Automatic screening for tuberculosis in chest radiographs: a survey
Introduction
Tuberculosis is an infectious disease caused by the bacillus Mycobacterium tuberculosis, which affects mainly the lung. While TB is less prevalent in industrialized nations, the death toll in developing countries is high. In 2011, 8.7 million people fell ill with TB, and 1.4 million died from TB (1). The emergence of new drug resistant strains is beginning to exacerbate the problem, rendering the existing drugs ineffective, and necessitating resolute action for any attempt to eradicate the disease to be successful. In addition, large numbers of patients with HIV/TB co-infections need to be X-rayed and screened for active TB to ensure a proper treatment of their infection(s). Taking standard chest X-rays (CXRs) is an inexpensive way to screen for the presence of TB. Unfortunately, the interpretation of CXRs is subject to human error and depends on the expertise of the reader (2-5). In addition, mass screening of a large population is a time-consuming and tedious task, which requires considerable effort when done manually. For this reason, there is considerable interest in developing computer-aided diagnostic systems (CAD) that can detect TB automatically in CXRs. These systems have the potential to reduce the risk of detection errors and increase the efficiency of mass screening efforts.
This paper is geared towards both computer scientists and radiologists. The former will gain medical insight, which will help them to solve the specific problems posed by TB, while the latter will learn about the state-of-the-art of computer-aided TB diagnostics. The structure of the paper is as follows: first, we describe the screening challenges involved when trying to screen CXRs automatically for TB. This includes a brief description of the many manifestations of TB in a CXR. We also discuss the specific problems of HIV/TB co-infections and the adjustments necessary in regions where TB is epidemic. Then, we briefly describe the technical advances in TB Imaging. The next section presents a detailed survey of the latest computational developments in image processing for TB screening systems, covering the pre-processing, feature computation, and classification techniques. Finally, a discussion and outlook conclude the paper.
Screening challenges
According to the World Health Organization (WHO), pulmonary TB is on the rise worldwide, and the largest impact of this increase relates to inadequate control in developing nations, particularly those with high endemic rates of HIV infection (6). Patients exposed to TB can be characterized clinically into two groups: (I) those with active TB disease; and, (II) those who have been exposed but do not have active disease. Exposed patients who do not have active disease may harbor small colonies of active bacilli sheltered within their bodies, particularly in their lungs, and are thus at risk for activation of TB, if their physical health state is compromised. This happens when immune surveillance and control systems fail in immuno-compromised states such as HIV/AIDS, as well as in iatrogenically introduced immune-compromised states from drugs to treat cancer or auto-immune and rheumatologic conditions.
The imaging features of active TB and inactive (sometimes called treated or “healed”) disease do have some unique features, but also overlap. Within the lung, imaging features of active pulmonary TB include but are not limited to the following manifestations:
• Air space consolidation small or large, that is segmental or lobar opacity in the lung, oftentimes reported as pneumonia or pneumonitis;
• Miliary pattern is a fine granular sandy or seed-like appearance throughout the entirety of both lungs, reported as diffuse bilateral infiltrates, sometimes (correctly) referred to as micronodular (7). This corresponds to hematogenous disease spread;
• Cavity formation, a finding in the lung with a detectible radiodense rim, which on a CXR may be continuous or discontinuous, differentiated from a mass as it has some central complete or relative radiolucency. This corresponds to caseous necrosis of lung tissue;
• Bronchiectasis or enlargement of airways can appear as tubular rings or cylinders of irregular diameter extending radially from the lung hila, with or without central radiolucency.
In addition to the lung findings, there are important findings in active TB outside of the lung parenchyma, including but not limited to abnormalities of the mediastinum and pleura:
• Lymph node enlargement or adenopathy is a smoothly marginated well-defined mass in close anatomic proximity to the mediastinum. These are silhouetted against the margins of normal structures such as the predictable pulmonary arteries and veins, which may facilitate their detection. The lack of discernability or detection of the lower third of the right tracheal wall [paratracheal stripe (7)] is also a specific feature of an enlarged lymph node frequently seen in patients with TB (8);
• Pleura can be thickened in TB, a finding that can be easily detected through careful survey of the periphery of the lungs just adjacent to ribs superiorly and laterally, and along the diaphragm inferiorly;
• Pleural effusions result in loss of visualization (indistinctness) of the lateral costophrenic and medial cardiophrenic sulci on PA radiographs.
In addition to these findings of active disease, classic features of TB exposure include calcified granulomata and apical pleural thickening.
Many of these characteristic findings occur in TB but also occur in many other diseases. The CXRs of some patients with active TB exhibit none of the above findings, and are normal to the human eye, and others do demonstrate very subtle abnormal findings, which sensitive interpreters will be able to detect, such as linear opacities (9).
The determination of definitive disease activity, and thus need for treatment is not radiographic, but is related to microscopic evaluation of extracted sputum from the lung. According to the WHO lime book, epidemiologic survey CXRs are tools used to “identify participants eligible for bacteriological exam”, serving both as a screening and measurement tool. As such, the primary interpretation of a CXR for screening surveys is different from clinical radiology (not for screening). The purpose of screening is to identify everything that is or could be related to a patient having TB. Both TB specific X-ray changes and those that are subtle and non-specific, and might typically be overlooked, are included or “intentionally over-read”, that is read with careful scrutiny and reported with a lower sensitivity for abnormality. The use of computerized image interpretation can lend a level of objectification to image analysis far exceeding that of a single or group of human radiologists, reducing intra and inter rater variability. This objectivity would have intrinsic value both to an individual patient in a screening study or in an epidemiologic survey, as well as to study design (10). In addition, the potential for immediate, on-the-spot interpretation of “normal”, which is entirely negative for TB or any pulmonary disease possibly related to TB; or “abnormal”, which is positive for TB or any other disease that could, even in the rarest of circumstances, have a statistical association with TB, is of potential value in a mobile screening program.
In a mobile screening program as defined by the WHO, all participants who either have CXRs “suspicious for TB” or whose X-rays are “minimally abnormal” should be “intentionally over-read” by the interpreter, and have an on-the-spot sputum collection. An embedded computerized program would allow this to occur during the primary encounter, limiting the resources required to identify the patient whose chest X-ray has been screened and interpreted as abnormal. In screening programs for endemic (frequently occurring) diseases, each patient has a reasonably high likelihood of having disease, or pre-test probability of having disease. Pre-test probability is considered as an input variable by human readers, and may bias their interpretation of images, towards “intentionally over-reading” or “under-calling”. In this screening context, a low false negative rate is desirable and a high false positive rate is tolerated. TB screening in endemic nations exemplifies this particular scenario. Computerized algorithms for “disease detection” of TB in this group may be most successful when casting the widest net possible. Their design could incorporate a high sensitivity, for abnormality and reproducibility, exceeding that of the human interpreter, particularly for subtle pathology. For a specific screening indication, the experience or judgment applied by any particular radiologist, who may have variable influences, may choose a different threshold systematically, or unpredictably. These different thresholds are measurable as inter-rater and intra-rater reproducibility. This variable human behavior could inappropriately decrease or increase the number of patients required to participate in the secondary test. The sensitivity of the interpretation required in a screening program in a region where TB is epidemic is considered higher than, for example, that exhibited by a US based hospital radiologist interpreting routine radiographs. The screening evaluation threshold for abnormal includes findings routinely identified and “downplayed” in the community setting. This practice is referred to as “intentionally over-reading” (WHO). These specific findings include the presence of calcified granulomata (well delineated highly opaque densities), minimal and or symmetrical apical pleural thickening (as an entity distinct from apical consolidation), and linear opacities near the periphery of the lung, which are typically perpendicular to the long axis of the pleura in that region, called “Kerley B” lines, sub pleural or linear scars. By extension, an ideal screening algorithm would include these as abnormal rather than normal (WHO).
In the evaluation of TB screening surveys in high prevalence HIV/AIDS regions, the role of “intentional over-reading” is also important (WHO). The relative frequency of typical TB-associated imaging features is different in patients with HIV. The common and easily visually identified patterns of lobar opacity, cavitary mass and pleural effusion are present in healthier subsets of the HIV positive population, who may not carry a diagnosis of AIDS prior to TB screening (11). Among those with advanced disease, patterns of disseminated or “miliary” disease and lymph node enlargement (lymphadenopathy) are more common, compared to those with more intact immune function and dual disease state (12). In clinical practice, comparison with prior radiographs is paramount in the HIV population, and any subtle change from baseline is, typically “intentionally over-read”, as radiographic stigmata of common opportunistic infections, such as pneumocystis carinii pneumonia (PCP) can be difficult to detect (13). Epidemiologic surveys typically lack prior studies for comparison, presenting unique challenges. In the designing of what radiologists refer to as an imaging “search pattern”, which is a hierarchical checklist of findings and contingencies that contribute to the decision algorithm in a region with a high endemic rate of TB and HIV, special care must be taken to pay attention to any subtle deviation from normal in the mediastinal contour (14). On a single CXR (PA or AP) this can be difficult to discern, and in clinical radiology this is far more detectable on the lateral view. In the one retrospective review of sputum positive TB patients with HIV, 13% of the chest X-rays were normal, or showed linear opacities or calcified granulomata only (9).
Technical advances in TB imaging
Although there have been many technical and image processing advances since the discovery of X-rays over a century ago; screening for TB and other lung processes on chest X-rays has lagged behind until very recently. Perhaps the landmark event that will lead to the largest public health development in this important area is the transition of film based systems to digitized radiography (15-17).
As digital chest radiography replaces film-based systems, image-processing capabilities will continue to proliferate. In addition to immediate storage and transmission for remote diagnostics and follow-up, image pre- and post- processing will be widespread. Although the first phase of going filmless was Computed Radiography (CR); more recent developments now with Digital Radiography (DR) allow instant review of images including portable imaging.
The historical challenge of overlying structures has recently been answered with Dual Energy (DE) radiography that allows for subtracting the ribs (for example) due to unique characteristics of energy absorption. Additionally, improved X-ray detectors and slot scanning to remove antiscatter continually allow improved resolution.
Open source, web based educational tools such as Robochest (7) and decision support (CXR CAD) allow for improved diagnostics; especially in regions of the world that do not have adequate expertise resources. Cavity and nodule detection along with pattern recognition can be assisted with Bayesian applications (18).
Computerized image analysis with image pre- and post-processing helps reduce perceptual and cognitive errors by highlighting abnormalities and characterizing lung patterns to assist physicians as virtual second opinions. Improved display is possible with gray-scale processing, edge enhancement and multifrequency processing for optimal display (19).
An economical method has been developed that is found to be successful in jails called miniature chest X-ray screening (10) and has been found to detect a majority of asymptomatic cases (20).
Lastly, temporal subtraction (14) allows for improved detection of subtle changes on serial CXR, with digital tomosynthesis (21) increases conspicuity of lung nodules; perhaps one day replacing or supplementing chest CT for cancer follow-up and/or screening. This would be a tremendous radiation dose-savings, reducing medical diagnostic costs, while allowing more widespread cancer detection and monitoring.
Pre-processing
A CAD system usually applies a series of pre-processing steps to an input image. The main goal of pre-processing is to enhance the image quality so that objects of interest, such as nodules or linear opacities consistent with scarring/fibrosis, become more evident. The quality of the pre-processing therefore strongly affects the performance of the subsequent processing steps. For X-ray screening, typical pre-processing steps are contrast enhancement, bone suppression, and lung boundary detection. This section will present the software implementations of these steps. For recent hardware advances in detector technology, we refer readers to (19).
Histogram equalization
Contrast enhancement techniques applied to X-rays can significantly improve the contrast at the lung boundary and on the tissue surface. To enhance the contrast in the anatomic area of interest, histogram analysis of the image data is a common technique. Histogram equalization increases the contrast for low-contrast regions by spreading out the most frequent intensity levels (22). Other enhancement techniques proposed in the literature include transformations based on wavelets and piecewise linear models (23,24). Another example is the energy normalization technique proposed in (25), which improved lung segmentation results for several X-ray sets.
Bone suppression
Bone suppression is an important preprocessing step for the lung segmentation and feature extraction stages. Especially the strong edges resulting from the rib cage and the clavicle bones produce local minima that cause problems for minimization-based segmentation algorithms, such as deformable models or graph cuts. Ribs and clavicle bones can occlude abnormalities in the lung regions, which complicates the feature extraction stage of a CAD system. Processing images with lower resolution may help suppress the rib cage and the edges of the clavicle bones. However, this simple solution is mostly suitable for the lung segmentation stage and may not be effective for other purposes. The better solution is to apply image-processing techniques that automatically detect/remove the bone structures in a CXR. Working with bone-suppressed images helps to detect TB, which very often manifests itself in the apical regions of the lung, and to prevent minimization-based approaches from being stuck in local minima (26). Readers can find more information on algorithms for detection and suppression of bones in X-rays here (27-30).
Besides running image processing algorithms on X-rays, bone suppression images can be generated by dual-energy imaging techniques, which acquire images at different energy levels to highlight the soft tissue (19,31); see also previous section.
Lung boundary detection
To confine feature computation to the lung region, most TB screening systems first apply a lung segmentation step (32). To detect the lung boundaries automatically, several techniques have been proposed in the literature. Early segmentation methods can be classified into four categories: rule-based methods, pixel classification methods, deformable models, and hybrid methods (33). Rule-based algorithms usually apply basic steps such as thresholding or morphological operations (34,35). In general, rule-based methods do not produce very accurate results. However, they can serve as an initialization stage for more robust segmentation algorithms. Pixel classification methods are more robust than rule-based methods (36-39). On public datasets, they outperform rule-based methods (40). Typical examples of deformable models are active shape models (ASM) (41) and active appearance models (AAM) (42). For example, these models have been successfully applied to lung boundary detection in (43,44). The flexibility of deformable models makes them suitable for segmentation of anatomical boundaries. Nevertheless, they require a proper initialization, and they are challenged by local minima especially at the ribs and clavicle bones, not dissimilar to radiologists. Finally, hybrid methods aim to produce better results by fusing several techniques (40). In (36), a rule-based and a pixel-based approach are combined. In (40), the authors propose three hybrid approaches, fusing deformation-based and pixel classification methods, and choose the best performing approach using majority voting. The quantitative results of early segmentation algorithms are reported in (33) on internal data sets, and in (40) on the publicly available JSRT dataset (32).
A more recent effort on lung boundary detection is based on ASM and SIFT descriptors (45,46). This approach describes the lung shape using population-based and patient-specific shape statistics. The authors report a high segmentation performance for their approach. Another recent method begins with a rule-based method followed by a deformable model approach (47). This method uses only low-level features to find the lung boundary, which can lead to a lower segmentation performance. In (48), the lung region is extracted using a combination of an intensity mask, a lung model mask derived from a training set, and a Log-Gabor mask. In (49), a lung mask derived from a training set in combination with a graph-cut algorithm is used to derive lung boundaries by minimizing the graph structure. Recently, a new algorithm has been proposed for emphysema detection, in which the lung boundaries are extracted using Kohonen networks (50).
In this survey, we only list the lung boundary extraction approaches applied to CXRs. Readers interested in the current state of general image segmentation approaches are referred to (51-55).
Features and classification methods
The complexity of TB screening lies in the large variety of TB manifestations encountered in practice, ranging from subtle miliary patterns to obvious effusions (56). Abnormal TB manifestations typically affect the texture and geometry of the lungs in a CXR. All the surveyed papers therefore describe methods to extract texture and geometry features to classify CXRs into abnormal or normal. Some papers try to address TB detection as a whole while others address only a specific TB manifestation. In the following, we introduce the reader to each of these papers. We believe that by doing so the reader will get a better idea of the underlying mathematical approaches used. This survey can serve as a starting point for future research by identifying new directions missed so far.
In (57), the authors propose a wavelet transform to decompose CXR images into high frequency and low frequency components. Thirty line profiles were obtained from a CXR image of a TB confirmed patient. These line profiles contained the 2nd, 3rd, 4th and 5th rib and were represented with Daubechies coefficients. A statistical analysis based on clustering was used to test whether these features can identify TB in a CXR.
In (58), an extensive work that combines advanced textural and geometrical features is proposed. The focus is on TB cavity detection. First, a coarse feature classification (SVM) using Gaussian model-based template matching (GTM), local binary pattern (LBP) and histogram of oriented gradients (HOG), extracts cavity candidates from CXR images. These candidates are further refined with image enhancement techniques, using the eigenvalues of the Hessian matrix (HIE) and an active contours snake-based technique (ACS). In the last stage, the enhanced cavity candidates are further narrowed down to reduce false positives using a classification (SVM) on a finer scale with the following features: circularity measure, gradient inverse coefficient of variation (GICOV), and Kullback-Leibler divergence (KLD).
In (59), the authors propose a method to locate focal opacities of TB in CXRs. They concentrate mainly on an initial extracting of the rib lines. Having located the ribs, they apply morphological operations (opening) and a seed growing method on the binary CXR image to locate the focal opacity.
In (26), a study compares automatic TB detection on regular CXR images and bone suppressed CXR images (BSI). The authors evaluate: (I) moments of intensity distributions of Gaussian-derivative filtered images, and (II) pixel relative locations inside the lung field. Overall, the classification (kNN) on BSI performs slightly better than the classification on regular CXR images.
In (60), a method based on phase congruency (PC) is proposed to classify TB. PC is a frequency-based edge model that is more robust against changes in illumination and contrast of an input image, and therefore less sensitive to noise. PC is computed over all pixels and four statistical measures are calculated on the PC values: average, variance, coefficient of variation, and maximum PC value from the control data. This feature space is used to measure the ground truth of the normal CXRs versus the CXRs with TB (training set). To classify CXRs, the Euclidian distance is calculated between the input feature vectors and the ground truth.
In (61), the authors propose a method based on local binary pattern (LBP) and Laplacian of Gaussian (LoG) to detect TB nodules in CXR images. Besides the texture extraction, their work focuses on the preprocessing part of lung segmentation, with rib suppression based on dual-energy subtraction.
In (62), a method based on template matching is used to identify miliary TB. Instead of performing template matching on the spatial domain, the authors transform the image and template into the frequency domain. This allows them to perform the correlation and template matching quickly. They correlate each image with 16 differently sized templates, each with its own threshold. To minimize the false positives and the false negatives in the training set, they set the thresholds appropriately.
In (63), a method to detect infiltrations and cavitations is proposed. The method includes mostly preprocessing algorithms, such as morphological operations, adaptive thresholding, orientations, and horizontal signatures, to identify anatomical structures: thoracic cage, diaphragm, spinal column, heart boundary, ribs, and clavicles. The extraction of these structures reduces the number of false positives. Furthermore, an iterative local contrast approach enhances suspected regions as cavitations or infiltrations.
In (64), the authors describe a methodology to segment lung fields on CXRs based on Watershed algorithm. They adjust the method by incorporating additional assumptions. For example, they assume that the lung and the background areas are greater than 20% and 30% of the image frame, respectively. For the detected lung field, a small fixed-sized window scan finds the average and the maximum gray levels of the pixels. An empirical intensity threshold helps to classify window centers as suspected nodules.
In (65), a clustering-based segmentation of the lungs is proposed. The extracted lung field is further enhanced by using a fuzzy-based contrast technique.
In (3), the authors propose a statistical interpretation technique to detect TB in CXR images. They first apply a wavelet transformation to the input CXR image. Then, they calculate 12 texture measures from the wavelet coefficients. Next, they perform a principal component analysis (PCA) on these texture measures to reduce dimensionality. Finally, they estimate misclassification probabilities by using probability ellipsoids and discriminant functions.
In (18), a method based on Bayesian classification detects TB cavities in CXRs. A gradient inverse coefficient of variation (GICOV) describes textures (region boundaries) and a circularity measure describes the shape of potential cavities.
In (66), a user-guided snake algorithm segments the lungs. Texture features (mean, variance, third moment, and entropy) are calculated on the intensity histograms of the segmented lungs. Decision trees perform the actual classification. This paper also indicates that features can show great disparities when computed separately on the left and right lung. The authors propose a TB index (TI) to discriminate between normal and abnormal CXRs based on their proposed texture features.
In (67), the authors focus more on local texture features to detect TB. They use distributions of texture features (central moments), measured in a local lung region, to describe a CXR image. Then, they compute the dissimilarities between the generated histograms and the saved histograms from the training set (prototypes). These dissimilarities are further classified using linear discriminant classifiers. Finally, a voting rule classifies the CXR as normal or abnormal based on the output of the classifiers.
In (68), a CAD system with graphical user interface allows users to load CXR images of the same patient and create subtraction images to see the differences. The system computes central moments on these subtraction images to detect whether the patient has improved or not.
In (48), we detect the lung boundary using a combination of different masks: an intensity mask, a statistical lung model mask that we trained on manually labeled ground-truth segmentations, and a Log-Gabor mask. As features, we use a mixture of shape and texture features based on the eigenvalues of the Hessian matrix and LBP. A support vector machine then distinguishes between normal and abnormal CXRs. We evaluated our system on images provided by a local TB control program, showing that the performance is reasonably close to human reading performance.
We created a comparison sheet (Table 1) providing an overview of various aspects of the surveyed papers. The sole purpose of this table is to work as a quick reference of research directions, without judging any of the works presented. The first row of the table contains the reference number and the name of the first author. For each approach, we list the methods used for preprocessing, feature computation, and classification. In addition, we list the size of the data sets used for training and testing, the reported accuracy, and the type of TB manifestations detected.
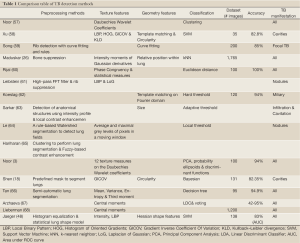
Full Table
The comparison table reveals several noticeable things: (I) there is no common dataset, (II) there is no specific classifier that performs best because each classifier uses its own training set, (III) the texture features usually tend to be wavelet coefficients, LBP, or moments, (IV) texture features are used more often than geometry characteristics, and (V) about half of the papers deal with TB manifestations in general while the other half focuses on a specific manifestation.
Discussion
Our survey shows that automatic TB detection has seen some progress over the last years. Several papers dealing either with a subset or directly with the whole gamut of TB manifestations have been published. However, it is virtually impossible to compare the performance of the proposed systems in a fair manner. The datasets used are not publicly available, and almost certainly contain CXRs taken under different conditions and with different disease severity. The methods used cover many of the imaging techniques already established in other areas. Thus, most of the systems follow traditional approaches and seem to obtain reasonable results. It remains to be seen how these systems will perform in practical field tests with larger data sets, and in regions where TB is epidemic. Screening tests in developing countries are currently in preparation (48,69), and will provide more insight into the practical capabilities of these systems. Independent observer tests will soon reveal whether these systems can reach a level of human performance, or if they may even be able to outperform expert radiologists in the near future. Finally, another important aspect is the integration of additional modalities into a CAD system, such as patient data (e.g., temperature, coughing, etc.). In a recent study, this meta-information led to a better automatic computer diagnosis (70).
Acknowledgements
This research is supported by the Intramural Research Program of the National Institutes of Health (NIH), National Library of Medicine (NLM), and Lister Hill National Center for Biomedical Communications (LHNCBC).
Disclosure: The authors declare no conflict of interest.
References
- WHO. Global Tuberculosis Report. World Health Organization, 2012.
- Nakamura K, Ohmi A, Kurihara T, et al. Studies on the diagnostic value of 70 mm radiophotograms by mirror camera and the reading ability of physicians. Kekkaku 1970;45:121-8.
- Noor NM, Rijal OM, Yunus A, et al. A statistical interpretation of the chest radiograph for the detection of pulmonary tuberculosis. In: Biomedical Engineering and Sciences (IECBES), 2010 IEEE EMBS Conference on, 2010:47-51.
- Schilham AM, van Ginneken B, Loog M. A computer-aided diagnosis system for detection of lung nodules in chest radiographs with an evaluation on a public database. Med Image Anal 2006;10:247-58.
- Toman K, Frieden T. Toman’s Tuberculosis: case detection, treatment, and monitoring: questions and answers. WHO, 2004.
- World Health Organization. Tuberculosis prevalence surveys: a handbook. World Health Organization, 2011.
- Folio LR. Robochest Web Teaching Tool. Available online: www.robochest.com (last accessed 10 April 2013)
- Lee KS, Song KS, Lim TH, et al. Adult-onset pulmonary tuberculosis: findings on chest radiographs and CT scans. AJR Am J Roentgenol 1993;160:753-8.
- Greenberg SD, Frager D, Suster B, et al. Active pulmonary tuberculosis in patients with AIDS: spectrum of radiographic findings (including a normal appearance). Radiology 1994;193:115-9.
- Jones TF, Schaffner W. Miniature chest radiograph screening for tuberculosis in jails: a cost-effectiveness analysis. Am J Respir Crit Care Med 2001;164:77-81.
- Long R, Maycher B, Scalcini M, et al. The chest roentgenogram in pulmonary tuberculosis patients seropositive for human immunodeficiency virus type 1. Chest 1991;99:123-7.
- Hill AR, Premkumar S, Brustein S, et al. Disseminated tuberculosis in the acquired immunodeficiency syndrome era. Am Rev Respir Dis 1991;144:1164-70.
- Kramer F, Modilevsky T, Waliany AR, et al. Delayed diagnosis of tuberculosis in patients with human immunodeficiency virus infection. Am J Med 1990;89:451-6.
- Tsubamoto M, Johkoh T, Kozuka T, et al. Temporal subtraction for the detection of hazy pulmonary opacities on chest radiography. AJR Am J Roentgenol 2002;179:467-71.
- Folio L, Stokes R, Frankfurter J. From film to filmless: military experience with teleradiology in Korea. Appl Radiol July 1995:36-9.
- Ravin CE, Chotas HG. Chest radiography. Radiology 1997;204:593-600.
- Summers RM. Road maps for advancement of radiologic computer-aided detection in the 21st century. Radiology 2003;229:11-3.
- Shen R, Cheng I, Basu A. A Hybrid Knowledge-Guided Detection Technique for Screening of Infectious Pulmonary Tuberculosis from Chest Radiographs. IEEE Trans Biomed Eng 2010;57:2646-56.
- McAdams HP, Samei E, Dobbins J 3rd, et al. Recent advances in chest radiography. Radiology 2006;241:663-83.
- Bonvin L, Zellweger JP. Mass miniature X-ray screening for tuberculosis among immigrants entering Switzerland. Tuber Lung Dis 1992;73:322-5.
- Dobbins JT 3rd, Godfrey DJ. Digital x-ray tomosynthesis: current state of the art and clinical potential. Phys Med Biol 2003;48:R65-106.
- Sherrier RH, Johnson GA. Regionally adaptive histogram equalization of the chest. IEEE Trans Med Imaging 1987;6:1-7.
- Matozaki T, Tanishita A, Ikeguchi T. Image enhancement of chest radiography using wavelet transform. Trans Inst Electron Inf Commun Eng D-II 2000;83:408-14.
- Shuyue C, Honghua H, Yanjun Z, et al. Study of automatic enhancement for chest radiograph. J Digit Imaging 2006;19:371-5.
- Philipsen RH, Maduskar P, Hogeweg L, et al. Normalization of chest radiographs. In: Proc. SPIE 8670, Medical Imaging 2013: Computer-Aided Diagnosis, 2013.
- Maduskar P, Hogeweg L, Philipsen R, et al. Improved texture analysis for automatic detection of tuberculosis (TB) on chest radiographs with bone suppression images. In: SPIE Medical Imaging, pages: 86700H-86700H. International Society for Optics and Photonics, 2013.
- Hogeweg L, Sánchez CI, de Jong PA, et al. Clavicle segmentation in chest radiographs. Med Image Anal 2012;16:1490-502.
- Karargyris A, Antani S, Thoma G. Segmenting anatomy in chest x-rays for tuberculosis screening. Conf Proc IEEE Eng Med Biol Soc 2011;2011:7779-82.
- Loog M, Ginneken B. Bony structure suppression in chest radiographs. In: Proceedings of the Second ECCV International Conference on Computer Vision Approaches to Medical Image Analysis, CVAMIA’06. Berlin, Heidelberg: Springer-Verlag, 2006:166-77.
- Suzuki K, Abe H, MacMahon H, et al. Image-processing technique for suppressing ribs in chest radiographs by means of massive training artificial neural network (MTANN). IEEE Trans Med Imaging 2006;25:406-16.
- MacMahon H, Li F, Engelmann R, et al. Dual energy subtraction and temporal subtraction chest radiography. J Thorac Imaging 2008;23:77-85.
- Shiraishi J, Katsuragawa S, Ikezoe J, et al. Development of a digital image database for chest radiographs with and without a lung nodule: receiver operating characteristic analysis of radiologists’ detection of pulmonary nodules. AJR Am J Roentgenol 2000;174:71-4.
- van Ginneken B, ter Haar Romeny BM, et al. Computer-aided diagnosis in chest radiography: a survey. IEEE Trans Med Imaging 2001;20:1228-41.
- Armato SG 3rd, Giger ML, MacMahon H. Automated lung segmentation in digitized posteroanterior chest radiographs. Acad Radiol 1998;5:245-55.
- Li L, Zheng Y, Kallergi M, et al. Improved method for automatic identification of lung regions on chest radiographs. Acad Radiol 2001;8:629-38.
- van Ginneken B, ter Haar Romeny BM. Automatic segmentation of lung fields in chest radiographs. Med Phys 2000;27:2445-55.
- Loog M, Ginneken B. Supervised segmentation by iterated contextual pixel classification. Proc. 16th Int. Conf. on Pattern Recognition, 2001:925-8.
- Loog M, van Ginneken B. Segmentation of the posterior ribs in chest radiographs using iterated contextual pixel classification. IEEE Trans Med Imaging 2006;25:602-11.
- Yue Z, Goshtasby A, Ackerman LV. Automatic detection of rib borders in chest radiographs. IEEE Trans Med Imaging 1995;14:525-36.
- van Ginneken B, Stegmann MB, Loog M. Segmentation of anatomical structures in chest radiographs using supervised methods: a comparative study on a public database. Med Image Anal 2006;10:19-40.
- Cootes TF, Taylor CJ, Cooper DH, et al. Active Shape Models-Their Training and Application. Comput Vis Image Understand 1996;61:38-59.
- Cootes TF, Edwards GJ, Taylor CJ. Active appearance models. IEEE Trans Pattern Anal Mach Intell 2001;23:681-5.
- van Ginneken B, Frangi AF, Staal JJ, et al. Active shape model segmentation with optimal features. IEEE Trans Med Imaging 2002;21:924-33.
- van Ginneken B, Katsuragawa S, ter Haar Romeny BM, et al. Automatic detection of abnormalities in chest radiographs using local texture analysis. IEEE Trans Med Imaging 2002;21:139-49.
- Shi Y, Qi F, Xue Z, et al. Segmenting lung fields in serial chest radiographs using both population-based and patient-specific shape statistics. IEEE Trans Med Imaging 2008;27:481-94.
- Shi Y, Shen D. Hierarchical shape statistical model for segmentation of lung fields in chest radiographs. Med Image Comput Comput Assist Interv 2008;11:417-24.
- Annangi P, Thiruvenkadam S, Raja A, et al. A region based active contour method for x-ray lung segmentation using prior shape and low level features. Biomedical Imaging: From Nano to Macro, 2010 IEEE International Symposium 2012:892-5.
- Jaeger S, Karargyris A, Antani S, et al. Detecting tuberculosis in radiographs using combined lung masks. Conf Proc IEEE Eng Med Biol Soc 2012;2012:4978-81.
- Candemir S, Jaeger S, Palaniappan K, et al. Graph-cut based automatic lung boundary detection in chest radiographs. In First IEEE Healthcare Technology Conference: Translational Engineering in Health & Medicine, Houston, USA, 2012:31-4.
- Coppini G, Miniati M, Monti S, et al. A computer-aided diagnosis approach for emphysema recognition in chest radiography. Med Eng Phys 2013;35:63-73.
- Arbeláez P, Maire M, Fowlkes C, et al. Contour detection and hierarchical image segmentation. IEEE Trans Pattern Anal Mach Intell 2011;33:898-916.
- Boykov Y, Funka-Lea G. Graph cuts and efficient n-d image segmentation. Int J Computer Vision 2006;70:109-31.
- Estrada FJ, Jepson AD. Benchmarking image segmentation algorithms. Int J Comput Vision 2009;85:167-81.
- Unnikrishnan R, Pantofaru C, Hebert M. Toward objective evaluation of image segmentation algorithms. IEEE Trans Pattern Anal Mach Intell 2007;29:929-44.
- Withey DJ, Koles ZJ. Medical image segmentation: Methods and software. Noninvasive Functional Source Imaging of the Brain and Heart and the International Conference on Functional Biomedical Imaging, 2007. NFSI-ICFBI 2007. Joint Meeting of the 6th International Symposium, 2007:140-3.
- Dailey CL, Gotway MB, Jasmer RM. Radiographic Manifestations of Tuberculosis. A primer for clinicians. San Francisco: Francis J. Curry National Tuberculosis Center, 2009.
- Noor NM, Rijal OM, Fah CY. Wavelet as features for tuberculosis (MTB) using standard x-ray film images. Signal Processing, 2002 6th International Conference, IEEE 2002;2:1138-41.
- Xu T, Cheng I, Long R, et al. Novel coarse-to-fine dual scale technique for tuberculosis cavity detection in chest radiographs. EURASIP Journal on Image and Video Processing 2013;3:1-18.
- Song YL. Localization algorithm and implementation for focal of pulmonary tuberculosis chest image. In: Machine Vision and Human-Machine Interface (MVHI), 2010 International Conference on, IEEE 2010:361-4.
- Rijal M, Ebrahimian H, Noor NM. Determining features for discriminating PTB and normal lungs using phase congruency model. In: Biomedical and Health Informatics (BHI), 2012 IEEE-EMBS International Conference on, IEEE 2012:341-4.
- Leibstein JM, Nel AL. Detecting tuberculosis in chest radiographs using image processing techniques. University of Johannesburg.
- Koeslag A, de Jager G. Computer aided diagnosis of miliary tuberculosis. Proceedings of the Pattern Recognition Association of South Africa, 2001.
- Sarkar S, Chaudhuri S. Automated detection of infiltration and cavitation in digital chest radiographs of chronic pulmonary tuberculosis. In: Engineering in Medicine and Biology Society, 1996. Bridging Disciplines for Biomedicine. Proceedings of the 18th Annual International Conference of the IEEE, IEEE 1997;3:1185-6.
- Le K. Automated detection of early lung cancer and tuberculosis based on x-ray image analysis. In: Proc. WSEAS International Conference on Signal, Speech and Image Processing, 2006:1-6.
- Hariharan S, Ray AK, and Ghosh MK. An algorithm for the enhancement of chest x-ray images of tuberculosis patients. In Industrial Technology 2000. Proceedings of IEEE International Conference on IEEE 2000;2:107-12.
- Tan JH, Acharya UR, Tan C, et al. Computer-assisted diagnosis of tuberculosis: a first order statistical approach to chest radiograph. J Med Syst 2012;36:2751-9.
- Arzhaeva Y, Hogeweg L, de Jong PA, et al. Global and local multi-valued dissimilarity-based classification: Application to computer-aided detection of tuberculosis. In Medical Image Computing and Computer-Assisted Intervention-MICCAI 2009. Springer, 2009:724-31.
- Lieberman R, Kwong H, Liu B, et al. Computer-assisted detection (CAD) methodology for early detection of response to pharmaceutical therapy in tuberculosis patients. In: SPIE Medical Imaging, International Society for Optics and Photonics, 2009:726030.
- Jaeger S, Antani S, Thoma G. Tuberculosis screening of chest radiographs, Available online: spie.org/x48510.xml (last accessed 10 April 2013). SPIE Newsroom, 2011.
- Van’t Hoog AH, Meme HK, Laserson KF, et al. Screening strategies for tuberculosis prevalence surveys: the value of chest radiography and symptoms. PLoS One 2012;7:e38691.


