Paediatric cerebrovascular CT angiography—towards better image quality
Introduction
CT angiography (CTA) is well established for the assessment of cerebrovascular disease and is increasingly performed in paediatric practice. CTA is quick, more practical than magnetic resonance angiography (MRA) in an unstable patient and may help avoid or defer invasive angiography (1,2). Image quality may be superior to that of MR angiography in certain situations with CTA being less susceptible to movement and flow artefacts (3,4). At our institution, CTA has become the first-line modality for vascular imaging in systemically compromised children with intracranial haemorrhage. CTA can be challenging to perform in paediatrics due to variability in cardiovascular physiology with age and because the speed of intracranial contrast distribution can be altered by disease, for example in arteriovenous shunting. There is currently no guidance on how to consistently achieve a good quality cerebrovascular CTA in children. This analysis aimed to identify the key factors which influence image quality.
Materials and methods
Institutional approval was obtained for a review of CTA studies performed at Great Ormond Street Hospital for Children. Intracranial and combined intracranial-extracranial studies were included. All scans were performed on a Siemens Somatom Definition 64-slice scanner (Siemens, Erlangen, Germany) using a manual triggering technique and cannula sizes ranging from 20 to 24 G.
Triggering technique
Automated bolus tracking is not currently performed in our department, as in our experience this increases the risk of a false trigger in children due to smaller vessel size, more rapid cardiovascular circulation and the risk of patient movement. The manual triggering technique relies on accurate observation and fast reaction by the radiographer. To begin with the manual technique, a monitoring slice through the unenhanced vessel is acquired prior to contrast injection. For an intracranial only scan, the monitoring slice was chosen in the proximal carotid artery, whereas for a combined extracranial-intracranial scan, the monitoring slice was chosen in the aortic arch. Once the injection commenced, the monitoring section was scanned every second. As soon as intravenous contrast became visible within the vessel, the scan was triggered. Of note, our CT scanner has an intrinsic lag of 2 seconds from pressing the start button to initiating the scan from caudal to cranial. All contrast injections were followed immediately by a ten millilitre 0.9% saline flush.
Patient demographics and scan information including trigger monitoring, contrast dose and flow rate were retrieved from the integrated radiology information system (GE Healthcare Centricity RIS-i version 5.0).
Patients were divided into three groups: all patients received Omnipaque 300 intravenous contrast containing 647 mg of iohexol equivalent to 300 mg of organic iodine per mL. Cases 1-13 were scanned using a 1 mL/kg contrast injection with flow rates of 1.5-2 mL/s. Cases 14 and 15 were scanned using 1 mL/kg contrast at a slightly higher flow rate (2.5 mL/s). Cases 16-20 were scanned using 2 mL/kg contrast a flow rate of 2.5-4 mL/s.
Radiological analysis
The diagnostic value of each study was assessed by two neuroradiologists in a consensus reading. Overall diagnostic scan quality was graded subjectively as, “good, satisfactory or poor”. Scan quality was defined as “good” if the observer was able to clearly distinguish arterial from venous circulation by density and arterial branches appeared sufficiently delineated to allow a confident diagnosis. “Satisfactory” was defined as a scan of diagnostic quality in which contrast density or the visibility of small detail could be further improved. “Poor” was defined as a scan, in which there was insufficient arterial contrast to confidently exclude an abnormality or too much venous contrast to distinguish arteries and veins. A degree of venous opacification was judged to be acceptable, possibly even desirable, as long as arterial opacification was greater.
Triggering adequacy was judged by assessing contrast density within the arterial and venous system in relation to scan quality. If there was sufficient enhancement and arterial greater than venous contrast, the scan was judged to be appropriately triggered. If maximum enhancement was visible in the systemic veins before sufficient contrast opacification of the intracranial arteries had occurred, triggering was categorised as early. If contrast opacification within the cerebral veins was equal or greater than in the arterial system, triggering was categorised as late.
A quantitative analysis using maximum Hounsfield Unit (HU) measurements was carried out by drawing a region of interest in the right terminal internal carotid artery (ICA), midsection of the basilar artery and right jugular bulb respectively.
Results
Twenty patients (n=11 females and n=9 males) aged 0 to 16 years with a mean of 8.2 years underwent cerebrovascular CTA in the study period. Sixty percent (n=12) of patients were scanned to evaluate for an underlying cause of spontaneous intracranial haemorrhage. The remaining patients received a scan for the following indications: to delineate arterial anatomy for surgical planning (n=2), for post-interventional assessment of an arteriovascular malformation (n=1), to evaluate cranial vascular tumours (n=2), to identify a cause of transient ischaemic attacks (n=1), following extra-intracranial carotid bypass surgery (n=1) and to assess arterial supply of a Vein of Galen malformation (n=1). Dose length product (DLP) values ranged from 104-529 mGy-cm per scan. To date, no national dose reference level (DRL) for paediatric CTA has been defined; however, none of the studies exceeded local or national reference levels for a non-contrast CT brain.
Using the qualitative visual scoring system, 75% of studies were judged to be of diagnostic quality (n=9 good, n=6 satisfactory) and 25% (n=5) were poor (see Table 1). Contrast opacification measurements within the arterial system varied between 133 and 593 HUs, and within the venous system from 62 to 358 HU (see Figure 1). For the scans where contrast opacification was judged subjectively to be poor, this corresponded to arterial measurements of less than 250 HU. For cases 1-13, good or satisfactory image quality was achieved in 10/13 patients. For cases 14-15, in which the flow rate was increased (2.5 mL/s), improved arterial opacification was observed. For cases 17 and 18, scanned with the higher contrast dose (2 mL/kg) and high flow rates (2.5-4 mL/s), the highest arterial attenuation was achieved. In cases 19 and 20, scan quality was poor despite the increased contrast dose and attempted higher flow rate due to wrong triggering.
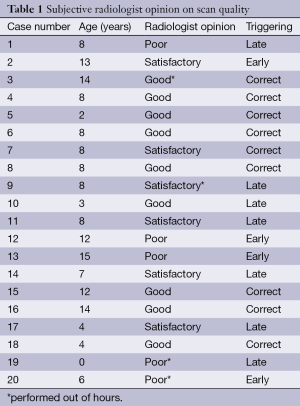
Full table
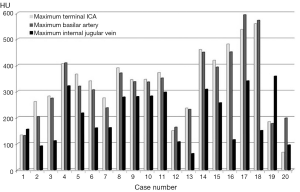
Triggering was judged to be appropriate in nine cases, early in four cases and late in seven cases. Of the four scans triggered early, three (75%) were poor (Figure 2). Of the seven scans triggered late, two (29%) were poor. Triggering was found to be wrongly timed more often in out-of-hours cases.
Discussion
Few centres perform paediatric cerebrovascular CTA in large numbers, respecting and reflecting the radiation sensitivity of developing organs. The authors clearly recognise the importance of radiation protection, especially in childhood. Certain vascular lesions, however, particularly when complicated by acute haemorrhage, may pose an immediate threat to life or long-term neurological outcome making the use of CT for accurate diagnosis relatively more justified (Figure 3). Our results indicate that a minimum arterial opacification of approximately 250 HU is required for a scan to be judged as good or satisfactory. In adults, it is widely known that high flow rates are desirable in achieving optimum CTA image quality (5).
Flow rate
For children there is limited evidence for an optimum flow rate, which is dependent on body size/age and limited by cannula diameter. In the literature, flow rates of 2.5-4 mL/s have been reportedly been used for paediatric CTA and CT perfusion (2,6). Poiseuille’s law provides guidance that for a small increase in cannula calibre flow rates may be altered significantly. There is evidence that the relationship between cannula diameter and flow rate may be complex and influenced by factors such as contrast viscosity (7,8). In this analysis, there was objectively improved contrast opacification with the higher flow rates (2.5-4 mL/s) emphasising the potential value of larger bore venous access, whereby maximum permissible flow rates may vary locally and with manufacturer recommendations. Cannula sizes were not specifically recorded as part of this study, although the maximum permissible flow rate was chosen for each child. In this analysis, there were no complications from extravasation or cannula occlusion. Insertion was undertaken in accordance with the Great Ormond Street Hospital Peripheral Venous Cannulation of Children Guideline (www.gosh.nhs.uk). To maximise the chances of a successful scan, liaison with the clinical team regarding the need for sufficient calibre access may be advisable. It is difficult to advocate specific cannula sizes, with choice being influenced by patient habitus, operator confidence and skill. No correlation was observed between scan quality and patient age. Although use of the higher contrast dose contributed to an increase in HU measurements, this alone was not sufficient to achieve good imaging. Case 19 represents a neonate, in whom the intended high flow rate was not achieved due to inability to use a pump injection. The combination of manual injection through narrow bore access and late triggering resulted in a suboptimal scan despite the increased contrast dose.
Triggering
From our results in cases 1-13, it appears possible to maintain sufficient image quality with a slower flow rate, as long as triggering is well timed. Correct triggering can be achieved through starting the scan immediately once contrast becomes visible in the artery of interest. This relies on the radiographer’s close observation during the monitoring phase of the injection. Hitting the scan button too early during the injection before contrast has entered the vessel, will result in early triggering. Uncertainty as to whether contrast opacification of the artery is sufficient typically causes delays in commencing the scan. The importance of triggering is highlighted by case 20, where despite the increased flow rate and contrast dose early triggering lead to a poor scan. In this analysis all but one of the four scans, which were triggered early resulted in poor image quality, suggesting early triggering may be more detrimental than late. One variable, which was not tested in this study, is a comparison of manual versus automated triggering. Using the currently available CT equipment, which represents current UK standard, experience at our institution was strongly in favour of the manual technique. To avoid unnecessary radiation by repeat scans, a manual versus automated comparison was therefore not undertaken. With technological improvements, development of an automated triggering system adapted to paediatric cerebrovascular CTA could be attempted.
In conclusion, high flow rates (2.5-4 mL/s) are desirable for paediatric CTA and appropriate triggering is crucial, particularly when flow rates are limited.
Disclosure: The authors declare no conflict of interest.
References
- Mason KP, Zurakowski D, Zucker EJ, Tracy DA, Lee EY. Image quality of thoracic 64-MDCT angiography: imaging of infants and young children with or without general anesthesia. AJR Am J Roentgenol 2013;200:171-6. [PubMed]
- Gatscher S, Brew S, Banks T, Simcock C, Sullivan Y, Crockett J. Multislice spiral computed tomography for pediatric intracranial vascular pathophysiologies. J Neurosurg 2007;107:203-8. [PubMed]
- Sugino T, Mikami T, Ohtaki S, Hirano T, Iihoshi S, Houkin K, Mikuni N. Assessment of moyamoya disease using multidetector row computed tomography. J Stroke Cerebrovasc Dis 2013;22:644-9. [PubMed]
- Perkins JA, Sidhu M, Manning SC, Ghioni V, Sze R. Three-dimensional CT angiography imaging of vascular tumors of the head and neck. Int J Pediatr Otorhinolaryngol 2005;69:319-25. [PubMed]
- Liu Q, Huang J, Degnan AJ, Chen S, Gillard JH, Teng Z, Lu J. Comparison of high-resolution MRI with CT angiography and digital subtraction angiography for the evaluation of middle cerebral artery atherosclerotic steno-occlusive disease. Int J Cardiovasc Imaging 2013;29:1491-8. [PubMed]
- Zebedin D, Sorantin E, Riccabona M. Perfusion CT in childhood stroke--initial observations and review of the literature. Eur J Radiol 2013;82:1059-66. [PubMed]
- Reddick AD, Ronald J, Morrison WG. Intravenous fluid resuscitation: was Poiseuille right? Emerg Med J 2011;28:201-2. [PubMed]
- Proctor RD, Beckett D, Oakes JL. Over the limit: use of peripheral venous cannulae above the manufacturer’s recommended flow rates. Clin Radiol 2011;66:456-8. [PubMed]


