
Preliminary application of CT perfusion source images for evaluating regional collateral circulation in unilateral Moyamoya disease
Introduction
Moyamoya disease (MMD) is characterized by chronic progressive stenosis or occlusion of the terminal portion and main branches of the bilateral internal carotid arteries (ICAs). If the angiographic findings were unilateral, the case would be considered unilateral MMD (1). By the time of diagnosis, these patients have developed collateral vessels to stabilize blood flow. However, these collaterals are imperfect and prone to bleeding, aneurysm, and thrombosis (2). Most adult patients with symptomatic MMD experience a chronic decrease in blood flow. Therefore, it is necessary to establish good collateral circulation to prevent the risks of ischemia and hemorrhage (3). Precise analysis of radiological findings including leptomeningeal collateralization and parenchymal perfusion is essential to make an accurate diagnosis, predict outcomes, and determine appropriate therapeutic strategies and follow-up (4). Digital subtraction angiography (DSA) is a commonly accepted method to evaluate the presence of collateral status (3), as it exhibits high spatial and temporal resolution of inflow dynamics, and has the ability to interrogate specific feeding vessels selectively. However, this is an invasive, time-consuming, and complex procedure. Thus, it would be helpful to have information regarding the collateral status using a noninvasive and easily accessible tomographic approach before and after revascularization therapy.
Noninvasive imaging of collaterals could be performed using computed tomography (CT) or magnetic resonance (MR) examination. CT has the advantages of wide availability and short scanning times. Currently, collateral grading scores on CT angiography (CTA) has been published (5). However, collateral vessels may not always be visible on standard single time-frame CTA because of delayed contrast arrival (6). Dynamic CT perfusion (CTP) imaging including images ranging from contrast agent infusion to the arterial phase, capillary phase, and venous phase can help overcome this limitation. Therefore, CTP source images (CTP-Sis) not only are used to derive perfusion maps such as cerebral blood flow but also contain information regarding the cerebral vasculature.
We devised an angiographic collateral grading system based on arterial and venous phase CTP-Sis that imitates the DSA scoring system. The purpose of this study was to assess the agreement between CTP-Sis and DSA for grading the collateral flow in patients with unilateral MMD.
Methods
Patient selection
We retrospectively studied 31 consecutive patients with unilateral MMD in Beijing Tiantan Hospital (April 2008 to November 2013). We included patients applying the following inclusion criteria: (I) diagnosed with MMD according to the Japanese MMD guideline (1); (II) involvement of only a unilateral middle cerebral artery (MCA); (III) had both CTP and DSA; and (IV) no prior surgical intervention such as a superficial temporal artery to MCA bypass. Three patients were excluded because of incomplete DSA images in other hospitals. The other four patients were excluded because CTP was performed in other hospitals with only perfusion parameter maps and no CTP-Sis. Finally, we included 24 patients who had CTP and DSA simultaneously as part of their preoperative assessment for a possible superficial temporal artery to MCA bypass. And all patients’ imaging data were from Beijing Tiantan Hospital. All patients were symptomatic, with the most common symptoms including a headache, dizziness, limb convulsions, and limb numbness or weakness. The study protocol was approved by the local institutional review board.
Imaging acquisition
Digital subtraction angiography
DSA was performed using a flat panel detector cerebral angiographic system (Artis Zee Floor; German Siemens Medical Systems). For all patients, images of the bilateral ICAs, external carotid arteries (ECAs), and bilateral vertebral arteries (VAs) were acquired using Seldinger’s transfemoral catheterization technique. Imaging through the entire arterial and venous phases was performed to evaluate slow-flowing collateral vessels.
CTP scanning
CTP examination was performed using 16-section spiral CT scanners (Somatom Volume Zoom; Siemens, Erlangen, Germany) or 64-slice spiral CT scanners (Discovery CT750HD; GE Healthcare Technologies, Milwaukee, WI, USA). Contiguous section CTP examinations at the level of the basal ganglia/internal capsules and the upper portion of the lateral ventricles were performed, including the anterior, posterior, and MCA territories. The acquisition parameters were as follows: 80 kV tube voltage, 209 mAs, 1.0 s rotation, 50-s scanning time, and 12-mm slice thickness (2 contiguous sections, 24-mm detection range) (Siemens); and 100 kV tube voltage, 150 mAs, 1.0 s rotation, 50-s scanning time, and 5-mm slice thickness (4 contiguous sections, 20-mm detection range) (GE). A bolus of 40-mL nonionic iodinated contrast agent (iobitridol, 300 mg/mL, Xenetix; Guerbet, Aulnay-sous-Bois, France) was administered into the antecubital vein at an injection rate of 5 mL/s (20-gauge intravenous cannula) using a power injector [EAZM Injection System; Ulrich Missouri (XD2501-C) injection system].
Image postprocessing for CTP-Sis
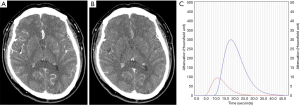
Image postprocessing software (5i3D; Neusoft Medical Systems, Shenyang, China) was used to select arterial and venous phase CTP-Sis (7). The time density curve was obtained from the anterior cerebral arterial and superior sagittal sinus. Arterial phase CTP-Sis (A-CTP-Si) and venous phase CTP-Sis (V-CTP-Si) were defined at the peak points on the arterial and venous time density curves, respectively, for further analysis (Figure 1).
Imaging analysis
Collateral assessment on angiography
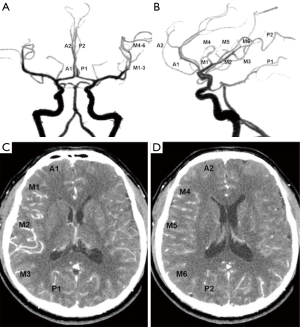
Guided by the Alberta Stroke Programme Early CT Score (ASPECTS) study (8), we divided the angiogram of the affected hemisphere into 10 anatomic sites based on the regional cortical vascular territories (Figure 2A,B). The collateral flow score was recorded for each of these 10 areas. The DSA studies were evaluated by two neuroradiologists (Z.Y.N and G.W.B.) using a 5-point collateral grading scale describing the intensity of collateral flow (American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology, ASITN/SIR) (9): 0, no collaterals were visible at the ischemic site; 1, slow collaterals at the periphery of the ischemic site with persistence of some defect; 2, rapid collaterals at the periphery of the ischemic site with persistence of some defect and to only a portion of the ischemic territory; 3, collaterals with slow but complete angiographic blood flow to the ischemic bed by the late venous phase; 4, complete and rapid collateral blood flow to the vascular bed in the entire ischemic territory by retrograde perfusion. Disagreements were resolved by consensus.
Collateral assessment on CTP-Sis
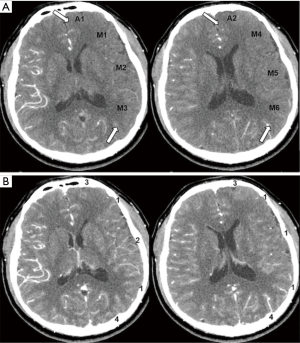
Arterial/venous phase CTP-Sis could be reviewed directly on a CT workstation. The measured sections were at the level of the basal ganglia, as well as at another level rostral to the ganglionic structures. Corresponding to the DSA scoring system, this grading was also performed in 10 regions on the affected side (10), as described in Figure 2C,D. CTP-Si was evaluated by another two neuroradiologists (Q.Y. and H.L.N.) who were blinded to the DSA study and grading results. The regional collateral circulation scoring system of CTP-Sis was also graded on a scale from 0 to 4: 0, no contrast enhancement of the ischemic site on either arterial or venous phase CTP-Si; 1, peripheral contrast enhancement of the ischemic site on V-CTP-Si; 2, peripheral contrast enhancement of the ischemic site on A-CTP-Si with persistence of some defect on V-CTP-Si; 3, complete contrast enhancement of the ischemic site on V-CTP-Si; and 4, complete contrast enhancement of the ischemic site on A-CTP-Si. Disagreements were also resolved by consensus. An example of the CTP-Sis collateral grading scale is shown in Figure 3.
Statistical analyses
The unit of analysis was the score of the two different methods for evaluating collateral circulation (CTP-Sis and DSA). Agreement, linear-weighted κ values, Kendall Tau-b measure of correlation and the exact Bowker test of symmetry (to determine any tendency for disagreements predominantly in 1 direction) were calculated for both the individual inter-reader scores and intermodality consensus scores. Additionally, the scores were divided into two categories: 0–2 (no perfusion or peripheral perfusion; poor or moderate collateral flow) and 3–4 (complete perfusion and good collateral flow) and were compared using unweighted κ, the Kruskal-Goodman γ measure of correlation, and the exact Bowker test of symmetry. DSA reading was considered the gold standard and, based on this, the specificity, positive predictive value, and negative predictive value of CTP-Sis for identifying collateral flow were determined. Significance was set at a two-sided P value of <0.05. All statistical analyses were performed using SAS (version 9.4, SAS Institute Inc., Cary, NC, USA).
Results
Patient characteristics
Twenty-four symptomatic patients (15 men and 9 women; mean age, 30.7±10.1 years; range, 14–50 years) with unilateral MMD were enrolled in this study. The mean interval between CTP and DSA was 14.7±10.3 days. All patients had an MCA on one side involved (17 on the left side; 7 on the right side). According to the modified thrombolysis in cerebral infarction (TICI) grading system (9), the MCA was occluded in 8 cases, had severe stenosis in 10 cases, and had moderate stenosis in 6 cases. A total of 240 corresponding anatomic areas on the affected side were evaluated and compared.
The relationship between the CTP-Sis and DSA collateral flow grading system
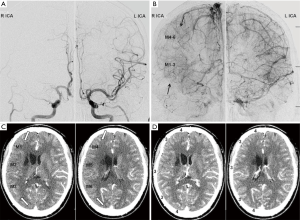
The unit of analysis was the score of the two different methods for evaluating collateral circulation (CTP-Sis and DSA). According to the CTP-Sis and DSA collateral scores, the number of lesions in 0–4 points was 2, 22, 18, 73, and 125, respectively (CTP-Sis), and 1, 17, 11, 86, and 125, respectively (DSA). Though some areas with DSA grade 3 were underestimated in CTP-Sis. There was good agreement between the two modalities as shown in Tables 1,2. It was more common for the CTP-Sis readers to agree on the exact score, as compared with the DSA readers (82.9% vs. 70.4%, respectively). This was also reflected in a higher-weighted κ for the CTP-Sis readers [κ=0.802; 95% confidence interval (CI), 0.743–0.861], as compared with the DSA readers (κ=0.633; 95% CI, 0.553–0.714). The weighted κ for the agreement between the consensus DSA and CTP-Sis scores was 0.768 (95% CI, 0.703–0.832). Figure 4 provides examples of both methods in a patient with unilateral MMD. Using a dichotomized scale [i.e., no or peripheral perfusion (score, 0–2) versus complete perfusion (score, 3–4)], the κ value was even higher (0.791; 95% CI, 0.682–0.899) that could be considered the substantial agreement. Based on the CTP-Sis and DSA consensus scores, 82% and 87%, respectively, the regions were completely perfused (collateral score 3–4). For the consensus CTP-Sis and DSA collateral scores, sensitivity, specificity, positive predictive value, and negative predictive value were 0.714 (95% CI, 0.578–0.851), 0.995 (95% CI, 0.985–1.00), 0.968 (95% CI, 0.906–1.00), and 0.943 (95% CI, 0.911–0.974), respectively. Table S1 shows the breakdown of the various collateral scores using the entire 5-point scale. Table S2 shows the breakdown of the various collateral scores using the Dichotomized Scores. The analysis of Kendall Tau-b indicated that the two collateral circulation grading systems had a statistical correlation (Kendall’s tau-b =0.80). The average scores for CTP-Sis were slightly higher than those of DSA.

Full table
Full table

Full table

Full table
Discussion
Collateral flow plays a critical role in stabilizing the cerebral circulation if the principal conduits fail (11). Comprehensive and accurate assessment of the structure and function of the cerebral collateral circulation is an essential prerequisite and the basis for individualized treatment in cerebrovascular disease. Preliminary studies have shown that the establishment of good collateral circulation could increase the benefit of acute recanalization (12) and reduce the risk of hemorrhagic transformation in patients with acute ischemic stroke. It also significantly reduces the risk of recurrent stroke in symptomatic intracranial artery stenosis (13) and reduces the number and volume of focal cerebral infarctions. Furthermore, recent studies (14,15) showed that an embolic mechanism plays an important role in ischemic attacks in patients with MMD and that a good collateral status in patients with MMD might be associated with the washout of emboli. Direct or indirect bypass surgery is recommended to improve the regional cerebral hemodynamics in patients with MMD who have a poor collateral status. For all these reasons, having the capability to estimate the location and intensity of collateral perfusion using a credible method would be desirable.
In this study, we developed a collateral grading system for CTP-Sis. Further, the new collateral grading system for CTP-Sis demonstrated strong agreement with the DSA measurements. Additionally, CTP-Sis can also differentiate between poor and robust collateral flow that is consistent with a DSA-based collateral grading scale. Dynamic CTP-Sis can provide time-resolved images of the arterial, parenchymal, and venous phases that are analogous to the dynamic measurement of DSA. Combined with arterial and venous phase CTP-Sis, the new model was established based on the DSA collateral assessment system (9), which was graded according to the speed and extent of collateral arrival in the ischemic region. MMD is characterized by progressive stenosis. At the time of diagnosis, some patients have established collateral vessels of the leptomeninges. If the collateral circulation was well established, the blood supply area of the stenosed vessels would be completely perfused during the venous phase, i.e., grade 3. If the area of responsible blood supply continues to be hypoperfusion until the venous phase, the collateral compensation is poor, which is grade 0 in the CTP-Sis scoring system. Some areas with DSA grade 3 were underestimated in CTP-SI. The reasons were analyzed. It was found that the underestimated areas were associated with subcortical white matter lesions or infarcts. Therefore, we believed that CTP-SIS could better reflect the brain parenchymal state of patients. In addition, this did not affect the determination of clinical protocols.
In our study, the most involved hemispheres had a good collateral status (3–4 scores) on both CTP-Sis and DSA (82% and 87%, respectively) that was in line with the results of Kim et al. (14). This may be because of the chronic course of MMD that allowed enough time to develop collaterals. The collateral vessels in MMD include dilated perforating arteries that may be small, weak, and prone to hemorrhage and thrombosis, as well as the leptomeningeal arteries that play a major role in maintaining blood flow. The new model used in our study was mainly performed to assess the leptomeningeal collateral circulation.
DSA has been regarded as the gold standard to evaluate collateral status. However, sometimes DSA is not feasible as a fast-diagnostic tool because it is resource intensive and has a higher rate of complications than noninvasive imaging modalities (16), and it cannot provide information regarding the status of the brain parenchyma. Compared with DSA, CTP-Sis can be acquired noninvasively and rapidly, with the entire scan protocol completed within five minutes. Moreover, CTP-Sis provides time-resolved images that are analogous to the dynamic measurement of DSA. Recent studies have shown that the regional angiographic grading scores for collateral flow correlated with cerebral infarction in stroke patients (17). According to the ASPECT partition method, CTP is a tomographic scan, which is more beneficial for revealing the local collateral circulation. In addition to validating these general results, the current study also examines how well different readers agree on both the CTP-Sis and DSA grading scales, showing that agreement is higher with the CTP-Sis method. Compared with the evaluation method for DSA, CTP-Sis is more easily mastered and applied by radiologists and clinicians.
Currently, several different grading scores have been published in assessing collateral circulation. A commonly used noninvasive marker to evaluate collateral status is single-phase CTA. However, this technique provides only a snapshot in time of the cerebral vasculature and has been shown to lead to an underestimation of the collateral status (6,18). CTP-Sis was more reliable than single-phase CTA that is attributable to its dynamic image capturing, thus providing more information. Dynamic CTA is increasingly used to assess collateral circulation, but its clinical value still needs to be verified (19). Compared with CTA, CTP-Sis requires a lower radiation dose and has been reported to be less sensitive to patient motion. As we know, poor imaging quality caused by motion artifacts can affect physicians’ judgment in clinical practice. Previous studies demonstrated that arterial spin-labeling (ASL), an MRI perfusion method requiring no contrast agent or radiation exposure, is also suited to evaluating collateral perfusion. However, its greatest flaw is its exquisite sensitivity to arterial arrival delays and long acquisition time (20). Compared with ASL, we encouraged CTP-Sis examinations for cases who had any metal in their body or cannot stand loud buzzing noises or confined spaces.
Our study had several limitations. First, there were difficulties applying the DSA collateral system (17) that was initially developed for acute ischemic stroke as part of the PROACT II study to a chronic cerebrovascular disease such as MMD. Second, our collateral scoring system is new, and the series was limited regarding the number of patients studied. Thus, it requires further validation using a larger sample size. Third, it was sometimes difficult to completely distinguish delayed pia mater arteries from cortical veins in the venous phase, as was some cases with DSA images. However, the regional circulation time of hypoperfusion in the affected side was slower than that in the contralateral normal brain tissue in unilateral MMD patients. The time of the venous phase was set at the time when the contrast medium of superior sagittal sinus reached its peak. At the moment, the venous phase CTP-Si represented the venous phase of normal brain tissue on the opposite side, while the venous phase was later on the affected side, so the enhancement of the affected side was mainly due to collateral circulation compensation. Fourth, the time point for the CTP investigation was discrepant. The interval between CTP-Sis and DSA ranged from 4 to 25 days for reasons beyond our control that might also represent a particular limitation of this study. However, by the time of diagnosis, patients had developed a collateral network of vessels at the base of the brain and had hardly any image changes in the short term.
Conclusions
We believe that time-resolved CTP-Sis is a relatively inexpensive, non-invasive, credible, and feasible method for collateral perfusion measurement. The present study represents an initial step in exploring the clinical value of CTP-Si in the management of Moyamoya, as compared with DSA. Further study with a large number of cases is warranted. Further investigation of this method in other cerebrovascular diseases including acute ischemic stroke can also be warranted.
Acknowledgements
We appreciated that this manuscript had been edited by native English-speaking experts from BioMed Proofreading LLC.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study protocol was approved by the local institutional review board.
References
- Ganesan V, Smith ER. Moyamoya: defining current knowledge gaps. Dev Med Child Neurol 2015;57:786-7. [Crossref] [PubMed]
- Park SE, Kim JS, Park EK, Shim KW, Kim DS. Direct versus indirect revascularization in the treatment of moyamoya disease. J Neurosurg 2018;129:480-9. [Crossref] [PubMed]
- Kuroda S. Cerebrovascular disease: New data on surgical therapy for pediatric moyamoya disease. Nat Rev Neurol 2010;6:242-3. [Crossref] [PubMed]
- Liebeskind DS, Tomsick TA, Foster LD, Yeatts SD, Carrozzella J, Demchuk AM, Jovin TG, Khatri P, von Kummer R, Sugg RM, Zaidat OO, Hussain SI, Goyal M, Menon BK, Al Ali F, Yan B, Palesch YY, Broderick JP. IMS III Investigators. Collaterals at angiography and outcomes in the Interventional Management of Stroke (IMS) III trial. Stroke 2014;45:759-64. [Crossref] [PubMed]
- Maas MB, Lev MH, Ay H, Singhal AB, Greer DM, Smith WS, Harris GJ, Halpern E, Kemmling A, Koroshetz WJ, Furie KL. Collateral vessels on CT angiography predict outcome in acute ischemic stroke. Stroke 2009;40:3001-5. [Crossref] [PubMed]
- Smit EJ, Vonken EJ, van Seeters T, Dankbaar JW, van der Schaaf IC, Kappelle LJ, van Ginneken B, Velthuis BK, Prokop M. Timing-invariant imaging of collateral vessels in acute ischemic stroke. Stroke 2013;44:2194-9. [Crossref] [PubMed]
- Wang XC, Gao PY, Xue J, Liu GR, Ma L. Identification of infarct core and penumbra in acute stroke using CT perfusion source images. AJNR Am J Neuroradiol 2010;31:34-9. [Crossref] [PubMed]
- Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet 2000;355:1670-4. [Crossref] [PubMed]
- Higashida RT, Furlan AJ, Roberts H, Tomsick T, Connors B, Barr J, Dillon W, Warach S, Broderick J, Tilley B, Sacks D. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke 2003;34:e109-37. [Crossref] [PubMed]
- Zaharchuk G, Do HM, Marks MP, Rosenberg J, Moseley ME, Steinberg GK. Arterial spin-labeling MRI can identify the presence and intensity of collateral perfusion in patients with moyamoya disease. Stroke 2011;42:2485-91. [Crossref] [PubMed]
- Albers GW. Impact of recanalization, reperfusion, and collateral flow on clinical efficacy. Stroke 2013;44:S11-12. [Crossref] [PubMed]
- Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, Roy D, Jovin TG, Willinsky RA, Sapkota BL, Dowlatshahi D, Frei DF, Kamal NR, Montanera WJ, Poppe AY, Ryckborst KJ, Silver FL, Shuaib A, Tampieri D, Williams D, Bang OY, Baxter BW, Burns PA, Choe H, Heo JH, Holmstedt CA, Jankowitz B, Kelly M, Linares G, Mandzia JL, Shankar J, Sohn SI, Swartz RH, Barber PA, Coutts SB, Smith EE, Morrish WF, Weill A, Subramaniam S, Mitha AP, Wong JH, Lowerison MW, Sajobi TT, Hill MD, Investigators ET. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015;372:1019-30. [Crossref] [PubMed]
- Fanou EM, Knight J, Aviv RI, Hojjat SP, Symons SP, Zhang L, Wintermark M. Effect of Collaterals on Clinical Presentation, Baseline Imaging, Complications, and Outcome in Acute Stroke. AJNR Am J Neuroradiol 2015;36:2285-91. [Crossref] [PubMed]
- Kim DY, Son JP, Yeon JY, Kim GM, Kim JS, Hong SC, Bang OY. Infarct Pattern and Collateral Status in Adult Moyamoya Disease: A Multimodal Magnetic Resonance Imaging Study. Stroke 2017;48:111-6. [Crossref] [PubMed]
- Sabarudin A, Subramaniam C, Sun Z. Cerebral CT angiography and CT perfusion in acute stroke detection: a systematic review of diagnostic value. Quant Imaging Med Surg 2014;4:282-90. [PubMed]
- Latchaw RE, Alberts MJ, Lev MH, Connors JJ, Harbaugh RE, Higashida RT, Hobson R, Kidwell CS, Koroshetz WJ, Mathews V, Villablanca P, Warach S, Walters B. American Heart Association Council on Cardiovascular R, Intervention SC, the Interdisciplinary Council on Peripheral Vascular D. Recommendations for imaging of acute ischemic stroke: a scientific statement from the American Heart Association. Stroke 2009;40:3646-78. [Crossref] [PubMed]
- Kim JJ, Fischbein NJ, Lu Y, Pham D, Dillon WP. Regional angiographic grading system for collateral flow: correlation with cerebral infarction in patients with middle cerebral artery occlusion. Stroke 2004;35:1340-4. [Crossref] [PubMed]
- Menon BK, Smith EE, Modi J, Patel SK, Bhatia R, Watson TW, Hill MD, Demchuk AM, Goyal M. Regional leptomeningeal score on CT angiography predicts clinical and imaging outcomes in patients with acute anterior circulation occlusions. AJNR Am J Neuroradiol 2011;32:1640-5. [Crossref] [PubMed]
- Beyer SE, Thierfelder KM, von Baumgarten L, Rottenkolber M, Meinel FG, Janssen H, Ertl-Wagner B, Reiser MF, Sommer WH. Strategies of collateral blood flow assessment in ischemic stroke: prediction of the follow-up infarct volume in conventional and dynamic CTA. AJNR Am J Neuroradiol 2015;36:488-94. [Crossref] [PubMed]
- Bhaskar S, Bivard A, Stanwell P, Parsons M, Attia JR, Nilsson M, Levi C. Baseline collateral status and infarct topography in post-ischaemic perilesional hyperperfusion: An arterial spin labelling study. J Cereb Blood Flow Metab 2017;37:1148-62. [Crossref] [PubMed]


