comparison of pyogenic postoperative and native vertebral osteomyelitis. Spine J 2019;19:880-7. [Crossref] [PubMed]
- Aagaard T, Roed C, Dragsted C, Skinhøj P. Microbiological and therapeutic challenges in infectious spondylodiscitis: a cohort study of 100 cases, 2006-2011. Scand J Infect Dis 2013;45:417-24. [Crossref] [PubMed]
- Berbari EF, Kanj SS, Kowalski TJ, Darouiche RO, Widmer AF, Schmitt SK, Hendershot EF, Holtom PD, Huddleston PM 3rd, Petermann GW, Osmon DR. Infectious Diseases Society of America. 2015 Infectious Diseases Society of America (IDSA) Clinical Practice Guidelines for the Diagnosis and Treatment of Native Vertebral Osteomyelitis in Adults. Clin Infect Dis 2015;61:e26-46. [Crossref] [PubMed]
- Zimmerli W. Clinical practice. Vertebral osteomyelitis. N Engl J Med 2010;362:1022-9. [Crossref] [PubMed]
- McHenry MC, Easley KA, Locker GA. Vertebral osteomyelitis: long-term outcome for 253 patients from 7 Cleveland-area hospitals. Clin Infect Dis 2002;34:1342-50. [Crossref] [PubMed]
- Numaguchi Y, Rigamonti D, Rothman MI, Sato S, Mihara F, Sadato N. Spinal Epidural Abscess: Evaluation with Gadolinium-Enhanced MR Imaging. Radiographics 1993;13:545-59. [Crossref] [PubMed]
- Darouiche RO, Hamill RJ, Greenberg SB, Weathers SW, Musher DM. Bacterial spinal epidural abscess. Review of 43 cases and literature survey. Medicine (Baltimore) 1992;71:369-85. [Crossref] [PubMed]
- Eastwood JD, Vollmer RT, Provenzale JM. Diffusion-weighted imaging in a patient with vertebral and epidural abscesses. AJNR Am J Neuroradiol 2002;23:496-8. [PubMed]
- Shah LM, Salzman KL. Imaging of spinal metastatic disease. Int J Surg Oncol 2011;2011:769753. [Crossref] [PubMed]
- Holtås S, Heiling M. Lönntoft. Spontaneous Spinal Epidural Hematoma: Findings at MR Imaging and Clinical Correlation. Radiology 1996;199:409-13. [Crossref] [PubMed]


