Atypical developmental venous anomaly associated with contrast enhancement and hyperperfusion in the surrounding basal ganglia
Introduction
Developmental venous anomalies (DVAs) are the most common type (>60%) of cerebral vascular malformations. They are generally accepted as variants of venous development (1,2). It is usually difficult to identify DVAs without administering a contrast medium, because DVAs mainly consist of small vessels with slow flow. T2*-weighted GE imaging is sensitive and able to detect small venous structures. However susceptibility-weighted imaging (SWI), a relatively new 3D gradient-echo MR imaging (MRI) technique with both phase and magnitude information, improves sensitivity to detect small vascular structures, which are invisible on conventional imaging (3). The purpose of this article is to illustrate DVA with atypical hemodynamic pattern and discuss the role of imaging for diagnostic workup of DVA.
Case report
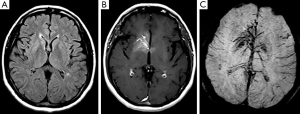
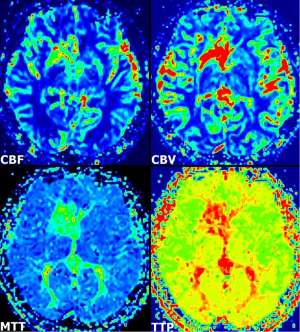
A 55-year-old woman presented with dizziness, vertigo, and disequilibrium. No neurologic deficits were present on physical examination. No abnormalities were reported on unenhanced cranial MRI performed at another facility 3 days previously (Figure 1A). Therefore, MRI was repeated with contrast enhancement at our institution. Post-contrast MR images showed DVA with classical caput medusa appearance in the right basal ganglia draining to the deep venous system (Figure 1B). In addition, there was contrast enhancement in the basal ganglia around DVA. DVA, which could not be seen on conventional MRI, was easily distinguished on SWI (Figure 1C). On perfusion MRI, there was increase in cerebral blood flow (CBF), cerebral blood volume (CBV), mean transit time (MTT), and time-to-peak (TTP) of both DVA and surrounding basal ganglia (Figure 2). There was no pathological finding other than DVA on MR venography. The symptoms of the patient resolved completely within a few days. The MRI findings were stable at her 6- and 12-month follow-up.
Discussion
Developmental venous anomalies classically appear as a caput medusa consisting of a radially arranged venous complex of small deep parenchymal veins that coalesce into a large draining vein converging on a centrally located venous trunk. These anomalies are frequently identified incidentally and may not be detected on conventional MRI sequences without contrast-medium administration (1,2). SWI has been reported to be the ideal technique in the identification and characterization of vascular malformations (4). SWI can show deep medullary veins and draining vein of the DVA, as was found in our case, which are hardly visible on conventional MR sequences without requiring contrast media. Reichenbach et al. (5) could identify the typical configuration of the abnormal veins in DVAs more precisely on SWI with unique functional and anatomical information not available with other imaging techniques. In a case of venous congestion associated with DVA, SWI demonstrated abnormal structures connected to DVA, not revealed on other sequences including contrast-enhanced T1-weighted images and these structures were considered to be presumably thrombosed veins (6). In our case, findings of DVA, far more extensive on SWI than on contrast-enhanced T1-weighted images, may be explained by these data.
The brain parenchyma surrounding the DVA has usually been reported as normal. A previous review showed subjacent parenchymal signal-intensity alterations in DVAs with an adjusted prevalence rate of 7.8% (7). The etiology of the signal-intensity changes is uncertain, but some possible causes include edema, gliosis, demyelination, leukoaraiosis, ischemia, glial metaplasia and/or any combination (7,8). In our case, there was no associated parenchymal signal intensity abnormality on MR images. On the other hand, increased perfusion patterns were detected within the drainage territory of the DVA on perfusion MRI, in agreement with a previous report in four patients with DVAs (1). In addition to these cases, a recent report described two patients with increased perfusion in the region of the DVA, on perfusion CT (2). Accordingly, two types of DVAs have been offered: atypical DVA with increased perfusion that may lead to tissue damage, and typical (uncomplicated) DVA with normal perfusion that does not affect the integrity of the surrounding parenchyma. A review of 34 subjects, the diffusion and perfusion MRI findings of the signal-intensity abnormalities associated with DVA suggested vasogenic edema with congestion and delayed perfusion as the underlying pathophysiologic characteristics (8). The abnormally large drainage vicinity of the DVA may result in relative volume overload and edema with congestion and/or chronic ischemia. Though no abnormality was observed on FLAIR MRI showing parenchymal damage surrounding DVA, parenchymal contrast enhancement seen in this case may be indicative of early manifestations of venous congestion and/or ischemia associated with DVA.
DVAs are limited to the venous structures, and implicated veins are often abnormally dilated and twisted and follow a chaotic pattern on histopathological analysis. The vascular anomalies associated with DVAs include DVA stenoses, dystrophic calcifications, and cerebral cavernous malformations (CCM) (2). Sharma et al. (9) revealed significant differences in perfusion parameters around DVAs with and without CCMs and proposed possible role of hemodynamic differences in the formation of CCMs. It has been suggested that an abnormal vascular bed of a DVA might cause altered hemodynamics or might be more vulnerable to result in microhemorrhage, in turn leading to angiogenic proliferation (7,9). In our case, there was contrast enhancement in the basal ganglia surrounding the DVA, in addition to abnormal perfusion pattern described in previous reports. This theory, which has been proposed to explain the association of CCMs and DVAs, may also explain the contrast enhancement and abnormal perfusion pattern in the territory of DVAs. We did not detect any interval change in a year. The possibility that these findings might represent early manifestations of a progressive process requires further follow-up.
As described above, DVAs have been commonly observed to be associated with altered hemodynamics on perfusion MRI (8-10). In a study of DVA-associated perfusion changes (10), perfusion abnormalities with DVAs were common on perfusion MRI but uncommon on arterial spin labeling (ASL). Most perfusion MRI changes appeared larger than the DVA itself, due to blooming of susceptibility effects related to gradient echo technique as well as the anatomy and physiology of DVAs, in agreement with previously published data. On the other hand, intrinsic or venous ASL signal in a small fraction of DVAs was interpreted to represent transitional or mixed malformations with arteriovenous shunting. DVAs coexisting with arteriovenous malformation (AVM) or an arteriovenous shunt have been defined as mixed or transitional vascular malformations, in the literature (10). Furthermore, DVA coexisting with a true AVM, treated by selective transarterial embolization while preserving the DVAs, has been reported in two patients and the cause of this rare presentation was attributed to the association of the AVM and DVA (11).
In our case, atypical DVA was likely unrelated to the clinical symptoms of the patient. However, it has been postulated that the increased perfusion pattern might have been a contributing factor to the associated hemorrhage, or to the development of a possible underlying cavernomatous venous malformation in one of the case reports (2). Therefore, perfusion imaging may be helpful to identify atypical DVAs with an increased risk of associated complications. More detailed studies are required to determine the clinical significance of abnormal perfusion pattern associated with a DVA.
In conclusion, DVAs may present with atypical imaging findings, such as contrast enhancement and increased perfusion in the surrounding parenchyma, probably due to anomalous venous drainage. These unusual perfusion patterns of the DVAs should be differentiated from other entities such as hypervascular brain tumors or ischemia with hemodynamic changes, which have different clinical management.
Disclosure: The authors declared that this study has received no financial support. No conflict of interest was declared by the authors.
References
- Camacho DL, Smith JK, Grimme JD, Keyserling HF, Castillo M. Atypical MR imaging perfusion in developmental venous anomalies. AJNR Am J Neuroradiol 2004;25:1549-52. [PubMed]
- Kroll H, Soares BP, Saloner D, Dillon WP, Wintermark M. Perfusion-CT of developmental venous anomalies: typical and atypical hemodynamic patterns. J Neuroradiol 2010;37:239-42. [PubMed]
- Mittal S, Wu Z, Neelavalli J, Haacke EM. Susceptibility-weighted imaging: technical aspects and clinical applications, part 2. AJNR Am J Neuroradiol 2009;30:232-52. [PubMed]
- Lee BC, Vo KD, Kido DK, Mukherjee P, Reichenbach J, Lin W, Yoon MS, Haacke M. MR high-resolution blood oxygenation level-dependent venography of occult (low-flow) vascular lesions. AJNR Am J Neuroradiol 1999;20:1239-42. [PubMed]
- Reichenbach JR, Jonetz-Mentzel L, Fitzek C, Haacke EM, Kido DK, Lee BC, Kaiser WA. High-resolution blood oxygen-level dependent MR venography (HRBV): a new technique. Neuroradiology 2001;43:364-9. [PubMed]
- Amemiya S, Aoki S, Takao H. Venous congestion associated with developmental venous anomaly: findings on susceptibility weighted imaging. J Magn Reson Imaging 2008;28:1506-9. [PubMed]
- Santucci GM, Leach JL, Ying J, Leach SD, Tomsick TA. Brain parenchymal signal abnormalities associated with developmental venous anomalies: detailed MR imaging assessment. AJNR Am J Neuroradiol 2008;29:1317-23. [PubMed]
- Jung HN, Kim ST, Cha J, Kim HJ, Byun HS, Jeon P, Kim KH, Kim BJ, Kim HJ. Diffusion and perfusion MRI findings of the signal-intensity abnormalities of brain associated with developmental venous anomaly. AJNR Am J Neuroradiol 2014;35:1539-42. [PubMed]
- Sharma A, Zipfel GJ, Hildebolt C, Derdeyn CP. Hemodynamic effects of developmental venous anomalies with and without cavernous malformations. AJNR Am J Neuroradiol 2013;34:1746-51. [PubMed]
- Iv M, Fischbein NJ, Zaharchuk G. Association of developmental venous anomalies with perfusion abnormalities on arterial spin labeling and bolus perfusion-weighted imaging. J Neuroimaging 2015;25:243-50. [PubMed]
- Erdem E, Amole AO, Akdol MS, Samant RS, Yaşargil GM. Developmental venous anomaly coexisting with a true arteriovenous malformation: a rare clinical entity. J Neurointerv Surg 2012;4:e19. [PubMed]


