Left ventricular deformation abnormalities in a patient with calpainopathy—a case from the three-dimensional speckle-tracking echocardiographic MAGYAR-Path Study
Introduction
Genetic background
Calpainopathy is the most common autosomal recessive limb-girdle muscular dystrophy (LGMD). Due to their heterogeneity, the occurrence of the LGMDs is difficult to assess. Estimates report a prevalence ranging from 1 in 14,500 to 1 in 123,000 (1-3). On the basis of immunohistochemical and genetic examinations, the overall frequency of the LGMDs is of 5–70 cases/million population (4-9). According to the inheritance pattern, LGMDs are classified to dominant (LGMD1) and recessive forms (LGMD2). Calpainopathy is defined as limb-girdle muscular dystrophy type 2A (LGMD2A). Depending on the population examined, calpainopathy accounts for about 26–30% of the LGMD cases. There are populations, such as that in the Basque region of Spain or Reunion Island, where it represents as many as 80%, whereas in Denmark, it accounts for only 6% of all of the LGMD cases (9).
LGMD2A is caused by mutations in the gene CAPN3 located on chromosome 15q15.1-q21.1. CAPN3 gene encodes the 94-kDa non-lysosomal calcium-dependent cysteine protease, calpain 3. More than 300 mutations of the gene have so far been identified; however, genotype-phenotype correlations could not yet be found. Intra- and interfamilial phenotypic variability have been described (3,10,11). The function of calpain 3 has not been completely elucidated. Calpain 3 has been suggested to be involved in the maturation of contractile elements after muscle degeneration (12). Calpain 3 interacts with titin and dysferlin, and is presumed to play a role in the function of the dysferlin protein complex. Calpain-deficiency has been shown to cause sarcomere abnormality and it leads to muscle fiber death, whereas its functional impairment affects muscle membrane remodeling and repair (13,14).
Disease course
The disease begins in childhood, usually by the age of 8–15, with the reported ages of onset ranging between 2 and 40 years. Early motor milestones are usually normal. Toe walking is common and can be the presenting sign, but the earliest signs are usually attributable to the involvement of the hip adductors and the gluteus maximus (9,10,13).
The most common appearance is that of scapular, pelvic girdle, and trunk weakness, with facial muscles being generally spared. Muscle weakness is generalized and symmetrical, being more pronounced proximally and relatively mild distally. Hip-girdle weakness is the most pronounced in the gluteus maximus and in the hip adductors, leading to a wide-based, lordotic gait, whereas the involvement of the scapular muscles leads to scapular winging (present in 83% of all cases). Abdominal muscles (i.e., rectus abdominis) are also involved (10). These features together with the involvement of the elbow flexors and the sparing of the respiratory muscles are considered specific for LGMD2A (15). The atrophy of the involved muscles is prominent. Hypertrophy can also occur in some patients. Contractures develop with time and are also considered characteristic. As the respiratory muscles are spared, the vital capacity rarely declines below 80% and no nocturnal hypoventilation has been observed. Cardiac involvement has not yet been reported (16). The progression of the disease is slow and the patients become wheelchair-bound at 11–28 years after the onset of the disease, generally by the age of 40. The earlier the onset of the disease, the more rapid the progression (10).
Several phenotypes have been described, including the limb-girdle type, the Leyden-Moebius type with pelvic-femoral involvement, the Erb dystrophy type with scapular-humeral involvement, and the Miyoshi muscular dystrophy phenotype (3,10). A severe Duchenne-like form and a form with significant contractures similar to that seen in Emery-Dreifuss muscular dystrophy have also been described (9). Distal muscle weakness (foot drop) has also been reported as the initial symptom of calpainopathy (17). Calpainopathy can also present as an asymptomatic hyperCKemia in 10–14% of cases (9,10).
Diagnosis and treatment
The diagnosis of the disease is based on the clinical presentation, the elevated CK levels, and the findings of electromyography, muscle biopsy, and molecular genetic testing. Muscle biopsy shows fiber-size variation, interstitial fibrosis, an enhanced internalization of nuclei, and occasionally the presence of eosinophils owing to the upregulation of interleukin-32 and immunoglobulin genes (13).
Immunohistochemical staining reveals a decreased immunoreactivity for the 60-kD and/or the 30-kD band of calpain 3 by Western blot. The reduction or the loss of staining for the 60-kD band is more specific and sensitive than that for the 30-kD band, whereas the loss of staining for both bands is highly specific for LGMD2A. However, about 25% of patients have normal staining. A decreased immunohistochemical reactivity for dysferlin can also occur in calpainopathy, whereas a reduced calpain 3 staining may be present in other LGMDs as well, such as 1C, 2B, 2I, J, and the dystrophinopathies (9). Genetic testing shows an autosomal recessive mutation in chromosome 15, at 15q15.1. More than 150 mutations have so far been identified, including missense mutations, early stop codons, splicing defects, small insertions or deletions, and large genomic deletions at various locations along the gene (10).
There is at present no treatment available for LGMDs. Supportive care aims at preserving muscle function, maintaining functional ability, delaying the development of skeletal abnormalities and contractures, prolonging ambulation, and increasing the life expectancy. If necessary, orthopedic procedures can be recommended to maintain ambulation and independence. Annual cardiological evaluation is advisable.
We present a case with calpainopathy in which cardiac involvement has been extensively investigated by routine Doppler echocardiography, extended with three-dimensional speckle-tracking echocardiography (3DSTE).
Case presentation
The 43-year-old male patient was first investigated at the age of 12 for an abnormal gait. His first complain was walking tiptoe. Later, proximal weakness occurred in the upper and lower extremities, with difficulty lifting arms, climbing stairs, and walking, accompanied by a waddling, lordotic gait. Muscle atrophy developed in the limb-girdle muscles. No muscle hypertrophy was observed. Achillotomy was performed at age 12, resulting in improved gait. He became wheelchair-bound by the age of 38. For another 2 years, he was still able to stand up with help, but was unable to walk. He also has mildly elevated levels of cholesterol and a mild hypertension. His family history is negative for muscle disease. On neurological examination, he currently presents with a symmetrical tetraparesis and muscle atrophy, the latter being more pronounced proximally. Muscle strength in the upper extremities is 2/5 proximally and in the elbow flexors, and 3/5 to 4/5 distally. In the lower extremities, muscle strength is 1/5 to 2/5 proximally, being the most pronounced in the hip adductors, and 3/5 in the distal muscles. Severe symmetrical muscle atrophy can be seen.
He was diagnosed at the age of 12 with limb-girdle muscle dystrophy. CK levels were mildly elevated, ranging between 488 and 700 U/L (normal up to 195 U/L). Muscle biopsy was first performed at age 12 and showed a myopathic pattern, with no specific changes. The second muscle biopsy performed at age 19 revealed a dystrophinopathy, and the diagnosis of Becker muscular dystrophy was presumed. Later (at age 38) the diagnosis was re-evaluated. The molecular genetic test performed did not confirm the presumed Becker muscular dystrophy and Pompe disease was also ruled out as an etiology underlying the muscle weakness. The clinical course of the disease raised the suspicion of calpainopathy. Molecular genetic testing revealed a homozygous deletion (C550delA) in exon 4 of the CAPN3 gene. The diagnosis of LGMD2A was established at age 39. The patient is regularly examined by a neurologist and has annual cardiology check-ups.
The patient was enrolled into the MAGYAR-Path Study (Motion Analysis of the heart and Great vessels bY three-dimensionAl speckle-tRacking echocardiography in Pathological cases), in which, among other factors, the potential diagnostic and prognostic significance of 3DSTE-derived variables were assessed in different pathological conditions (18). Routine cardiological examination included physical examination, ECG, 2D Doppler echocardiography, and 3DSTE. In addition to routine cardiac echocardiographic examination using a Toshiba Artida ultrasound machine (Toshiba Medical Systems, Tokyo, Japan), a 3D dataset was digitally collected for offline chamber quantification using a PST-25SX matrix-array transducer with 3DSTE capability. Description of 3DSTE analysis of the left ventricular (LV) was detailed in a previous study (19). Briefly, three short-axis views and the apical 4-chamber (AP4CH) and 2-chamber (AP2CH) views extracted from 3D echocardiographic datasets were analyzed offline using the 3D Wall Motion Tracking software version 2.5 (Toshiba Medical Systems). Following the manual marking of two points in the endocardium at the edges of the mitral valve and one point at the apex on the AP4CH and AP2CH views, the endocardial 3D surface was automatically detected, tracked, and reconstructed throughout the cardiac cycle. Using the created LV-3D model, several strain and rotational curves were generated for the quantification of LV global and segmental deformations from the volumetric data (Figure 1).
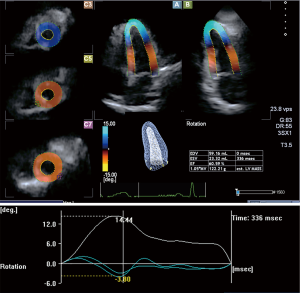
No chest pain or any signs suggestive of cardiovascular abnormalities were found in the past medical history of the patient or revealed on physical examination. On ECG, normofrequent sinus rhythm with normal repolarization was found, and echocardiography revealed normal chamber dimensions with an ejection fraction (EF) of 61%. Diastolic dysfunction could not be confirmed (E/A =67/45, E wave deceleration time =168 ms) and only low-grade mitral and aortic regurgitations were demonstrated by Doppler echocardiography. LV hypertrophy was not observed. 3DSTE-derived LV global radial, longitudinal, circumferential, and area strains were reduced (−20.2%, −10.8%, −25.6%, and −37.1%, respectively). Apical LV rotation was found to be severely increased (14.4 degree), whereas basal LV rotation proved to be normal (−3.8 degree), resulting in an increased LV twist (18.2 degree) in this case.
Discussion
Advances have been made in the diagnosis of muscle dystrophies, and in cases without previously established genetic diagnosis, re-evaluation of the diagnosis should be considered. In the present case, the presumed diagnosis was that of Becker muscular dystrophy; however, this could not be proven by molecular genetic testing. On the basis of the clinical picture and the clinical course suggestive of calpainopathy, molecular genetic testing was performed, revealing a homozygous deletion (C550delA) in exon 4 of the CAPN3 gene. Based on a large study, in the case of a characteristic phenotype, the probability that a patient has LGMD2A is 90% when there is no calpain detectable in the Western blot. However, the characteristic phenotype was found to be a more accurate predictor compared to the Western blot in case of patients for whom either the phenotype or the protein analysis was suggestive of calpainopathy (20). This emphasizes the importance of the examination and follow-up by an experienced neurologist in the diagnostic work-up of patients with muscle dystrophies. Our patient undergoes annual cardiological evaluation aiming at the assessment of cardiac involvement by 3DSTE.
3DSTE is a novel development in echocardiography, allowing the parallel assessment of volumetric cyclic changes and the analysis of deformation via strain assessments of heart chambers, by the use of the same 3D model created from the same digitally acquired 3D dataset (18). Exact validated volumetric and functional parameters can be assessed at the same time within a relatively short period of time, followed by a detailed analysis of the volumetric and functional features of the atria and ventricles (21). In addition to volumetric measurements, deformation parameters, including the uni- and multidirectional strains of the LV, quantitative features of LV contractility, can be assessed together with parameters characterizing LV rotational mechanics. In the past, only different invasive techniques, including microsonometry or tagged magnetic resonance imaging were suitable for the quantification of LV rotations and twist; however, due to their invasive nature, availability, and cost, their determination remained problematic (22). 3DSTE solved this problem, allowing the assessment of quantitative deformation features and rotational mechanics at the same time (18). Normally, the LV base rotates clockwise, whereas the LV apex rotates counterclockwise, due to the fact that myocardial fibers on the subepicardial side run in a left-handed direction (22,23). Their net sum is called the LV twist, which is responsible for approximately 40% of the LV pumping function during systole, as evidenced by physiological studies. However, myocardial fibers on the subendocardial side run in a right-handed direction, and the contraction of these fibers causes an opposite rotation: the base rotates counterclockwise whereas the apex rotates clockwise. The radius of the rotation of the subepicardium is greater than that of subendocardium, providing a greater torque, which results in a significantly expressed rotation of the subepicardium. In case of a subepicardial dysfunction, the rotation decreases, whereas in case of a subendocardial dysfunction, the rotation increases. There are confusing results about LV rotational mechanics in heart failure with preserved EF (22). Some authors found hyperrotation in association with abnormal relaxation (subendocardial dysfunction) and hyporotation in association with advanced diastolic dysfunction (24), whereas other authors reported that LV twisting does not decrease with diastolic dysfunction (25). In the presented case with calpainopathy, routine echocardiographic examination did not confirm systolic or diastolic heart failure. However, over reduced LV strains, significantly increased LV apical rotation and LV twist were found indicating asymptomatic subclinical (latent) LV dysfunction (26-29).
To the best of the authors’ knowledge, this is the first study in which the investigation of the cardiac involvement in calpainopathy revealed reduced LV strains and increased LV apical rotation and twist. We found no factors that could theoretically underlie the observed alteration in LV rotational mechanics in the present case, but the effect of comorbidities, the applied medications or other factors could not be fully excluded. Further examinations are warranted to confirm our findings and to find a correct pathophysiological explanation.
Acknowledgements
We acknowledge Mr. Levente Szalárdy, MD for the linguistic correction of the manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- van der Kooi AJ, Barth PG, Busch HF, de Haan R, Ginjaar HB, van Essen AJ, van Hooff LJ, Höweler CJ, Jennekens FG, Jongen P, Oosterhuis HJ, Padberg GW, Spaans F, Wintzen AR, Wokke JH, Bakker E, van Ommen GJ, Bolhuis PA, de Visser M. The clinical spectrum of limb girdle muscular dystrophy. A survey in The Netherlands. Brain 1996;119:1471-80. [Crossref] [PubMed]
- Urtasun M, Sáenz A, Roudaut C, Poza JJ, Urtizberea JA, Cobo AM, Richard I, García Bragado F, Leturcq F, Kaplan JC, Martí Massó JF, Beckmann JS, López de Munain A. Limb-girdle muscular dystrophy in Guipúzcoa (Basque Country, Spain). Brain 1998;121:1735-47. [Crossref] [PubMed]
- Pegoraro E, Hoffman EP. Limb-Girdle Muscular Dystrophy Overview. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE. editors. Seattle (WA): University of Washington, Seattle, 2000:1993-2016. Available online: http://www.ncbi.nlm.nih.gov/books/NBK1408/
- Moore SA, Shilling CJ, Westra S, Wall C, Wicklund MP, Stolle C, Brown CA, Michele DE, Piccolo F, Winder TL, Stence A, Barresi R, King N, King W, Florence J, Campbell KP, Fenichel GM, Stedman HH, Kissel JT, Griggs RC, Pandya S, Mathews KD, Pestronk A, Serrano C, Darvish D, Mendell JR. Limb-girdle muscular dystrophy in the United States. J Neuropathol Exp Neurol 2006;65:995-1003. [Crossref] [PubMed]
- van der Kooi AJ, Frankhuizen WS, Barth PG, Howeler CJ, Padberg GW, Spaans F, Wintzen AR, Wokke JH, van Ommen GJ, de Visser M, Bakker E, Ginjaar HB. Limb-girdle muscular dystrophy in the Netherlands: gene defect identified in half the families. Neurology 2007;68:2125-8. [Crossref] [PubMed]
- Lo HP, Cooper ST, Evesson FJ, Seto JT, Chiotis M, Tay V, Compton AG, Cairns AG, Corbett A, MacArthur DG, Yang N, Reardon K, North KN. Limb-girdle muscular dystrophy: diagnostic evaluation, frequency and clues to pathogenesis. Neuromuscul Disord 2008;18:34-44. [Crossref] [PubMed]
- Guglieri M, Magri F, D'Angelo MG, Prelle A, Morandi L, Rodolico C, Cagliani R, Mora M, Fortunato F, Bordoni A, Del Bo R, Ghezzi S, Pagliarani S, Lucchiari S, Salani S, Zecca C, Lamperti C, Ronchi D, Aguennouz M, Ciscato P, Di Blasi C, Ruggieri A, Moroni I, Turconi A, Toscano A, Moggio M, Bresolin N, Comi GP. Clinical, molecular, and protein correlations in a large sample of genetically diagnosed Italian limb girdle muscular dystrophy patients. Hum Mutat 2008;29:258-66. [Crossref] [PubMed]
- Fanin M, Nascimbeni AC, Aurino S, Tasca E, Pegoraro E, Nigro V, Angelini C. Frequency of LGMD gene mutations in Italian patients with distinct clinical phenotypes. Neurology 2009;72:1432-5. [Crossref] [PubMed]
- Lopate G. Limb-Girdle Muscular Dystrophy Treatment & Management. Available online: http://emedicine.medscape.com/article/1170911-treatment
- Limb-girdle muscular dystrophy (LGMD) syndromes. Available online: http://neuromuscular.wustl.edu/musdist/lg.html
- Schessl J, Walter MC, Schreiber G, Schara U, Müller CR, Lochmüller H, Bönnemann CG, Korinthenberg R, Kirschner J. Phenotypic variability in siblings with Calpainopathy (LGMD2A). Acta Myologica 2008;27:54-8. [PubMed]
- Hauerslev S, Sveen ML, Duno M, Angelini C, Vissing J, Krag TO. Calpain 3 is important for muscle regeneration. Evidence from patients with limb girdle muscular dystrophies. BMC Musculoskelet Disord 2012;13:43. [Crossref] [PubMed]
- Gallardo E, Saenz A, Illa I. Limb-girdle muscular dystrophy 2A. Handb Clin Neurol 2011;101:97-110. [Crossref] [PubMed]
- Huang Y, de Morrée A, van Remoortere A, Bushby K, Frants RR, den Dunnen JT, van der Maarel SM. Calpain 3 is a modulator of the dysferlin protein complex in skeletal muscle. Hum Molec Genet 2008;17:1855-66. [Crossref] [PubMed]
- Groen EJ, Charlton R, Barresi R, Anderson LV, Eagle M, Hudson J, Koref MS, Straub V, Bushby KM. Analysis of the UK diagnostic strategy for limb girdle muscular dystrophy 2A. Brain 2007;130:3237-49. [Crossref] [PubMed]
- Quick S, Schaefer J, Waessnig N, Schultheiss T, Reuner U, Schoen S, Reichmann H, Strasser R, Speiser U. Evaluation of heart involvement in calpainopathy (LGMD2A) using cardiovascular magnetic resonance. Muscle Nerve 2015;52:661-3. [Crossref] [PubMed]
- Burke G, Hillier C, Cole J, Sampson M, Bridges L, Bushby K, Barresi R, Hammans SR. Calpainopathy presenting as foot drop in a 41 year old. Neuromuscul Disord 2010;20:407-10. [Crossref] [PubMed]
- Nemes A, Kalapos A, Domsik P, Forster T. Three-dimensional speckle-tracking echocardiography - a further step in non-invasive three-dimensional cardiac imaging. Orv Hetil 2012;153:1570-7. [Crossref] [PubMed]
- Kalapos A, Domsik P, Forster T, Nemes A. Left ventricular strain reduction is not confined to the noncompacted segments in noncompaction cardiomyopathy - Insights from the three-dimensional speckle tracking echocardiographic MAGYAR-Path Study. Echocardiography 2014;31:638-43. [Crossref] [PubMed]
- Sáenz A, Leturcq F, Cobo AM, Poza JJ, Ferrer X, Otaegui D, Camaño P, Urtasun M, Vílchez J, Gutiérrez-Rivas E, Emparanza J, Merlini L, Paisán C, Goicoechea M, Blázquez L, Eymard B, Lochmuller H, Walter M, Bonnemann C, Figarella-Branger D, Kaplan JC, Urtizberea JA, Martí-Massó JF, López de Munain A. LGMD2A: genotype-phenotype correlations based on a large mutational survey on the calpain 3 gene. Brain 2005;128:732-42. [Crossref] [PubMed]
- Urbano-Moral JA, Patel AR, Maron MS, Arias-Godinez JA, Pandian NG. Three-dimensional speckle-tracking echocardiography: Methodological aspects and clinical potential. Echocardiography 2012;29:997-1010. [Crossref] [PubMed]
- Nakatani S. Left ventricular rotation and twist: why should we learn? J Cardiovasc Ultrasound 2011;19:1-6. [Crossref] [PubMed]
- Nemes A, Kalapos A, Domsik P, Forster T. Left ventricular rotation and twist of the heart. Clarification of some concepts. Orv Hetil 2012;153:1547-51. [Crossref] [PubMed]
- Park SJ, Miyazaki C, Bruce CJ, Ommen S, Miller FA, Oh JK. Left ventricular torsion by two-dimensional speckle tracking echocardiography in patients with diastolic dysfunction and normal ejection fraction. J Am Soc Echocardiogr 2008;21:1129-37. [Crossref] [PubMed]
- Wang J, Khoury DS, Yue Y, Torre-Amione G, Nagueh SF. Left ventricular untwisting rate by speckle tracking echocardiography. Circulation 2007;116:2580-6. [Crossref] [PubMed]
- Tavakoli V, Sahba N. Assessment of age- related changes in left ventricular twist by 3-dimensional speckle-tracking echocardiography. J Ultrasound Med 2013;32:1435-41. [Crossref] [PubMed]
- Kleijn SA, Pandian NG, Thomas JD, Perez de Isla L, Kamp O, Zuber M, Nihoyannopoulos P, Forster T, Nesser HJ, Geibel A, Gorissen W, Zamorano JL. Normal reference values of left ventricular strain using three-dimensional speckle tracking echocardiography: results from a multicentre study. Eur Heart J Cardiovasc Imaging 2015;16:410-6. [Crossref] [PubMed]
- Nemes A, Földeák D, Domsik P, Kalapos A, Sepp R, Borbényi Z, Forster T. Different patterns of left ventricular rotational mechanics in cardiac amyloidosis-results from the three-dimensional speckle-tracking echocardiographic MAGYAR-Path Study. Quant Imaging Med Surg 2015;5:853-7. [PubMed]
- Nemes A, Kovács Z, Kalapos A, Domsik P, Forster T. Left ventricular rotational mechanics in identical twins with juvenile idiopathic arthritis (from the MAGYAR-Twin Study). Quant Imaging Med Surg 2017;7:138-9. [Crossref] [PubMed]


