
Focus on the annuloplasty in aortic valve repair: implications from a quantitative multislice computed tomography analysis
Introduction
Aortic valve (AV) repair in young patients evolved towards a promising alternative to AV replacement in the last decade and has several major advantages (1). However, recurrent AV regurgitation is still a major issue in patients after AV repair as compared to those after surgical AV replacement (2). AV annulus diameter >28 mm and lack of annuloplasty in the setting of dilated ventriculo-aortic junction (VAJ) has been reported to be a risk factor for recurrent AV regurgitation and redo surgery after AV repair (3). Therefore, different annuloplasty techniques have been proposed to address VAJ dilatation and to improve durability of AV repair (4). However, annuloplasty in AV repair is insufficiently standardized and the most appropriate surgical technique has still lacking. Furthermore, there are some inherent limitations of currently available AV annuloplasty techniques. Proper positioning of annuloplasty device at the VAJ level is crucial to achieve an appropriate AV annular stabilization (5,6). Consequently, deep surgical dissection of the aortic root is required for all external annuloplasty techniques, including reimplantation procedure and external ring annuloplasty to reach the nadirs of aortic cusps (6,7). Surgical dissection of AV annular plane is especially demanding at the level of the right coronary sinus where the right ventricular outflow tract myocardium merges with the muscular sleeve of left ventricular outflow tract (LVOT), thereby limiting the surgical accessibility of the aortic annulus (5).
Given this anatomic complexity of AV annular structure, precise knowledge and understanding of aortic annular anatomy is crucial to develop more durable techniques in AV repair (8). Modern imaging techniques such as cardiac multi-slice computed tomography (MS-CT) are routinely used for precise aortic root measurements and preprocedural planning of the catheter-based AV procedures (9). Having said that, MS-CT may similarly provide valuable information for AV repair by facilitating quantitative measurements of AV annular structures and thereby leading to a more standardized and reproducible AV annuloplasty (4).
Therefore, we aimed to analyse the functional anatomy of AV annulus based on the preoperative MS-CT data and to compare AV annular structures in patients presenting with the different types of tricuspid AV disease.
Methods
Patient population
We retrospectively identified 326 consecutive patients with tricuspid AV disease who underwent preprocedural cardiac MS-CT scans from January 2000 until January 2017. Preoperative MS-CT were performed for the planning of catheter-based aortic and/or mitral valve procedures in all patients. Subsequently, we subdivided our study population into three cohorts according to the underlying tricuspid AV disease:
- Twenty-five patients (mean age 73.0±10.8 years, 68% male) with severe AR who underwent MS-CT-scan in preparation for transcatheter AV replacement (AR subgroup). All 25 patients had degenerative tricuspid AV regurgitation and were high-risk/inoperable for open surgery, as consented by our institutional heart-team.
- Two hundred forty three patients (mean age 79.3±6.9 years, 58% male) with severe symptomatic tricuspid AV stenosis and aortic valve area (AVA) <1 cm2 who underwent preprocedural MS-CT scan in preparation for TAVR (AS subgroup).
- Fifty-eight patients (mean age 76.0±6.5 years, 36% male) who underwent MS-CT scan prior to transcatheter mitral valve repair in the absence of AV disease (normal AV subgroup). All these patients had a secondary mitral regurgitation with a predominant ring dilatation/leaflet tenting (i.e., type I and type IIIb MR) and no relevant degenerative mitral leaflet disease.
Exclusion criteria were previous AV interventions (e.g., prior AV replacement) and insufficient quality of MS-CT scan.
Our study protocol has been approved by the local Ethics Committee, which is in accordance with the Helsinki declaration. Individual patient consent was waived in this retrospective analysis.
MS-CT measurements
We analysed ECG-gated cardiac MS-CT data using 3-mensio Structural Heart™ imaging program (Version 8.1: Pie Medical Imaging, Maastricht, Netherlands) which is routinely used for TAVR planning at our center. According to our institutional TAVR evaluation protocol, all measurements were performed in the mid-systole (i.e., 30% of the cardiac cycle).
Measurements of the anatomic AV-annulus
We measured AV annular diameters at the level of the hinge points (nadir) of AV cusps (so-called basal ring plane). Maximal and minimal transverse basal ring diameters were measured (Figure 1A) and the AV annular eccentricity index [(max AV annulus ×100/min AV annulus) − 100] was calculated.
Next, we focussed on the three-dimensional AV annular shape (Figure 1B). For precise anatomic AV annular measurements, we manually identified the semilunar insertion line of AV cusps and measured the anatomic AV annular perimeter (Figure 1B). Briefly, anatomic insertion line of AV cusps was tracked manually using the 3-mensio Structural Heart™ imaging program. Annulus insertion line was defined by successively selecting 20 control points in the long-axis plane which were tracked all the way up to the top of the commissures. Furthermore, we calculated the projected AV annular area and perimeter at the level of basal ring (Figure 2).
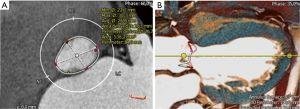
Measurement of the coronary ostia distances and the intramuscular plane in the right coronary sinus
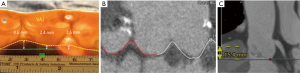
We assessed the distance of left and right coronary orifice in relation to the basal AV annular plane. Additionally, we defined the so-called “intramuscular plane in the right coronary sinus” which represents the distance between the merging point of RVOT and LVOT and the basal AV annular plane in the right coronary sinus as displayed in the Figure 2.
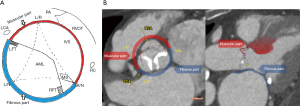
Measurements of the fibrous and muscular components of ventricular-aortic junction
Following the definition of 3-dimensional anatomic AV annulus (Figure 2), we subdivided AV annular perimeter into two separate components of the fibrous and myocardial tissue. Definition of tissue components was performed as shown in Figure 3. We separated fibrous vs. muscular component of AV annulus based on two criteria: (I) anatomic landmarks of aortic root—the region of aorto-mitral continuity (i.e., in between right and left fibrous trigone) and the area of membranous septum were assigned to the fibrous component of AV-annulus, while the region of the right coronary sinus and the left-right commissural area were treated as the muscular component of AV-annulus; (II) color-coding according to Hounsfield units was additionally used to support the differentiation between fibrous and muscular tissue. Color-coding of LV apex myocardium was used as a reference for the muscular component, while membranous interventricular septum served as a reference for the fibrous component. The relative perimeter of the fibrous and myocardial component of the AV annulus was calculated as shown in Figure 3. The fibrous and myocardial component of AV annulus were expressed as a percentage of the total annular perimeter.
Statistical analysis
Normally distributed continuous variables are presented as mean ± standard deviation; categorical variables are expressed as number and frequencies. Data were tested for normal distribution using the Kolmogorov-Smirnov test. All statistical analyses were performed using IBM SPSS 23 software (IBM Corp., Armonk, NY, USA). Comparisons of differences were accomplished using the one-way analysis of variance (ANOVA). Testing of differences between each group was performed using Bonferroni post-hoc analysis. All P values <0.05 were considered statistically significant.
Results
Measurements of the anatomic AV-annulus
AV annular measurements revealed a significantly larger maximal AV annulus diameter in AR subgroup vs. AS subgroup (28.6±2.1 vs. 26.4±2.5 mm, P<0.001) (Table 1). The minimal AV annulus diameter was significantly smaller in the normal AV subgroup as compared to the AR subgroup (21.3±2.4 vs. 22.4±1.7 mm, P=0.029).

Full table
AV annular eccentricity index was significantly better preserved in the normal AV-subgroup as compared to the AR-subgroup (32.7% ±10.7% vs. 27.8% ±9.2%; P=0.048) and the AS-subgroup (32.7% ±10.7% vs. 20.4% ±8.8%; P<0.001).
In addition, we found significant differences in the AV annular perimeter and AV annular area between the subgroups (Figure 4). Anatomic AV annular perimeter was significantly larger in the AR subgroup as compared to the normal AV subgroup (107.4±14.5 vs. 99.3±10.7 mm; P=0.011) (Table 2). Likewise, the projected AV perimeter (i.e., at the level of basal ring) was significantly larger in the AR subgroup as compared to the AS subgroup (88.4±14.5 vs. 82.4±11.8 mm; P<0.001) and to the normal AV subgroup (88.4±14.5 vs. 80.0±6.7 mm; P<0.001). Furthermore, projected AV annular area was significantly increased in the AR subgroup as compared to the AS subgroup (5.7±1.0 vs. 5.1±0.8 cm2; P=0.003) as well as compared to the normal AV subgroup (5.7±1 vs. 4.8±0.8 cm2; P<0.001).

Full table
Measurement of the coronary ostia distances and the intramuscular plane in the right coronary sinus
The distance from the AV basal ring plane to the right coronary artery (RCA) orifice was significantly different among the three study subgroups. The RCA distance was greater in the AR subgroup as compared to the AS subgroup (18.2±3.0 vs. 16.3±3.3 mm; P=0.018) as well as to the normal AV subgroup (18.2±3.0 vs. 15.9±3.2 mm; P=0.011). The distance between AV basal ring plane and the left coronary artery ostium was comparable between the three study groups (Table 3).

Full table
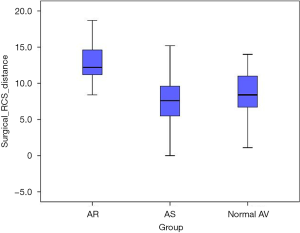
The intramuscular plane in right coronary sinus (RCS) distance was significantly increased in the AR subgroup as compared to the AS subgroup (12.8±2.7 vs. 7.5±3.6 mm; P<0.001) and as compared to the normal AV subgroup (12.8±2.7 vs. 8.7±3.0 mm; P<0.001) (Figure 5).
Measurements of the fibrous and muscular components of ventricular-aortic junction
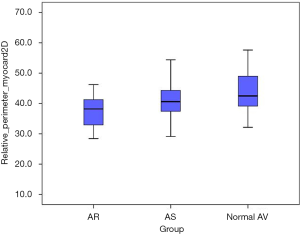
We found a significant difference in the relation of muscular vs. fibrous component of the anatomic AV annulus when comparing the three study cohorts. Myocardial component of the anatomic AV annulus was significantly smaller in the AR subgroup when compared to the AS subgroup (37.5% ±5.1% vs. 40.5% ±5.5%; P=0.039) and the normal AV subgroup (37.5% ±5.1% vs. 44.3% ±10.2%; P=0.001) (Figure 6).
Discussion
Precise understanding of the functional AV annulus anatomy is crucial when performing annuloplasty in AV repair surgery. Quantitative preoperative imaging, and in particular MS-CT, facilitate the procedural planning in cardiovascular interventions and help to define the most appropriate surgical approach (10). Routine measurements of AV annular diameters by transthoracic or transoesophageal echocardiography provide only limited information regarding AV annular structures and therefore are insufficient to design an optimal annuloplasty in AV repair. In contrast, quantitative analysis of 3D MS-CT imaging data provide very detailed information on the functional anatomy of AV annulus (10,11). Therefore, we aimed to examine detailed AV annulus structure by means of quantitative MS-CT analysis in different tricuspid AV pathologies with the special focus on patients with tricuspid aortic regurgitation (AR).
Previous anatomic aortic root studies demonstrated that AV annulus has a complex scalloped three dimensional structure which is very different from a complete ring or circle (12). The formation consisting of the three semilunar insertion lines of the aortic cusps is more likely to appreciate the true anatomic circumstances (13). Considering excellent spatial and time resolution of MS-CT imaging through the whole cardiac cycle, we aimed to measure AV annulus respecting this three-dimensional crown-like structure.
Three novel and the most important findings in our current study are the following: (I) three-dimensional anatomic AV annular perimeter is significantly increased in patients with tricuspid AR; (II) intramuscular plane in the right coronary sinus is significantly increased in AR patients; (III) relation between fibrous and muscular AV annular component differs significantly between tricuspid AR patients and those with a normal AV function.
Our study revealed important differences in the functional AV annulus anatomy in AR patients. Anatomic and projected AV annulus perimeter as well as AV annular area were all significantly increased in the AR subgroup as compared to the patients with normally functioning AV and in those with AS. These findings substantiate the association of AV annular dilatation with AR and, simultaneously, obviate the need of annuloplasty in AV repair. We previously introduced the term of AV-annular eccentricity index which describes the percentual difference between maximal and minimal AV annular diameter during the cardiac cycle (14). Although maximal AV annulus diameter was quite comparable between AR patients and those with normal AV function, there was a significant difference in the minimum AV annulus diameter between the two groups (Table 1). Consequently, eccentricity index of AV annulus was significantly reduced in AR patients as compared to the normal AV subgroup (Table 1). In other words, the ovality of basal AV ring is significantly diminished in AR patients, which may indicate the potential pathophysiological impact of diastolic AV annular dysfunction in the genesis of AR. In addition to dilatation of AV basal ring in the AR setting, there is an increased circularity of AV basal ring, which potentially contributes to AR progression. This finding reinforces furthermore the concept of flexible annuloplasty device which would allow systolic circularity and simultaneously support diastolic ovality of AV basal ring plane, mimicking the physiological change of AV basal ring shape during the cardiac cycle (14).
Another important insight from our quantitative MS-CT data analysis is the finding of an increased intramuscular plane between RVOT and LVOT to the level of basal AV ring in the region of right coronary sinus in AR patients (Table 3, Figure 5). The comparison of intramuscular plane in the right coronary sinus between the three study subgroups revealed that the distance was the most extensive (i.e., mean distance of 13 mm) in the AR subgroup as compared to the normal AV subgroup and the AS subgroup. This quantitative anatomic information is relevant for AV repair surgery, since annuloplasty device must reach the level of the basal AV ring. In the surgical setting of external annuloplasty (i.e., reimplantation procedure or external ring annuloplasty) the external aortic root dissection should reach the level of the basal ring, which corresponds the virtual plane connecting the nadirs of aortic cusps (5). The technical difficulty of the proximal dissection line is due to the fact that the basal ring plane is overlaid by RVOT in the region of right coronary sinus and at the level of commissure between the right and left coronary cusp (5). Our MS-CT-based analysis demonstrates that muscular plane overlays the level of the basal ring in the whole segment of the right coronary sinus (Figure 5). Furthermore, the distance of surgical dissection through the muscular plane required to reach basal AV ring in the right coronary sinus seems to be more extensive in our MS-CT analysis as compared to the previous ex-vivo anatomic studies (5,6). From the practical point of view, our quantitative MS-CT analysis indicates that an extensive surgical dissection >10 mm in the muscular plane between RVOT and LVOT is required in AR patients for an appropriate positioning of an external annuloplasty device in the region of the right coronary sinus.
Furthermore, AV annuloplasty should take into account the functional asymmetry of aortic root (15). Previous anatomic studies revealed that 60% VAJ consist of fibrous tissue, which forms the central fibrous skeleton of the human heart (12). However, VAJ portion in the region of right coronary sinus and in the anterior part of the left coronary sinus is composed of the LV myocardial tissue (15). Our study revealed for the first time that the relation between fibrous and muscular component of the VAJ differs significantly between AR patients and those with a normal AV function (Figure 6). VAJ dilatation in the AR subgroup was accompanied with a percentual decrease of the muscular component as compared to the AS subgroup and the normal AV subgroup.
Limitations
This is a retrospective single centre study with all well-known limitations of such study design. No between-group matching for age, gender and pre-existing comorbidities was performed, since MS-CT data was obtained from consecutive patients scheduled for TAVR or other catheter-based heart interventions. Patients in the normal AV subgroup had normal AV function in combination with a functional mitral regurgitation and, therefore, cannot be considered as completely healthy individuals. Furthermore, with respect to AR, diastolic measurements of AV annulus and comparison between systolic and diastolic measurements would be of additional value. However, MS-CT scans were performed according to the protocol for transcatheter AV replacement and therefore only mid-systolic images (i.e., 30% cardiac cycle) were available.
Another important limitation of our current study is the fact that herein we analyze an elderly patient’s cohort with a tricuspid aortic valve (TAV) which is quite different from a typical population of AV repair patients who are usually younger and frequently have a congenital AV disease (i.e., bicuspid or unicuspid aortic valve). Therefore, measured parameters apply only to an elderly patient cohort with a tricuspid AR which may be quite different as compared to the usual young-aged AV repair candidates. Nonetheless, the focus of current analysis was AV annulus and reconsideration of annular stabilization concepts and we still had a cohort of 83 (25%) patients with a non-calcified TAV, which allowed us to analyze aortic annulus dynamics. Therefore, we feel that MS-CT measurements, even in these elderly patients who are not optimal candidates for AV repair, are still relevant for a functional AV annulus analysis, at least in patients with a TAV.
Conclusions
Although quantitative MS-CT analysis is routinely used for catheter-based cardiovascular interventions only, it provides valuable information on the functional anatomy of AV annular structures required for AV annuloplasty. Elderly tricuspid AR patients differ significantly regarding their AV annular dimensions and basal ring morphology as compared to the AS patients and those with a normal AV function. Our current findings should stimulate further improvements of the annuloplasty strategies in AV repair.
Acknowledgments
Presented at the Annual Meeting of German Society of Cardiothoracic and Vascular Surgery, Leipzig, 2019.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/qims.2020.03.16). The authors have no conflicts of interest to declare.
Ethical Statement: Our study protocol has been approved by the local Ethics Committee, which is in accordance with the Helsinki declaration. Individual patient consent was waived in this retrospective analysis.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Boodhwani M, El Khoury G. Aortic valve repair: indications and outcomes. Curr Cardiol Rep 2014;16:490-96. [Crossref] [PubMed]
- Arabkhani B, Mookhoek A, Di Centa I, Lansac E, Bekkers JA, De Lind Van Wijngaarden R, Bogers AJ, Takkenberg JJ. Reported Outcome After Valve-Sparing Aortic Root Replacement for Aortic Root Aneurysm: A Systematic Review and Meta-Analysis. Ann Thorac Surg 2015;100:1126-31. [Crossref] [PubMed]
- David TE. Aortic valve repair and aortic valve-sparing operations. J Thorac Cardiovasc Surg 2015;149:9-11. [Crossref] [PubMed]
- Komiya T. Aortic valve repair update. Gen Thorac Cardiovasc Surg 2015;63:309-19. [Crossref] [PubMed]
- de Kerchove L, Jashari R, Boodhwani M, Duy KT, Lengelé B, Gianello P, Mosala Nezhad Z, Astarci P, Noirhomme P, El Khoury G. Surgical anatomy of the aortic root: implication for valve-sparing reimplantation and aortic valve annuloplasty. J Thorac Cardiovasc Surg 2015;149:425-33. [Crossref] [PubMed]
- Khelil N, Sleilaty G, Palladino M, Fouda M, Escande R, Debauchez M, Di Centa I, Lansac E. Surgical anatomy of the aortic annulus: landmarks for external annuloplasty in aortic valve repair. Ann Thorac Surg 2015;99:1220-6. [Crossref] [PubMed]
- Cheng A, Dagum P, Miller DC. Aortic root dynamics and surgery: from craft to science. Philos Trans R Soc Lond B Biol Sci 2007;362:1407-19. [Crossref] [PubMed]
- Schäfers HJ. Aortic annuloplasty: The panacea of valve-preserving aortic replacement? J Thorac Cardiovasc Surg 2017;153:1043-1044. [Crossref] [PubMed]
- Chourdakis E, Koniari I, Kounis NG, Velissaris D, Koutsogiannis N, Tsigkas G, Hauptmann KE, Sontag B, Hahalis G. The role of echocardiography and CT angiography in transcatheter aortic valve implantation patients. J Geriatr Cardiol 2018;15:86-94. [PubMed]
- Thériault-Lauzier P, Spaziano M, Vaquerizo B, Buithieu J, Martucci G, Piazza N. Computed Tomography for Structural Heart Disease and Interventions. Interv Cardiol 2015;10:149-54. [Crossref] [PubMed]
- Regeer MV, Kamperidis V, Versteegh MI, Klautz RJ, Scholte AJ, Bax JJ, Schalij MJ, Marsan NA, Delgado V. Aortic valve and aortic root features in CT angiography in patients considered for aortic valve repair. J Cardiovasc Comput Tomogr 2014;8:299-306. [Crossref] [PubMed]
- Ho SY. Structure and anatomy of the aortic root. Eur J Echocardiogr 2009;10:i3-i10. [Crossref] [PubMed]
- Anderson RH. The surgical anatomy of the aortic root. Multimed Man Cardiothorac Surg 2007;2007:mmcts.2006.002527.
- Petersen J, Voigtländer L, Schofer N, Neumann N, von Kodolitsch Y, Reichenspurner H, Girdauskas E. Geometric changes in the aortic valve annulus during the cardiac cycle: impact on aortic valve repair. Eur J Cardiothorac Surg 2018;54:441-5. [Crossref] [PubMed]
- Loukas M, Bilinsky E, Bilinsky S, Blaak C, Tubbs RS, Anderson RH. The anatomy of the aortic root. Clin Anat 2014;27:748-56. [Crossref] [PubMed]



