Haemostatic material (Surgicel®) mimicking residual tumour: magnetic resonance imaging findings in operated pediatric neuro-oncology cases
Introduction
Surgical resection is the first line treatment in the majority of pediatric brain tumors. Even in cases when total resection is not possible, a subtotal debulking is used to reduce mass effect and to provide tissue for histological and molecular diagnosis, necessary to guide oncologic therapies, to assess risk stratification and to predict prognosis (1-3).
Control of intraoperative hemorrhage is a critical point in neurosurgical procedures and is routinely accomplished using chemical hemostatic agents, often preferred to ‘mechanical hemostasis’ (vascular clips, suture ligations, manual pressure) or electrocoagulation, due to brittleness of the brain capillaries (4-8). Oxidized regenerated cellulose (polyanhydroglucuronic acid -Ethicon, Surgicel®; Johnson and Johnson Medical, Arlington, TX) is one of the most widely used bioabsorbable topical hemostatic material (5,6,9). Surgicel® is a sterile, inert and bioabsorbable substance and it can be left in the surgical bed. However its presence and the possible associated granulomatous reaction (Textiloma/Gossypiboma) may lead to misinterpretation of post-operative MRI images (9-12). This eventuality is very rare, however some cases of residual hemostatic material mimicking tumoral residuum or abscess have been described in the literature (5,7,9,13-16). Neuroimaging, in particular MRI imaging, has an important role in such cases, providing accurate evaluation of intracranial lesions before and after surgery, identifying postoperative complications, and assessing therapy related changes (6,8,17). Familiarity with these postoperative appearances (especially in the unlikely event of granulomatous reaction to Surgicel®) and to differentiate them from tumor residuum/recurrence is critical as it allows proper risk stratification and assist in the development of an appropriate management plan.
The aims of this paper is to report three neuro-oncologic pediatric cases where the presence of Surgicel® in the surgical bed caused a diagnostic challenge and was initially misdiagnosed as tumor residuum/recurrence.
Case 1
A 7-year-old girl presented with a 12–18 months history of excessive thirst, frontal headache for 3 weeks and sudden onset of visual loss. Initial MRI showed a mixed solid-cystic retro-chiasmatic suprasellar mass centered at the hypothalamus with compression of the optic chiasm. The solid component of the mass demonstrated enhancement post gadolinium administration. The normal posterior pituitary bright spot was also not appreciated (Figure 1, panels A,B,C).
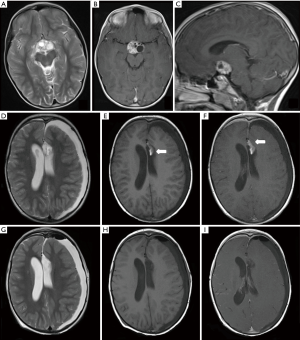
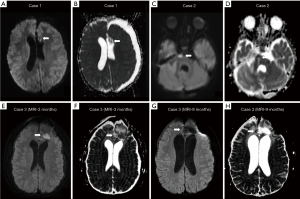
Debulking surgery was performed to relieve the compression upon the optic chiasm and obtain tissue for histopathological analysis. Elevated beta-hCG levels in the serum and cerebrospinal fluid was suggestive of a secreting non-germinomatous germ cell tumor (NGGCT), subsequently confirmed on histopathological examination. The patient underwent adjuvant chemotherapy, followed by a second surgical resection a year later to remove the residual neoplasm. No immediate post-surgical complications were detected. Follow up MRI a month later (Figure 1D,E,F) revealed an ill-defined lesion of heterogeneous signal intensity along the medial aspect of the surgical cavity, adjacent to the left frontal horn, with areas of T1 shortening, and diffusion restriction (Figure 2, panels A,B), as well as a focal area of enhancement anteriorly. Due to the diagnostic dilemma between maturing blood clot and residual/recurrent tumour, a second-look surgery was performed. Histological analysis showed lack of tumoral cells and evidence of foreign material with granulomatous reaction. MRI after the surgery revealed no residual abnormal tissue (Figure 1G,H,I).
Case 2
A 14-month-old girl presented with 1 month history of progressive weakness, frequent falling (to the right), vomiting and lethargy. These symptoms were followed by development of nystagmus and quadriparesis. This prompted the clinician to perform CT and MR imaging of the brain which showed a large right sided posterior fossa mass with infiltrative pattern and invasion of adjacent structures, as well as extension through the right Luschka foramen, suggestive of an ependymoma (Figure 3A,B). The mass showed heterogeneous signal intensities on T1- and T2-weighted images, with no significant diffusion restriction and inhomogeneous contrast enhancement.
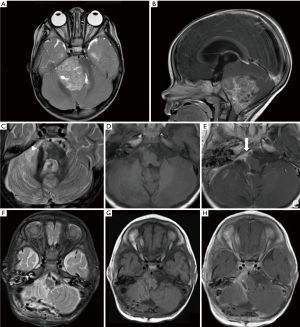
After surgical resection anaplastic ependymoma was confirmed on pathology. Follow-up MRI showed a residual nodular area of contrast enhancement, with diffusion restriction (Figure 3C,D,E and Figure 2C,D), adherent to the right anterior aspect of the brainstem and extending anteriorly along the clivus. In suspicion of disease residuum, a second-look surgery was performed, with histopathological diagnosis of foreign material with associated granulomatous reaction. No evidence of residual disease on post-surgical MRI was observed (Figure 3F,G,H).
Case 3
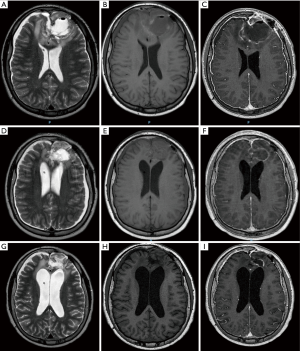
A 14-year-old male was referred to our institution following surgical resection of a frontal dural-based mass lesion, performed in an external hospital. Neuropathological examination revealed a meningeal fibrosarcoma. Brain MRI performed at our institution 2 months after surgical removal of the mass was suggestive of residual/recurrent disease. Therefore, the patient underwent a second surgical intervention, which confirmed the presence of residual tumour. Post-operative MRI performed 1 week after the second surgery showed a large surgical cavity containing hyper-proteinaceous fluid. There was also heterogeneous signal along the surgical margins, likely related to haemorrhagic and hemostatic materials (Figure 4A,B,C). There was no definite evidence of residual disease and the patient underwent radiotherapy (RT). Follow-up MRI scan performed 3 months after completion of RT showed reduced volume of the surgical cavity with peripheral reactive linear enhancement and hemosiderosis. Within the surgical cavity there was new evidence of amorphous tissue with restricted diffusion without contrast-enhancement (Figure 4D,E,F and Figure 2E,F). Close follow-up was performed in the following months, demonstrating progressive reduction in size of the surgical cavity and disappearance of the amorphous tissue with restricted diffusion 6 months later, suggestive of foreign body inflammatory reaction to the hemostatic material (Figure 4G,H,I and Figure 2G,H).
Discussion
Hemostatic substances are routinely used during intracranial surgery; one of the most frequently used material is Oxidized Cellulose Polymer (Surgicel®; Johnson and Johnson Medical, Arlington, TX); a sterile and bioabsorbable thrombogenic material. Surgicel comprises of polyanhydroglucuronic acid, with dissolved pure cellulose in an alkaline solution, subsequently regenerated into continuous solid fibers, knitted into gauze and oxidized. It is usually placed along the surgical margins to accelerate the hemostatic process and usually progressively reabsorbed and eliminated. However, occasionally it may cause a significant foreign body reaction, inducing the formation of granulomatous tissue (5,6,9,13,14). On pathology, Surgicel appears as a nidus of fibers in alternating crossed and longitudinal sections. In the native status these fibers are solid, while in surgical bed they appear as empty “ghost” fibers surrounded by blood products and/or necrotic changes. The following terms have been historically used to describe foreign-body related inflammatory pseudo-tumors: (I) textiloma (from Latin “textile”, a woven fabric, plus the suffix “oma”, meaning swelling or tumor); (II) gossypiboma (from Latin “Gossypium”, the genus of cotton plants; (III) gauzoma (from surgical gauze); and (IV) muslinoma (from muslin) (9,10,15). When inflammatory granuloma is formed, foreign material appears surrounded by a variable amount of acute and chronic inflammatory cells, collagen deposition, reactive vascular proliferation, and foreign body giant cells (9), giving rise to the heterogenous appearances on imaging, delineated in Table 1.

Full table
On MRI, Surgicel® alone appears homogeneously isointense to slightly hyperintense (relative to the face muscles) on T1-weighted images, and iso- to hypointense on T2-weighted images. In some cases it may also show T2 prolongation with a low signal rim (5). However, when in contact with fresh blood, Surgicel® tends to demonstrate mixed signal intensities on T1-weighted images. Spiller et al. (18) reported that Surgicel® causes a significant decrease in the pH of heparinized blood, with consequent premature lysis of red blood cells. They postulated that the early T1-shortening seen in relation to Surgicel® may be related to both the low pH and premature lysis of RBCs (result in early appearance of methemoglobin, which is a paramagnetic substance), as seen in case 1 (Figure 4E). This is however not a constant finding in all cases, as Surgicel® without associated clot formation had been reported, as early as in 1999 (5). This is seen in case 2 (Figure 3D) and 3 (Figure 4E). Surgicel®-clot interaction also results in variable imaging appearances on T2-weighted image. Both T2 shortening (5,8) and relative T2 hyperintense signal and hypointense peripheral rim (5,11,15) have been described.
It was demonstrated that Surgicel® alone does not show enhancement, but, when placed in the surgical bed, it causes lowering of the pH with resultant vasoconstriction, tissue inflammation and possible formation of a Textiloma/Gossypiboma (characterized by prominent reactive vascular proliferation) (6,8,9,12,15,16). This may explain the contrast enhancement seen in two of our described cases (case 1 Figure 1F; case 2 Figure 3E). This may occur from 24 hours to many years after surgery (11,15,19).
All our cases showed restricted diffusion within or in the proximity of the surgical bed (Figure 2). Particularly in the third patient the core of the surgical cavity demonstrated marked and fairly homogeneous diffusion restriction mimicking disease recurrence or less commonly an inclusion dermoid cyst (Figure 2E,F). Previous reports stated that Surgicel® should not cause diffusion restriction (2). However, recently one case has been described with evidence of diffusion restriction along the surgical margins (11), postulated to be secondary to exudative inflammatory process. However, in our opinion, the presence of Surgicel®-related clot formation may also play a role.
The occurrence of Surgicel®-associated granulomatous reaction is extremely rare, explaining the limited evidence in literature, especially regarding appearance on diffusion-weighted imaging (DWI), as most of the previously reported cases were described before DWI was widely used. The variable appearances on DWI often cause confusion on postsurgical imaging. However, it may be useful in the context when the original tumour does not show diffusion restriction. For instance, in case 2, the tumor (anaplastic ependymoma) did not show evidence of restricted diffusion. Hence, the focal area of restricted diffusion seen within the surgical bed is more likely related to Surgicel®-associated granulomatous reaction and clot formation. DWI may result in confusion if the primary tumour shows restricted diffusion, as in case 1 and 3. In such cases, areas of restricted diffusion can be due to either postsurgical changes or tumour residuum.
The main importance of post-surgical imaging is to determine the presence of residual tumour as it impacts subsequent management plan. For instance, in the context of NGGCT, adjuvant radiotherapy or surgical resection of residual tumour may be considered after chemotherapy when residual tumour is detected on follow up imaging (20). The presence of residual tumour also greatly impacts prognostication. In pediatric patients with ependymoma, it has been reported that complete resection correlates positively with greater survival (21). In case of primary intracranial meningeal fibrosarcoma (case 3) only a complete or maximum possible resection can give a chance of longer disease free survival (22).
Conclusions
Surgicel®-associated clot formation and granulomatous reaction result in variable imaging appearances on follow up MRI, including focal areas of enhancement and restricted diffusion, both of which may simulate residual disease. Awareness of this phenomenon is crucial as the determination of the presence of residual disease greatly impacts management plan and prognostication. The authors recommend the inclusion and careful evaluation of diffusion-weighted sequence in all post-operative imaging protocols, especially if the primary tumour shows no evidence of restricted diffusion.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Mueller S, Chang S. Pediatric brain tumors: current treatment strategies and future therapeutic approaches. Neurotherapeutics 2009;6:570-86. [Crossref] [PubMed]
- Smith JS, Cha S, Mayo MC, McDermott MW, Parsa AT, Chang SM, Dillon WP, Berger MS. Serial diffusion-weighted magnetic resonance imaging in cases of glioma: distinguishing tumor recurrence from postresection injury. J Neurosurg 2005;103:428-38. [Crossref] [PubMed]
- Chhabda S, Carney O, D’Arco F, Jacques TS, Mankad K. The 2016 World Health Organization Classification of tumours of the Central Nervous System: what the paediatric neuroradiologist needs to know. Quant Imaging Med Surg 2016;6:486-9. [Crossref] [PubMed]
- Fiss I, Danne M, Stendel R. Use of gelatin-thrombin matrix hemostatic sealant in cranial neurosurgery. Neurol Med Chir (Tokyo) 2007;47:462-7. [Crossref] [PubMed]
- Oto A, Remer EM, O’Malley CM, Tkach JA, Gill IS. MR characteristics of oxidized cellulose (Surgicel). AJR Am J Roentgenol 1999;172:1481-4. [Crossref] [PubMed]
- Kothbauer KF, Jallo GI, Siffert J, Jimenez E, Allen JC, Epstein FJ. Foreign body reaction to hemostatic materials mimicking recurrent brain tumor. Report of three cases. J Neurosurg 2001;95:503-6. [Crossref] [PubMed]
- Yao HH, Hong MK, Drummond KJ. Haemostasis in neurosurgery: what is the evidence for gelatin-thrombin matrix sealant? J Clin Neurosci 2013;20:349-56. [Crossref] [PubMed]
- Brundage CM, Packer RA, Jones MD. Magnetic resonance imaging appearance and mechanism of action of five hemostatic agents used in neurosurgery. Vet Surg 2016;45:996-1004. [Crossref] [PubMed]
- Ribalta T, McCutcheon IE, Neto AG, Gupta D, Kumar AJ, Biddle DA, Langford LA, Bruner JM, Leeds NE, Fuller GN. Textiloma (gossypiboma) mimicking recurrent intracranial tumor. Arch Pathol Lab Med 2004;128:749-58. [PubMed]
- Van Goethem JW, Parizel PM, Perdieus D, Hermans P, de Moor J. MR. J Comput Assist Tomogr 1991;15:1000-3. [Crossref] [PubMed]
- Kumar R, Nadarajah J, Kumar A, Gamanagatti S. Misery of neurosurgeon: Gauzoma causing foreign body granuloma-role of radiologist. Asian J Neurosurg 2016;11:74-5. [Crossref] [PubMed]
- Anderson MD, Raghunathan A, Gilbert MR. Textiloma resembling anaplastic progression of an isocitrate dehydrogenase 1 (IDH1) mutant, low grade glioma. J Neurooncol 2013;111:377-9. [Crossref] [PubMed]
- Atay M, Ahmad IC, Bilgin M, Kocakoc E. Gossypiboma/textiloma mimicking as tumour recurrence. J Pak Med Assoc 2014;64:708-10. [PubMed]
- Buckley SC, Broome JC. A foreign body reaction to Surgicel(R) mimicking an abscess or tumour recurrence. Br J Neurosurg 1995;9:561-3. [Crossref] [PubMed]
- Akpinar A, Ucler N, Ozdemir CO. Textiloma (gossypiboma) mimicking recurrent intracranial abscess. BMC Res Notes 2015;8:390. [Crossref] [PubMed]
- Jang SW, Kim SJ, Kim SM, Lee JH, Choi CG, Lee DH, Kim EJ, Lee JK. MR spectroscopy and perfusion MR imaging findings of intracranial foreign body granuloma: a case report. Korean J Radiol 2010;11:359-63. [Crossref] [PubMed]
- Iv M, Telischak N, Feng D, Holdsworth SJ, Yeom KW, Daldrup-Link HE. Clinical applications of iron oxide nanoparticles for magnetic resonance imaging of brain tumors. Nanomedicine (Lond) 2015;10:993-1018. [Crossref] [PubMed]
- Spiller M, Tenner MS, Couldwell WT. Effect of absorbable topical hemostatic agents on the relaxation time of blood: an in vitro study with implications for postoperative magnetic resonance imaging. J Neurosurg 2001;95:687-93. [Crossref] [PubMed]
- Apter S, Hertz M, Rubinstein ZJ, Zissin R. Gossypiboma in the early post-operative period: a diagnostic problem. Clin Radiol 1990;42:128-9. [Crossref] [PubMed]
- Afzal S, Wherrett D, Bartels U, Tabori U, Huang A, Stephens D, Bouffet E. Challenges in management of patients with intracranial germ cell tumor and diabetes insipidus treated with cisplatin and/or ifosfamide based chemotherapy. J Neurooncol 2010;97:393-9. [Crossref] [PubMed]
- Wu J, Armstrong TS, Gilbert MR. Biology and management of ependymomas. Neuro Oncol 2016;18:902-13. [Crossref] [PubMed]
- Chopra R, Bhardwaj M, Premsagar IC. Fibrosarcoma of the meninges. Rare Tumors 2010;2. [Crossref] [PubMed]


