
A modified semi-quantitative (mSQ) grading scheme for osteoporotic vertebral fracture in elderly women
While osteoporotic vertebral fracture (oVF) is very common and has important clinical relevance, until recently there has been limited evidences to validate published criterium by which to make an accurate diagnosis of an oVF based on radiographs (1). Purely morphometric approach without qualitative, i.e., radiological, assessment of vertebral morphology can lead to both ‘over-call’ and ‘under-call’ of oVFs (1). In the early 90’s, Genant and colleagues at University of California at San Francisco (UCSF) proposed the semi-quantitative (SQ) grading scheme to evaluate osteoporotic vertebral deformity (oVD) (2). It is based on a visual assessment of both quantitative and qualitative characteristics of the vertebral body. OVDs are graded by estimation, from grade 0 (normal and <20% height reduction); grade 1 (20–25% in height reduction and in area of 10–20%); grade 2 (25–40% height reduction and in area of 20–40%); to grade 3 (>40% in height and area). A vertebra is considered to be SQ grade-0.5 when it has qualitatively suspected oVD without meeting height loss ≥20% criterium (2). Congenital or acquired non-osteoporotic vertebral deformities, which may mimic oVD in appearances, are systemically excluded (2). As it is difficult to estimate vertebral lateral area reduction, the percentage area reduction requirement which was described in Genant’s original article, has been mostly dropped by users of SQ criteria over the years (3). The qualitative/radiological aspect of SQ assessment has been repeatedly emphasized, though sometimes still ignored by many users (2-4).
Recent reports emphasize the importance of identifying osteoporotic vertebral endplate and/or cortex fracture [ECF, or ABQ fracture as defined by Jiang et al. (5)] (5-14). In addition to vertebral height, particular attention is paid to the fracture of endplates and vertebral anterior cortex. Recent UCSF publications such as Fink et al. (11) and Cawthon et al. (12) described that, in their Osteoporotic Fractures in Men (MrOS) study, they used the ‘Genant semi-quantitative (SQ) grading method to rate each vertebral level for fracture, with the modification that grade-1 fractures had to exhibit cortical discontinuity or depression of most of either the superior or inferior endplate to be considered fractured’. In our Osteoporotic Fractures in women (MsOS Hong Kong) study, we demonstrated that, compared with vertebrae without Genant SQ-oVD, ECF(−) mild and moderate oVDs did not have a higher short term (4 years) future risk for new incident oVD; however, these vertebrae with deformity had a higher risk of short term future turning to ECF(+) (15). Within the same mild/moderate VD grades, compared with the subjects without ECF, the subjects with ECF are associated with a higher short-term future risk of oVD progression and new incident oVD. OVD without ECF is clinically relevant, as oVD itself is associated with future risk of the vertebra turning into ECF(+) (15).
Considering the recent progresses in the understanding of oVD/oVF particularly the association between oVD and ECF (1,8,9,15), and based on Genant’s original SQ criteria (2), hereby we propose a modified semi-quantitative (mSQ) grading scheme for oVD/oVF evaluation in elderly women. It is our aim that this mSQ will be: (I) pathophysiologically relevant; (II) practical to apply in clinics; (III) has as much consistence with the existing SQ scheme as possible. We propose that, concerning the aspect of with or without oVD/oVF and its severity, vertebrae T4-L4 can be radiographically classified into:
- Grade-0: without oVD, without ECF.
- Mild (grade-1) oVD: qualitative osteoporotic deformity and vertebral height loss <20% (Figure 1).
- Mild (grade-1) oVF: mild oVD together with ECF, or ECF only.
- Moderate (grade-2) oVD: qualitative osteoporotic deformity and 20–34% vertebral height loss.
- Moderate (grade-2) oVF: moderate oVD together with ECF.
- Severe (grade-3) oVF: >34% vertebral height loss, always with ECF.
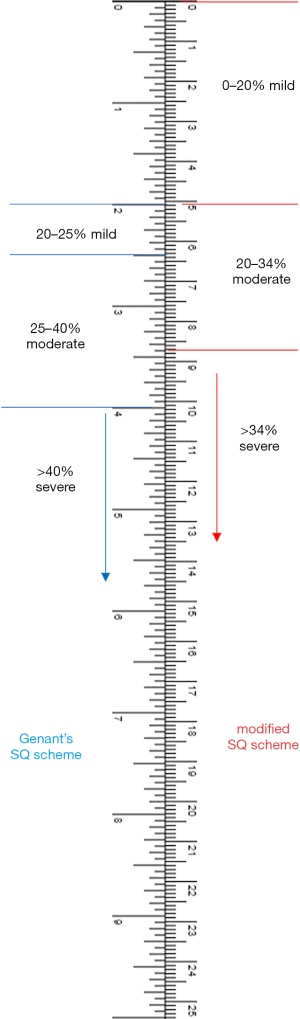
With this mSQ, only the percentage vertebral height reduction is retained and the percentage area reduction requirement is dropped. Percentage vertebral height reduction can be estimated visually during clinical practice, and measured for research or clinical trials. The osteoporotic VD morphological assessment has already been extensively described, and we suggest to follow Genant’s original descriptions (2-5). Genant et al. noted that ‘aside from morphometric features, most vertebral fractures are readily distinguished by the presence of endplate deformities and buckling of cortices, by the lack of parallelism of endplates, and by the loss of vertical continuity of vertebral morphology… Subtle distinctions between a fractured endplate and the deformity of Schmorl’s nodes or the remodeling of the vertebral bodies due to degenerative disk disease and scoliosis can frequently be made qualitatively by an experienced or trained observer’ (2). Genant and Jergas further noted that ‘in addition to height reductions, the reader pays careful attention to alterations in the shape and configuration of the vertebrae relative to adjacent vertebrae and expected normal appearances. These features add a strong qualitative, sometimes subjective aspect to the interpretation’ (4). Genant’s criteria do not require a conventional ‘fracture’ sign, we feel oVD may be the appropriate term for qualitative VD with radiographical deformity. If an ECF exists, then it will be reasonable to call the involved vertebra as having a ‘fracture’, i.e., oVF. Based on our assessment in 2,000 females at baseline (mean age: 72 years) and 1,533 of them at year 4 follow-up, an oVD with >34% height loss is always associated with radiographically identifiable vertebral fracture signs (15,16), therefore a severe oVD is always an oVF.
It has been generally accepted that oVD/oVF does not necessarily have a height loss (1,5,17). Lentle et al. noted that ‘relative reductions in vertebral height may not be a necessary nor sufficient criterion by which to diagnose a fracture’ (17). With the mSQ, as long as a qualitative oVD is diagnosed by an experienced reader, the 20% height loss requirement is removed for mild VD. It is also our experience that mild oVD/OVF usually do have some extent of height loss. According to Genant’s original description, definite oVD without meeting 20% height loss threshold should be classified as SQ grade-0.5. However, despite SQ grade-0.5 may be quite common, they are probably under-reported. The new mSQ may be able to resolve this under-reporting. To our knowledge, some practitioners also knowingly grade some oVDs with less than 20% height loss as Genant SQ grade-1. In our experience, if the readers have sufficient training in reading oVD/oVF, then a high degree of agreement can be achieved regarding whether or not a mild oVD exits, this is the same for the assessment of the existence of ECF (in our experience as well as literature reports) (5,10,18). Without the requirement of a height loss may also direct the attention of readers to the actual deformity, as Genant et al. emphasized (2-4), and avoid over-relying on morphometric height loss. We suggest that a distinctive mild oVD should be reported. The clinical management of such cases would depend on the clinical data such as BMD or other fragility fracture history (19). Though these mild oVDs may not have immediate fragility fracture consequence, they may be a risk factor further developing ECF, thus indicating they are a biomarker of compromised bone quality. The real-life importance of mild oVD may depend on patients individually, and a wait-and-see strategy with follow-up radiographs may be sufficient for many cases.
Our experience is that consistently and precisely estimating the extent of height loss according to Genant’s criteria is difficult. This has been echoed by Lentle et al. (1). In practice, an oVD with 18% height loss can be easily graded as SQ grade-1 (erroneously), a VD with 23% height loss can be easily graded as SQ grade-2 (erroneously). The narrow range of Genant’s SQ grade-1 height loss, i.e., 20–25%, can cause much inconsistency and difficulties for population studies. It is suspected that many reported epidemiological differences between regions and ethnic groups were largely due to methodology imprecision and inconsistency. Though higher degree of vertebral height loss is overall associated with higher ECF(+) prevalence, our data show, compared with SQ grade-2 oVDs, SQ grade-1 oVDs do not show distinctively lower ECF(+) prevalence; indeed, Genant grade-1 VDs show ECF(+) prevalence similar to VDs with 25–34% height loss [estimated from Table 6 of (9) and Figure 1 of (16)]. Thus we propose to group oVDs with 20–34% height loss together as moderate grade (mSQ grade-2). Since vertebral height loss >34% is always associated with ECF, we propose to group oVFs of 34–40% together with oVFs of >40% height loss, and designate them as the severe grade (mSQ grade-3). This mSQ scheme may be more practical, such as, if height loss is more than 1/3 of the vertebra, then it is severe grade (approximately >34%); if height loss is less than 1/5 of the vertebra, then it is mild grade (<20%).
We choose 20% vertebral height loss as the demarcation threshold between mild and moderate VDs mainly for the reason of being consistent with the Genant’s criteria. However, it is likely that some cases of 18% vertebral height loss is clinically as relevant as some cases of 22% vertebral height loss. This mSQ scheme also allows some convertibility with the existing SD scheme, as original SQ grade-0.5 will be mild (grade-1) in the new scheme, and original SQ grade-1 will be moderate (grade-2) in the new scheme (Figure 1).
Finally, it should be noted that oVDs/oVFs of elderly men and elderly women have distinctly different features. For example, our Ms OS (Hong Kong) year-0 baseline study (9) demonstrated that while the overall oVD prevalence is only slightly lower in elderly men than in elderly women (i.e., 13.2% vs. 16.1%), ECF prevalence is substantially lower in men than women (i.e., 5.88% vs. 11.93%). Moreover, 63.2% of the oVDs in men were Genant’s SQ grade-1, while only 30.5% of the oVDs in women were Genant’s SQ grade-1. OVDs in males with 25–34% height loss rarely have ECF, while it is common for oVDs in females with 25–34% height loss to be associated with ECF (16). In proportion, lower endplate in elderly males is much less likely to have osteoporotic fracture than in elderly females (20). The mSQ scheme proposed hereby is intended to be used for elderly women’s oVD/oVF evaluation. Moreover, different imaging technologies have difference sensitivity and specificity for detecting and classifying vertebral fracture. Our own experience is that, compared with cross-sectional imaging techniques such as CT, projectional radiograph is relatively insensitive in detecting ECF. However, radiograph remains the recommended technique for evaluating oVD/oVF (4), and likely is sufficient for majority of oVD/oVF cases as mild oVD/oVF may not need to be managed urgently (19).
Acknowledgements
I thank Professor Timothy C. Y. Kwok and Professor Ping Chung Leung for getting me engaged in the MsOS (Hong Kong) and MrOS (Hong Kong) studies; thank Dr. Min Deng, Dr. Lai-Chang He, and Mr. Nazmi Che-Nordin for reading seven thousand sets of spine radiographs with me; and thank Dr. Xiao-Guang Cheng for the discussions on Genant’s SQ criteria.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Lentle B, Koromani F, Brown J, Oei L, Ward L, Goltzman D, Rivadeneira F, Leslie WD, Probyn L, Prior J, Hammond I, Cheung A, Oei E. The Radiology of Osteoporotic Vertebral Fractures Revisited. J Bone Miner Res 2019. Epub ahead of print. [Crossref] [PubMed]
- Genant HK, Wu CY, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 1993;8:1137-48. [Crossref] [PubMed]
- Schwartz EN, Steinberg D. Detection of vertebral fractures. Curr Osteoporos Rep 2005;3:126-35. [Crossref] [PubMed]
- Genant HK, Jergas M. Assessment of prevalent and incident vertebral fractures in osteoporosis research. Osteoporos Int 2003;14 Suppl 3:S43-55. [Crossref] [PubMed]
- Jiang G, Eastell R, Barrington NA, Ferrar L. Comparison of methods for the visual identification of prevalent vertebral fracture in osteoporosis. Osteoporos Int 2004;15:887-96. [Crossref] [PubMed]
- Yoshida T, Nanba H, Mimatsu K, Kasai T. Treatment of osteoporotic spinal compression fractures. Conservative therapy and its limitation. Clin Calcium 2000;10:53-8.
- Fabreguet I, Fechtenbaum J, Briot K, Paternotte S, Roux C. Lumbar disc degeneration in osteoporotic men: prevalence and assessment of the relation with presence of vertebral fracture. J Rheumatol 2013;40:1183-90. [Crossref] [PubMed]
- Lentle BC, Berger C, Probyn L, Brown JP, Langsetmo L, Fine B, Lian K, Shergill AK, Trollip J, Jackson S, Leslie WD, Prior JC, Kaiser SM, Hanley DA, Adachi JD, Towheed T, Davison KS, Cheung AM, Goltzman D. CaMos Research Group. Comparative Analysis of the radiology of osteoporotic vertebral fractures in women and men: cross-sectional and longitudinal observations from the Canadian Multicentre Osteoporosis Study (CaMos). J Bone Miner Res 2018;33:569-79. [Crossref] [PubMed]
- Deng M, Zeng XJ, He LC, Leung JCS, Kwok AWL, Griffith JF, Kwok T, Leung PC, Wáng YX. Osteoporotic Vertebral Fracture Prevalence in Elderly Chinese Men and Women: A Comparison of Endplate/Cortex Fracture-Based and Morphometrical Deformity-Based Methods. J Clin Densitom 2017. Epub ahead of print. [Crossref] [PubMed]
- Oei L, Koromani F, Breda SJ, Schousboe JT, Clark EM, vanMeurs JB, Ikram MA, Waarsing JH, van Rooij FJ, Zillikens MC, Krestin GP, Oei EH, Rivadeneira F. Osteoporotic vertebral fracture prevalence varies widely between qualitative and quantitative radiological assessment methods: the Rotterdam Study. J Bone Miner Res 2018;33:560-8. [Crossref] [PubMed]
- Fink HA, Litwack-Harrison S, Ensrud KE, Shen J, Schousboe JT, Cawthon PM, Cauley JA, Lane NE, Taylor BC, Barrett-Connor E, Kado DM, Cummings SR, Marshall LM. Osteoporotic Fractures in Men (MrOS) Study group. Association of incident, clinically undiagnosed radiographic vertebral fractures with follow-up back pain symptoms in older men: the Osteoporotic Fractures in Men (MrOS) Study. J Bone Miner Res 2017;32:2263-8. [Crossref] [PubMed]
- Cawthon PM, Haslam J, Fullman R, Peters KW, Black D, Ensrud KE, Cummings SR, Orwoll ES, Barrett-Connor E, Marshall L, Steiger P, Schousboe JT. Osteoporotic Fractures in Men (MrOS) Research Group. Methods and reliability of radiographic vertebral fracture detection in older men: the osteoporotic fractures in men study. Bone 2014;67:152-5. [Crossref] [PubMed]
- Wang YX, Santiago RF, Deng M, Nogueira-Barbosa MH. Identifying osteoporotic vertebral endplate and cortex fractures. Quant Imaging Med Surg 2017;7:555-91. [Crossref] [PubMed]
- Wang YX, Deng M, He LC, Che-Nordin MN, Santiago RF. Osteoporotic vertebral endplate and cortex fractures: a pictorial review. J Orthop Translat 2018;15:35-49. [Crossref] [PubMed]
- Wáng YXJ, Che-Nordin N, Deng M, Leung JC, Kwok AWL, He LC, Griffith JF, Kwok TCY, Leung PC. Osteoporotic vertebral deformity with endplate/cortex fracture is associated with higher further vertebral fracture risk: the Ms. OS (Hong Kong) study results. Osteoporos Int 2019. Epub ahead of print. [Crossref] [PubMed]
- Deng M, Kwok TCY, Leung JCS, Leung PC, Wang YX. All osteoporotically deformed vertebrae with >34% height loss have radiographically identifiable endplate/cortex fracture. J Orthop Translat 2018;14:63-6. [Crossref] [PubMed]
- Lentle B, Trollip J, Lian K. The Radiology of Osteoporotic Vertebral Fractures Redux. J Clin Densitom 2016;19:40-7. [Crossref] [PubMed]
- Ferrar L, Jiang G, Schousboe JT, DeBold CR, Eastell R. Algorithm-based qualitative and semiquantitative identification of prevalent vertebral fracture: agreement between different readers, imaging modalities, and diagnostic approaches. J Bone Miner Res 2008;23:417-24. [Crossref] [PubMed]
- Szulc P. Vertebral fracture: diagnostic difficulties of a major medical problem. J Bone Miner Res 2018;33:553-9. [Crossref] [PubMed]
- Che-Nordin N, Deng M, Griffith JF, Leung JCS, Kwok AWL, Zhu YQ, So RHY, Kwok TCY, Leung PC, Wáng YX. Prevalent osteoporotic vertebral fractures more likely involve the upper endplate than the lower endplate and even more so in males. Ann Transl Med 2018;6:442. [Crossref] [PubMed]


