Ring-enhancing lesions in neonatal meningitis: an analysis of neuroradiology pitfalls through exemplificative cases and a review of the literature
Introduction
Neonatal meningitis (NM) represents an inflammatory response to many types of cerebrospinal fluid (CSF) and pia-arachnoid infections of the central nervous system (CNS). NM can be classified as early or late depending of their occurrence by vertical transmission during the first week of life, or by interpersonal/nosocomial transmission as a late onset presentation of neonatal sepsis (1,2). Gram-positive NM represents the majority of cases, with Group B streptococcus (GBS) as the leading causative pathogen in this class (3). Gram-negative NM is usually related to Escherichia Coli, Klebsiella, Enterobacter, Salmonella and Klebsiella (Table 1). Proteus Mirabilis represents only 4% of cases in this class (4).

Full table
High clinical suspicion, prompt diagnosis, immediate institution of therapy and early recognition and management of complications can make a huge difference in the neurological outcome as well as decreased mortality (3).
Very often the clinical course of NM is characterized by sudden worsening, usually caused by convulsive states, but at times associated with cerebrovascular complications and cerebral strokes. Clinically it can be challenging to evaluate the severity, exact location, and degree of insult to the brain; for this reason, an immediate radiological recognition of these pathological patterns in patients admitted to Neurointensive Care Units (NICU) is pivotal in providing clinicians with useful information to guide the medical treatments (2).
Despite its limitations, mainly related to costs, logistics and safety issues, magnetic resonance imaging (MRI) represents the best modality imaging to investigate those conditions: in fact it provides excellent image definition, accuracy and quality. Neuroradiologists need to be aware of the possible pitfalls that may be encountered in this clinical scenario, as such we herein provide two exemplificative cases of NM complicated with ring enhancing lesions. Differentiating between evolving ischemic strokes or brain abscesses can become a real neuroradiology challenge; solving it appropriately, by achieving the correct diagnosis and timely choosing the best medical or surgical treatment is of paramount importance for the appropriate management of those sick neonates.
Exemplificative case 1: evolving intracerebral hematoma
A female baby, born at term to a healthy mother, presented at day 9 with lethargy, poor feeding, seizures and fever. Her anterior fontanelle was soft and head circumference within the 50th and 85th centile. Admission to NICU was promptly arranged; the analysis of a CSF tap resulted positive for GBS, and antibiotic treatment was timely started on grounds of meningitis as the most likely cause for her clinical presentation. A cranial ultrasound was also performed during the first day of hospitalization and ruled out intraventricular/intraparenchymal bleeding or hydrocephalus; though an electroencephalography demonstrated irritation of the right hemisphere, pointing at the temporal lobe as possible lesional area. An MRI brain performed within 24 hours from admission revealed in fact confluent areas of ischemic strokes involving the right temporal and occipital lobes, and the left occipital lobe with some associated hemorrhagic changes (Figure 1A,B). Other smaller areas of cerebral ischemia were noted in the right deep cerebral white matter, and a small area of diffusion restriction was also seen in the occipital horn of the right lateral ventricle in keeping with small amount of pyogenic exudate (Figure 2A,B). Due to initial concerns of contrast medium toxicity the scan was unenhanced.
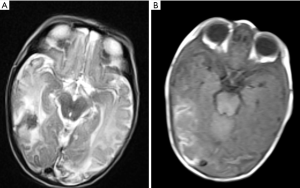
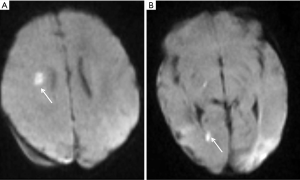
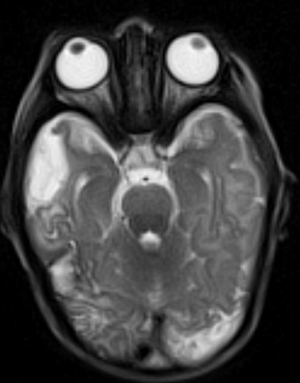
Follow-up enhanced MRI brain at day 22 showed expected evolution of the infarctions with encephalomalacic changes (Figure 3) and interval resolution of the intraventricular exudate. However an organized smooth closed ring-enhancing lesion with core of diffusion restriction was noted in the right temporal lobe (Figure 4). This finding raised the suspicion of cerebral abscess, and only an accurate review of the imaging data allowed to clarify its nature and guide the clinicians to the optimal medical management. In fact on one hand, as the neonatal brain is not myelinated yet at that age, the absence of perilesional edema, usually expected in case of abscess, could not rule out the infective nature of the lesion; on the other hand, the ring-enhancing lesions always showed a very low signal on T2 gradient, which is typical of blood products (Figure 5). Indeed those abnormalities were consequences of a vasculitic process, nonetheless given the septic state the areas of diffusion restriction were initially interpreted as acute cerebritis, in fact the latter can cause restriction and thus trigger the diagnostic dilemma (5). The case was discussed in the neuroradiology multidisciplinary meeting, and the neuroradiology advise was to consider the lesion as expression of intracerebral hematoma, and the ring enhancement as transitory evidence of its subacute radiological evolution. Hence, a neurosurgical intervention was not deemed appropriate on the base of the radiological opinion. Further clinical follow-up eventually confirmed this diagnosis.
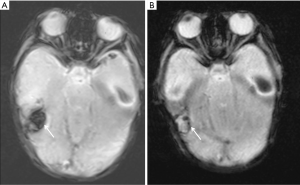
Exemplificative case 2: intraparenchymal abscess
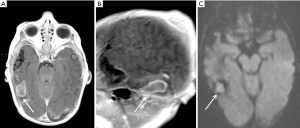
A 15-day-old baby boy was admitted to NICU with high fever and cardiovascular dysfunction requiring fluid resuscitation and inotropic support. Blood test demonstrated raised inflammatory markers and an initial CSF analysis showed pleocytosis. Given the initial diagnosis of NM with septic shock a broad-spectrum empiric antibiotic treatment was timely started. The baby developed seizures overnight and underwent an MRI brain which showed two large space occupying lesions (SOL) localized in the frontal lobes, both surrounded by perilesional edema, with the right one exerting significant mass effect on the corpus callosum with initial midline shift. Diffusion weighted imaging (DWI) sequences demonstrated peripheral and inhomogeneous diffusion restriction, therefore raising suspicion of a late stage cerebritis (Figure 6A,B). The MRI study was supplemented with susceptibility weighted imaging (SWI) sequences which showed mildly hypointense rim surrounding the lesions, in keeping with the presence of free radicals (Figure 6C). In fact, it is known that SWI phase images showing the hypointensity rim delineate the periphery of the lesion, suggesting an increased venous vasculature (paramagnetic deoxy-hemoglobin), blood products from hemorrhages, or presence of paramagnetic free radicals released by macrophages (6,7).
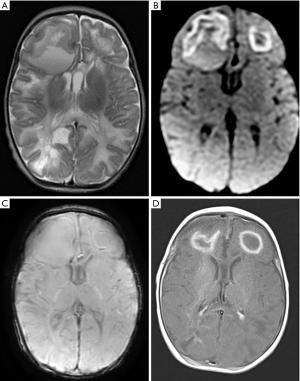
Given the mass effect suggestive of raised intracranial pressure the case was discussed with the neurosurgical team on call and the patient underwent a prompt stereotactic evacuation of the right frontal lesion. The surgical procedure allowed to aspirate almost 15 cc of purulent material. The antibiotic treatment was optimized in the postoperative days due to the results obtained from blood culture and one of the intraoperative samples of the purulent collection sent to the microbiology laboratory. Both specimens in fact showed the presence of Proteus Mirabilis; of note, the culture of the initial CSF sample gave no growth. The postoperative course was uneventful and an MRI brain with contrast performed on the 6th postoperative day confirmed a progressive involution of the lesions which were characterized by the typical rim enhancing capsule and hypointense core (Figure 6D).
Discussion
NM is usually caused by an initial bacteremia, followed by CNS infection. An association between high-level bacteremia and development of NM has been suggested for both Gram-positive and Gram-negative pathogens from experimental models of hematogenous meningitis (8,9). This implies that bloodstream survival in an important virulence trait of meningeal pathogens to avoid immune clearance by complement-mediated or antibody-mediated phagocytic killing mechanisms of host immune cells (10).
Two mechanisms of breakdown of the integrity of the blood brain barrier (BBB), the structural and functional barrier that maintains the homeostasis of the neutral microenvironment by impending the passage of virtually all molecules except those that are small or lipophilic, have been demonstrated: transcellular and paracellular translocation (10,11). Those penetration mechanisms are very similar in both infectious and oncological spreading to the CNS, and experimental studies are currently carried out to exploit these mechanisms and optimize the distribution of contrast agents for MRI and nuclear medicine through the BBB (12-15). In NM, the transcellular and paracellular translocation are usually mediated by a complex interplay between increased expression of inflammatory cytokines/chemokines and adhesion molecules promoting BBB permeability (15-17). This initial process is usually followed by infection of the pia and arachnoid mater including the vessels traversing the subarachnoid space. This stage creates the basis for the accumulation of inflammatory exudates within the sulci, a condition typical of the in the early phases of NM, also known as subependymal gliosis (18,19).
Parenchymal complications in NM may occur for two reasons: (I) abscess formation, characterized by early cerebritis with focal accumulation of pathogens evolving into a central area of necrosis surrounded by a perilesional astrocytosis, or (II) vasculitic process, causing infarction and subsequent liquefactive ischemic necrosis involving the white matter (20,21). In both instances, the resulting SOL may appear with a ring enhancement on CT and MRI investigations (22). From a clinical perspective, the neuroradiology evidence of an intracranial ring enhanced SOL is considered as evidence of brain abscess until proved otherwise (22,23). Nonetheless, a ring enhancement may also occur in the late subacute stage of evolving cerebral hematomas, despite this being less commonly encountered in clinical practice (24). This radiological finding may persist for weeks up to 2 or 3 months (22). Identify the correct diagnosis in this clinical scenario often represents one of the finest challenges for neuroradiologists.
Similarly to the four stages of abscess formation (Table 2), the proposed mechanisms of ring enhancement in evolving hematomas are: (I) the breakdown of the BBB (early phase), and (II) the formation of vascular granulation tissue (late phase) (25). On DWI the core of brain hematoma is hyperintense in the hyperacute and late subacute stages: the process of clotting, in fact, involves transformation of a fluid to a semisolid with consequent restriction of the water diffusion. Noteworthy, DWI signal is not increased in the intervening acute and early subacute phases because of T2-induced hypointensity of clot (“T2 dark-through”) (25). This specific image pattern simulates cerebral pyogenic abscesses that also show restricted core (pyogenic exudate) and peripheral enhancement (capsule) particularly in setting of bacterial meningitis (23,26). The main key features to be considered at time of differential diagnosis between these two entities are: (I) the history of stroke and vascular distribution, favoring hematoma; versus (II) the presence of multiple rings and satellite lesions and diffuse perilesional edema, favoring abscesses (27).

Full table
The two exemplificative cases of NM reported above presented exceptional challenges for the clinicians. In fact, on one hand bacterial NM is followed by abscess formation in only 13% of cases (28); whereas, on the other hand GBS meningitis can often be associated with cerebrovascular complications including strokes (3). In the first case this lead to hemorrhagic transformation of some areas of infarction; unfortunately though, that specific evolution made the differential diagnosis very difficult since no previous scans were available for comparison. Therefore, the very low signal on T2 gradient and the absence of perilesional edema led to consider an evolving hematoma as the most reasonable explanation for the neuroradiological pattern identified on the initial MRI. In the second case, the fact that a rare Gram-negative bacteria, Proteus Mirabilis, was eventually demonstrated as the causative pathogen responsible for septic state and abscess formation made the case unusually challenging, noteworthy only few tens of Proteus Mirabilis—confirmed brain abscess have been reported in the literature so far (29). Furthermore, clinicians should always bear in mind that the appearance and evolution of abscesses in neonates with septic state is much more unpredictable than in older children due to the lack of proper immune response (23,30).
Conclusions
This educational article focuses on imaging key features useful in the diagnosis of neonates presenting with NM and ring-enhancing cerebral SOL. Out of 1.2 million cases of bacterial NM diagnosed every year, up to 170,000 are fatal, and survivors are left with permanent neurological sequelae (10). Understanding that evolving hematomas have to be considered in the differential with cerebral abscesses is particularly important in some specific groups of NM. Indeed, identifying the neuroradiological peculiarities of those two clinical entities can be particularly difficult especially in cases of brain infections associated with strokes such as in the case of GBS NM. Furthermore, a better knowledge of the pathological basis of these conditions is of paramount importance in rapidly achieving the correct diagnosis through a timely prescription of the most appropriate investigations and medical treatment, reserving only selected cases for neurosurgical consultations and stereotactic biopsy/drainage.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the next of kin of the patients for publication of this article and any accompanying images.
References
- Tack DM, Holman RC, Folkema AM, Mehal JM, Blanton JD, Sejvar JJ. Trends in encephalitis-associated deaths in the United States, 1999-2008. Neuroepidemiology 2014;43:1-8. [Crossref] [PubMed]
- Gupta N, Grover H, Bansal I, Hooda K, Sapire JM, Anand R, Kumar Y. Neonatal cranial sonography: ultrasound findings in neonatal meningitis-a pictorial review. Quant Imaging Med Surg 2017;7:123-31. [Crossref] [PubMed]
- Hernández MI, Sandoval CC, Tapia JL, Mesa T, Escobar R, Huete I, Wei XC, Kirton A. Stroke patterns in neonatal group B streptococcal meningitis. Pediatr Neurol 2011;44:282-8. [Crossref] [PubMed]
- Heath PT, Nik Yusoff NK, Baker CJ. Neonatal meningitis. Arch Dis Child Fetal Neonatal Ed 2003;88:F173-8. [Crossref] [PubMed]
- Tung GA, Rogg JM. Diffusion-weighted imaging of cerebritis. AJNR Am J Neuroradiol 2003;24:1110-3. [PubMed]
- Lai PH, Chang HC, Chuang TC, Chung HW, Li JY, Weng MJ, Fu JH, Wang PC, Li SC, Pan HB. Susceptibility-weighted imaging in patients with pyogenic brain abscesses at 1.5T: characteristics of the abscess capsule. AJNR Am J Neuroradiol 2012;33:910-4. [Crossref] [PubMed]
- Toh CH, Wei KC, Chang CN, Hsu PW, Wong HF, Ng SH, Castillo M, Lin CP. Differentiation of pyogenic brain abscesses from necrotic glioblastomas with use of susceptibility-weighted imaging. AJNR Am J Neuroradiol 2012;33:1534-8. [Crossref] [PubMed]
- Moxon ER, Ostrow PT. Haemophilus influenzae meningitis in infant rats: role of bacteremia in pathogenesis of age-dependent inflammatory responses in cerebrospinal fluid. J Infect Dis 1977;135:303-7. [Crossref] [PubMed]
- Ferrieri P, Burke B, Nelson J. Production of bacteremia and meningitis in infant rats with group B streptococcal serotypes. Infect Immun 1980;27:1023-32. [PubMed]
- van Sorge NM, Doran KS. Defense at the border: the blood-brain barrier versus bacterial foreigners. Future Microbiol 2012;7:383-94. [Crossref] [PubMed]
- Ganau M. Tackling gliomas with nanoformulated antineoplastic drugs: suitability of hyaluronic acid nanoparticles. Clin Transl Oncol 2014;16:220-3. [Crossref] [PubMed]
- Ganau M, Prisco L, Pescador D, Ganau L. Challenging New Targets for CNS-HIV Infection. Front Neurol 2012;3:43. [Crossref] [PubMed]
- Ganau L, Paris M, Ligarotti GK, Ganau M. Management of Gliomas: Overview of the Latest Technological Advancements and Related Behavioral Drawbacks. Behav Neurol 2015;2015:862634. [PubMed]
- Ganau M, Syrmos NC, D'Arco F, Ganau L, Chibbaro S, Prisco L, Ligarotti GKI, Ambu R, Soddu A. Enhancing contrast agents and radiotracers performance through hyaluronic acid-coating in neuroradiology and nuclear medicine. Hell J Nucl Med 2017;20:166-8. [PubMed]
- Sukumaran SK, Shimada H, Prasadarao NV. Entry and intracellular replication of Escherichia coli K1 in macrophages require expression of outer membrane protein A. Infect Immun 2003;71:5951-61. [Crossref] [PubMed]
- Santi I, Scarselli M, Mariani M, Pezzicoli A, Masignani V, Taddei A, Grandi G, Telford JL, Soriani M. BibA: a novel immunogenic bacterial adhesin contributing to group B Streptococcus survival in human blood. Mol Microbiol 2007;63:754-67. [Crossref] [PubMed]
- Mittal R, Krishnan S, Gonzalez-Gomez I, Prasadarao NV. Deciphering the roles of outer membrane protein A extracellular loops in the pathogenesis of Escherichia coli K1 meningitis. J Biol Chem 2011;286:2183-93. [Crossref] [PubMed]
- Ye Q, Shao WX, Shang SQ, Shen HQ, Chen XJ, Tang YM, Yu YL, Mao JH. Clinical Value of Assessing Cytokine Levels for the Differential Diagnosis of Bacterial Meningitis in a Pediatric Population. Medicine (Baltimore) 2016;95:e3222. [Crossref] [PubMed]
- Rosenberg HK, Levine RS, Stoltz K, Smith DR. Bacterial meningitis in infants: sonographic features. AJNR Am J Neuroradiol 1983;4:822-5. [PubMed]
- Foreman SD, Smith EE, Ryan NJ, Hogan GR. Neonatal Citrobacter meningitis: pathogenesis of cerebral abscess formation. Ann Neurol 1984;16:655-9. [Crossref] [PubMed]
- Yikilmaz A, Taylor GA. Sonographic findings in bacterial meningitis in neonates and young infants. Pediatr Radiol 2008;38:129-37. [Crossref] [PubMed]
- Osborn A. Imaging of Intracranial Parenchymal Hemorrhage. In: Osborn's Brain: Imaging Pathology and Anatomy. Salt Lake City: Amirsys, 2012.
- Tan RMR, Ganau M, Jeelani NU, Tahir Z, Mankad K, Kachramanoglou C, Prabhakar P, Goulden N, Samarasinghe S. Central nervous system aspergillosis resembling haemorrhagic brain infarct in a paediatric leukaemia patient. Br J Haematol 2017;178:642-5. [Crossref] [PubMed]
- Wong AA, Henderson RD, O'Sullivan JD, Read SJ, Rajah T. Ring enhancement after hemorrhagic stroke. Arch Neurol 2004;61:1790. [Crossref] [PubMed]
- Shah N, Reichel T, Fleckenstein JL. Diffusion Findings in Blood Clot: The Last Word? AJNR 2004;25:157-8. [PubMed]
- Muccio CF, Caranci F, D'Arco F, Cerase A, De Lipsis L, Esposito G, Tedeschi E, Andreula C. Magnetic resonance features of pyogenic brain abscesses and differential diagnosis using morphological and functional imaging studies: a pictorial essay. J Neuroradiol 2014;41:153-67. [Crossref] [PubMed]
- Weisburg LA. Peripheral rim enhancement in supratentorial intracerebral hematoma. Comput Tomogr 1980;4:145-54. [Crossref] [PubMed]
- Pong A, Bradley JS. Bacterial meningitis and the newborn infant. Infect Dis Clin North Am 1999;13:711-33. [Crossref] [PubMed]
- Phan H, Lehman D. Cerebral abscess complicating Proteus mirabilis meningitis in a newborn infant. J Child Neurol 2012;27:405-7. [Crossref] [PubMed]
- Neonatal Brain Infection. Available online: http://www.mrineonatalbrain.com/index.php


