
Compared with SonoVue® LR-5, Sonazoid® modified LR-5 has better diagnostic sensitivity for hepatocellular carcinoma: a systematic review and meta-analysis
Introduction
Hepatocellular carcinoma (HCC) is the most prevalent form of liver cancer and the third highest cause of cancer-related deaths worldwide (1,2). Fortunately, HCC can be diagnosed non-invasively by imaging methods. As a radiation-free, real-time, and cost-effective imaging modality, contrast-enhanced ultrasound (CEUS) has been recommended for the screening of high-risk populations and has been shown to exhibit good diagnostic performance in diagnosing HCC (3,4).
SonoVue® and Sonazoid® are two contrast agents (CAs) that are widely used in clinical practice for liver imaging (5). SonoVue® is a pure vascular agent that cannot pass through endothelial cells (6). Conversely, Sonazoid® can be phagocytosed by Kupffer and reticuloendothelial cells. This property allows Sonazoid® to provide detailed information about both the vascular phase and the post-vascular Kupffer phase (KP), thus providing additional diagnostic assistance (7). Some non-inferiority studies have revealed that the diagnostic accuracy of the two CAs is comparable (8,9), while other studies have indicated that in terms of diagnostic accuracy, Sonazoid® outperforms SonoVue® (10). However, these studies mainly focused on comparing the image characteristics of different time phases and did not specifically explore the diagnostic efficiency of the CEUS liver imaging reporting and data system (LI-RADS) algorithms between the two CAs.
To standardize reporting and enhance communication, the American College of Radiology (ACR) introduced the CEUS LI-RADS as a standardized system for reporting liver nodules in patients at high risk of HCC in 2016, and the system was further updated in 2017 (11). Under this system, liver lesions are categorized based on the potential risk of HCC. Patients with LI-RADS category 5 (LR-5) lesions can be treated for HCC without undergoing a biopsy, while those with LI-RADS category M (LR-M) lesions undergo further examination via a pathological assessment. However, the current version of the CEUS LI-RADS is only recommended for pure blood pool agents such as SonoVue®. With the increasing use of Sonazoid® in clinical practice, there have been proposals to modify the CEUS LI-RADS algorithms based on the distinct characteristics of Sonazoid® in the KP. It is crucial to compare the two algorithms to determine whether the modified Sonazoid® LI-RADS is worthy of popularization. To address this issue, we conducted a meta-analysis to compare the diagnostic efficiency of the LI-RADS algorithms between SonoVue® and Sonazoid®. We present this article in accordance with the PRISMA-DTA reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1616/rc) (12).
Methods
Study protocol and search strategy
We registered the protocol for this study on the PROSPERO platform (CRD42023434246). A thorough search of the PubMed, Medline, Web of Science, Embase, and Cochrane Library databases was conducted to retrieve original research articles investigating the diagnostic performance of the CEUS LI-RADS algorithms using SonoVue® and/or Sonazoid®. The search terms focused on three main concepts: LI-RADS, CEUS, and HCC. Table S1 lists all the search terms used. The search was restricted to human subjects and English-language studies published from January 2016 to June 2023. Additionally, the reference lists of the included studies were manually reviewed to identify any additional relevant studies.
Eligibility criteria
To be eligible for inclusion in this meta-analysis, the articles had to meet the following inclusion criteria: (I) include patients at high risk of HCC; (II) have the full text available that could be assessed and appraised; (III) concern studies that sought to detect lesions by CEUS using SonoVue® and/or Sonazoid®; (IV) include two-by-two tables that could be extracted to show the diagnostic efficiency of the LI-RADS for suspected liver nodules; and (V) use histopathology or imaging follow-up as the reference standards.
Articles were excluded from this meta-analysis if they met any of the following exclusion criteria: (I) examined patients with liver nodules who had already received treatment; (II) the total number of lesions examined in the study was less than 50; (III) lacked sufficient information for the pooled analysis; (IV) were duplicates or overlapping publications; and/or (V) concerned case reports, editorials, letters to the editor, reviews, or conference abstracts.
Study selection and data extraction
Two reviewers independently reviewed the articles to determine their eligibility and conducted the data extraction. A senior author was consulted if any disagreements arose.
The following information was extracted: (I) study characteristics: first author, publication year, country, medical center (single center or multi-center), study design (prospective or retrospective), study type (cohort study or case-control study), and reference standard (pathology or imaging follow-up); (II) patient characteristics: patient number, mean age, and gender; (III) lesion characteristics: number of observations and final diagnosis (HCC, non-HCC malignancy, or benign lesion); (IV) CEUS characteristics: CA (SonoVue® or Sonazoid®), LI-RADS version (ACR LI-RADS or modified LI-RADS); and (V) study outcomes: true positives (TPs), false positives (FPs), true negatives (TNs), and false negatives (FNs) for the CEUS LI-RADS. If an article contained multiple sets of diagnostic performance data (TPs, FPs, TNs, and FNs) from different reviewers, the data of the most experienced reviewer were selected for the meta-analysis if information about the reviewers’ relevant clinical experience was provided; if no information was provided about the reviewers’ experience, the average results were used to reduce bias.
Quality assessment
The Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool was used to assess the overall methodological quality and risk of bias of the articles by the two reviewers independently, and any disagreement was resolved by discussion. The QUADAS-2 tool includes the following four aspects: patient selection, index test, reference standard, and flow and timing.
Statistical analysis
The meta-analysis was performed using Meta-DiSc 1.4 software (Clinical Biostatistics unit, Madrid, Spain) and Stata 14.0 software (Stata Corporation, College Station, TX, USA). The threshold effect was tested by the Spearman correlation coefficient; a P value <0.05 was considered statistically significant. A bivariate random-effects model was used to determine the pooled sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic odds ratio (DOR), and the areas under the summary receiver operating characteristic (SROC) curves, and their 95% confidence intervals (CIs). The variance of the logit-transformed percentage method was used to test the difference between the pooled sensitivity and specificity, and the Z-value test was used to test whether the areas under the SROC curves (AUCs) were significantly different between the two LI-RADS algorithms; a P value <0.05 was considered statistically significant. Cochran’s Q test and the I2 statistic were used to quantitatively assess the heterogeneity; a P value <0.1 and an I2 value ≥50% indicated significant heterogeneity. A meta-regression analysis was conducted to explore the potential causes of heterogeneity, and a sensitivity analysis was conducted to evaluate the stability of the results. Deeks’ funnel-plot asymmetry test was used in the diagnostic meta-analysis to evaluate the possible presence of publication bias when there were more than 10 studies; a P value <0.10 indicated a significant possibility of publication bias.
Results
Literature selection
Figure 1 illustrates the process employed for the literature search and study selection. The search strategy yielded 1,177 articles. After removing the duplicate articles, 852 articles remained. Among these, an additional 814 articles were excluded by screening titles and abstracts because they did not meet the inclusion criteria or met the exclusion criteria, and 38 further articles were further excluded after the full-text review. Ultimately, 26 articles were included in the meta-analysis.
Study characteristics
Table 1 summarizes the study and patient baseline characteristics of the included studies. The 26 studies comprised a total of 8,495 patients with 9,244 lesions. Of the studies, 17 used SonoVue® as the CA (13-29), seven used Sonazoid® (30-36), and two used both SonoVue® and Sonazoid® (37,38). Three studies were prospective (13,19,38) and 23 were retrospective (14-18,20-37). Of the studies, 10 used histopathology only as the reference standard (15,16,19,21,23-25,27,30,33) and 16 used both histopathology and imaging follow-up as the reference standard (13,14,17,18,20,22,26,28,29,31,32,34-38).
Table 1
| Study (ref., year) | Country | Study design | Study type | Center | No. of patients | Age, y [range] | Male/female | No. of nodules | Nodule size, mm [range]* | No. of HCCs | No. of non-HCC malignancies | Benign | Contrast agent | LI-RADS | Reference standard |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Schellhaas B (13) (2017) | Germany | Prospective | Cohort | Single center | 100 | 66.1 [42–85] | 85/15 | 100 | 52.2 [10–290] | 87 | 6 [ICC] | 7 | SonoVue | ACR v2016 | Pathology or imaging follow-up |
| Terzi E (14) (2018) | Italy | Retrospective | Cohort | Multi-center | 848 | 70 [31–89] | 457/391 | 1,006 | 20 [5–150] | 820 | 53 [ICC: 40; CHC: 9; Others: 4] | 133 | SonoVue | ACR v2016 | Pathology or imaging follow-up |
| Chen LD (15) (2019) | China | Retrospective | Case-control | Single center | 210 | 55†, 54‡ [32–84] | 163/47 | 210 | NA [≤30 mm: 25; 31–50 mm: 47; >50 mm: 138] | 105 | 105 [ICC] | 0 | SonoVue | ACR v2017 | Pathology |
| Li J (16) (2019) | China | Retrospective | Cohort | Single center | 1,366 | 52.3 [18–90] | 1,097/269 | 1,366 | 47 [5–200] | 985 | 139 [ICC: 59; CHC: 14; M: 62; Others: 4] | 242 | SonoVue | ACR v2017 | Pathology |
| Zheng W (17) (2020) | China | Retrospective | Cohort | Single center | 1,826 | 54 [44–62] | 1,642/184 | 2,020 | NA | 1,514 | 138 [ICC: 57; CHC: 24; M: 53; Others: 4] | 368 | SonoVue | ACR v2017 | Pathology or imaging follow-up |
| Wang JY (18) (2020) | China | Retrospective | Cohort | Single center | 258 | 52 [21–82] | 192/66 | 355 | 25 [6–183] | 115 | 5 [ICC: 2; CHC: 1; Others: 2] | 235 | SonoVue | ACR v2017 | Pathology or imaging follow-up |
| Zhou H (19) (2022) | China | Prospective | Cohort | Multi-center | 96 | 58.8 [35–87]†/ 61.8 [45–79]‡ |
81/15 | 96 | 42.7 | 67 | 22 | 7 | SonoVue | ACR v2017 | Pathology |
| [9.9–169]†/ 46.3[9.8–100.0]‡ |
|||||||||||||||
| Li S (20) (2021) | China | Retrospective | Cohort | Single center | 84 | 57.3 [26–86] | 67/17 | 86 | 25.7 [5.5–96.2] | 53 | 1 [ICC] | 32 | SonoVue | ACR v2017 | Pathology or imaging follow-up |
| Huang Z (21) (2021) | China | Retrospective | Case-control | Single center | 158 | 53 [28–75]†/ 55 [37–67]‡ |
143/15 | 158 | NA | 106 | 52 [ICC: 32; M: 10; CHC: 4; Others: 6] | 0 | SonoVue | ACR v2017 | Pathology |
| Zuo DS (22) (2021) | China | Retrospective | Cohort | Single center | 215 | NA | 151/67 | 218 | NA | 99 | 27 [ICC: 10; Others: 17] | 92 | SonoVue | ACR v2017 | Pathology or imaging follow-up |
| Ding J (23) (2021) | China | Retrospective | Cohort | Single center | 264 | 59.4 [26–80] | 202/62 | 264 | 32 [10–95] | 223 | 23 [ICC: 16; CHC: 3; M: 4] | 18 | SonoVue | ACR v2017 | Pathology |
| Lv K (24) (2021) | China | Retrospective | Cohort | Single center | 250 | 61.32 [24–87] | 186/64 | 259 | 52.39 [6–158] | 172 | 61 | 26 | SonoVue | ACR v2017 | Pathology |
| Yang D (25) (2022) | China | Retrospective | Cohort | Single center | 205 | 52 [19–83] | 162/43 | 205 | 34 [10–50] | 142 | 26 [ICC: 13; M: 8; Others: 5] | 37 | SonoVue | ACR v2017 | Pathology |
| Vidili G (26) (2022) | Italy | Retrospective | Cohort | Single center | 269 | 69 [43–88] | 219/50 | 511 | 24 [5–200] | 423 | 29 [ICC: 23; M: 3; Others: 3] | 59 | SonoVue | ACR v2017 | Pathology or imaging follow-up |
| Huang W (27) (2023) | China | Retrospective | Cohort | Single center | 179 | 53.2 [28–80] | 147/32 | 194 | 21 [9–30] | 144 | 29 [ICC: 11; CHC: 4; M: 9; Others: 5] | 21 | SonoVue | ACR v2017 | Pathology |
| Ghiuchici AM (28) (2021) | Romania | Retrospective | Cohort | Single center | 382 | 63 [24–89] | 261/121 | 464 | 46 [8–170] | 359 | 37 | 68 | SonoVue | ACR v2017 | Pathology or imaging follow-up |
| Pan JM (29) (2021) | China | Retrospective | Cohort | Single center | 534 | NA | 460/74 | 545 | NA | 503 | 29 | 13 | SonoVue | ACR v2017 | Pathology or imaging follow-up |
| Liao W (30) (2023) | China | Retrospective | Cohort | Single center | 137 | 51 [43–58] | 117/20 | 140 | 35.5 [23.8–61.3] | 119 | 15 [ICC: 6; CHC: 3; M: 2; Others: 4] | 6 | Sonazoid | ACR v2017/Modified LI-RADS | Pathology |
| Hwang JA (31) (2022) | Korea | Retrospective | Cohort | Multi-center | 123 | 61.5 [21–86] | 98/25 | 123 | 25 [10–130] | 77 | 15 [ICC: 11; CHC: 2; Others: 2] | 31 | Sonazoid | ACR v2017/Modified LI-RADS | Pathology or imaging follow-up |
| Hwang JA (32) (2021) | Korea | Retrospective | Cohort | Single center | 203 | 61.3 [32–83] | 159/44 | 122§ | 15 [7–50] | 89 | NA | NA | Sonazoid | ACR v2017/Modified LI-RADS | Pathology or imaging follow-up |
| Li L (33) (2023) | China | Retrospective | Cohort | Single center | 171 | 54 | 140/31 | 171 | 47 [9–105] | 114 | 43 | 14 | Sonazoid | Modified LI-RADS | Pathology |
| Li L (34) (2022) | China | Retrospective | Cohort | Single center | 293 | 55 | 140/31 | 304 | 43 [6–158] | 274 | 14 [ICC: 8; CHC: 1; M: 5] | 16 | Sonazoid | ACR v2017/Modified LI-RADS | Pathology or imaging follow-up |
| SugimotoK (35) (2020) | Japan | Retrospective | Cohort | Single center | 104 | 70.0 [54.5–78.0] | 74/30 | 104 | 17.9 [13.1–28.2] | 64 | 16 [ICC: 6; M: 9; Others: 1] | 24 | Sonazoid | Modified LI-RADS | Pathology or imaging follow-up |
| Takahashi H (36) (2022) | Japan | Retrospective | Cohort | Single center | 102 | 71 [63–78] | 64/48 | 102 | 25.5 [16.8–44.3] | 52 | 36 [ICC: 10; M: 26] | 14 | Sonazoid | ACR v2017/Modified LI-RADS | Pathology or imaging follow-up |
| Huang J (37) (2023) | China | Retrospective | Cohort | Single center | 59 | 54 [51–57] | 49/10 | 62 | 35 [10–105] | 55 | 3 [CHC: 1; ICC: 1; M: 1] | 4 | SonoVue/Sonazoid | ACR v2017/Modified LI-RADS | Pathology or imaging follow-up |
| Kang HJ (38) (2020) | Korea | Prospective | Cohort | Single center | 59 | 65 [49–86] | 47/12 | 59 | 28 [11–100] | 43 | 10 [CHC: 3; ICC: 6; Others: 1] | 6 | SonoVue/Sonazoid | ACR v2017 | Pathology or imaging follow-up |
*, the data are presented as the mean or median value with the range in brackets; †, the data are presented as the mean values for the HCC patients; ‡, the data are presented as the mean values for the non-HCC patients; §, only 122 nodules ≥1 cm and had Sonazoid modified LI-RADS category. HCC, hepatocellular carcinoma; LI-RADS, liver imaging reporting and data system; ICC, intrahepatic cholangiocarcinoma; ACR, American College of Radiology; v2016, version 2016; CHC, combined hepatocellular cholangiocarcinoma; NA, not available; v2017, version 2017; M, metastases.
Nineteen studies (13-29,37,38) with 8,178 nodules examined the diagnostic performance of SonoVue® LR-5 in detecting HCC, of which, two studies employed the 2016 version (13,14), 17 studies employed the 2017 version (15-29,37,38). One of the nine studies using Sonazoid® as the CA evaluated the diagnostic performance of Sonazoid® using the ACR LR-5 criteria and thus was not included in the further analysis. Eight studies with 1,128 nodules examined the diagnostic efficiency of Sonazoid® modified LR-5 (30-37), of which, two used version 1 and six used version 2. The detailed criteria of SonoVue® LR-5 and Sonazoid® modified LR-5 are set out in Table 2.
Table 2
| Criteria | Definition |
|---|---|
| ACR CEUS LR-5 | ≥1 cm: APHE, late and mild washout |
| Modified LR-5 (version 1) | ≥1 cm: APHE (not rim and not peripheral globular) and KP defect; APHE (not rim and not peripheral globular), early washout, and mild KP defect |
| Modified LR-5 (version 2) | ≥1 cm: APHE (not rim and not peripheral globular) and KP defect |
ACR, American College of Radiology; CEUS, contrast-enhanced ultrasound; LR-5, LI-RADS category 5; LI-RADS, liver imaging reporting and data system; APHE, arterial phase hyperenhancement; KP, Kupffer phase.
In terms of the LR-M algorithms, 15 studies (13-17,20-28,38) examined the diagnostic performance of ACR LR-M of SonoVue®. However, only three studies (30,34,35) examined the diagnostic performance of the modified LR-M in diagnosing non-HCC malignancies, and reported 457 HCCs, 45 non-HCC malignancies, and 46 benign lesions.
Diagnostic performance of the LR-5 algorithms for HCC
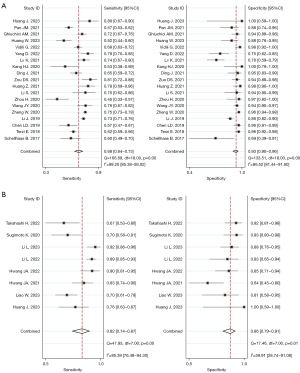
The Spearman correlation coefficients for SonoVue® LR-5 and Sonzaoid® modified LR-5 were 0.133 and 0.190, with P values of 0.586 and 0.651, respectively; thus, no threshold effect was found in our study. Figure 2 shows the forest plot of the pooled sensitivity and specificity of SonoVue® LR-5 and Sonazoid® modified LR-5. The pooled sensitivity values of SonoVue® LR-5 and Sonazoid® modified LR-5 were 0.68 (95% CI: 0.64–0.73, I2=89.20%; P<0.01) and 0.82 (95% CI: 0.74–0.87, I2=85.39%; P<0.01) (P<0.05), respectively. The pooled specificity values of SonoVue® LR-5 and Sonazoid® modified LR-5 were 0.93 (95% CI: 0.90–0.96, I2=86.52%; P<0.01) and 0.86 (95% CI: 0.79–0.91, I2=59.91%; P=0.01) (P<0.05), respectively. The PLRs of SonoVue® LR-5 and Sonazoid® modified LR-5I were 9.99 (95% CI: 6.51–15.34, I2=80.10%; P<0.01) and 5.89 (95% CI: 3.85–9.00, I2=28.81%; P=0.01), and the NLRs were 0.34 (95% CI: 0.30–0.39, I2=88.07%; P<0.01) and 0.21 (95% CI: 0.15–0.30, I2=82.12%; P<0.01), respectively (Figure S1). The DORs of SonoVue® LR-5 and Sonazoid® modified LR-5 were 29.36 (95% CI: 18.05–47.74, I2=100.00%; P<0.01) and 27.67 (95% CI: 15.40–49.70, I2=98.43%; P<0.01), respectively (Figure S2). The AUC values of SonoVue® LR-5 and Sonazoid® modified LR-5 were 0.86 (95% CI: 0.82–0.89) and 0.91 (95% CI: 0.88–0.93), respectively (Figure 3). The Z value of SonoVue® LR-5 and Sonazoid® modified LR-5 was 2.057>1.96 (P<0.05).

Diagnostic performance of the LR-M algorithms for non-HCC
A meta-analysis of the ability of the LR-M algorithms to diagnose non-HCC malignancies was conducted in accordance with the above-mentioned procedure, and the results are set out in Table 3. The pooled sensitivity and specificity of Sonazoid® modified LR-M for non-HCC malignancies were comparable to those of SonoVue® LR-M. The area under the SROC curve values for Sonazoid® modified LR-M and SonoVue® LR-M were 0.93 and 0.93, respectively, and the Z value was 0.0481<1.96 (P>0.05); however, the difference between the LR-M algorithms for the non-HCC malignancies was not significant.
Table 3
| Diagnostic performance | SonoVue LR-M | Sonazoid modified LR-M | |||
|---|---|---|---|---|---|
| Value (95% CI) | I2 | Value (95% CI) | I2 | ||
| Pooled sensitivity | 0.82 (0.79–0.84) | 88.7% | 0.89 (0.76–0.96) | 70.6% | |
| Pooled specificity | 0.86 (0.86–0.87) | 97.4% | 0.89 (0.86–0.92) | 91.1% | |
| PLR | 6.36 (4.41–9.17) | 95.4% | 8.13 (2.88–22.94) | 91.5% | |
| NLR | 0.20 (0.12–0.33) | 90.4% | 0.12 (0.02–0.72) | 67.2% | |
| DOR | 38.59 (19.96–74.61) | 82.7% | 69.99 (5.46–897.41) | 79.5% | |
LR-M, liver imaging reporting and data system definite or probable malignancy, not specific for hepatocellular carcinoma; HCC, hepatocellular carcinoma; 95% CI, 95% confidence interval; PLR, positive likelihood value; NLR, negative likelihood value; DOR, diagnostic odds ratio.
Meta-regression and sensitivity analysis
The I2 statistics revealed that substantial heterogeneity was present in this study, and a meta-regression analysis was performed to explore the causes of heterogeneity. Among the various covariates in this study, we analyzed the study design (prospective vs. retrospective), the study type (cohort vs. case-control), the number of medical centers (single center vs. multi-center), the subject enrollment (consecutive vs. selective), the number of lesions (<100 vs. ≥100), the LI-RADS version (version 2016 vs. version 2017 for SonoVue®, and version 1 vs. version 2 for Sonazoid®), and the reference standard (pathology vs. pathology and imaging follow-up).
The meta-regression results for SonoVue® LR-5 are set out in Table S2. Among the seven covariates, the study design was the source that contributed to the heterogeneity of the sensitivity, and the subject enrollment method was the source that significantly influenced the heterogeneity of the specificity. In relation to the reference standard, the results of the meta-regression analysis showed that the sensitivity and specificity of the studies using pathology and imaging follow-up as the reference standards were significantly higher than those using only pathology as the reference standard (sensitivity: 0.70 vs. 0.67, P=0.01; specificity: 0.95 vs.0.90, P=0.01).
As all of the eight studies had retrospective designs and employed consecutive subject enrollment, four covariates were included in the meta-regression analysis, and the results are set out in Table S3. The results indicated that the number of lesions was the source that significantly influenced the heterogeneity of the specificity. However, among the included eight studies, only one study reported <100 lesions.
The sensitivity analysis was conducted by removing each study one by one, and the results (Figure S3) indicated that no single study had a significant effect on the overall pooled estimates.
Publication bias and quality assessment
Based on the results of the Deeks’ funnel plot (Figure S4), no significant publication bias was observed in the literature related to SonoVue® LI-RADS (P=0.26); the funnel plot for Sonazoid® LI-RADS was omitted, as there were <10 publications on Sonazoid® LI-RADS. The results concerning the overall quality of the included studies are presented in Figure 4. The results of the index test and reference standard domain were satisfactory. In relation to the patient selection domain, two studies had a high risk of selection bias due to their case-control study designs (15,21), and seven studies had a unclear risk, as the inclusion criteria for the nodule size was not specified (13,22,25,27,32,33,39). In relation to the flow and timing domain, the quality of the included studies was relatively low, which might be due to the use of mixed reference standards (pathology and imaging diagnosis or pathology only) and unclear the time interval between the index test(s) and reference standards.

Discussion
The ACR CEUS LI-RADS was introduced to standardize the clinical management (i.e., assessment, communication, and recommendation) of HCC based on the final classification of liver nodules in patients at risk for HCC, and it is only recommended for pure blood pool CAs such as SonoVue®. The combined blood pool and KP CA Sonazoid® is also useful for the diagnosis of hepatic nodules, and modified LI-RADS algorithms were first proposed in 2020 (35). Compared with the SonoVue® LI-RADS, research on the Sonazoid® modified LI-RADS is limited. Thus, we conducted this meta-analysis to compare the diagnostic performance of the two algorithms.
The CEUS LR-5 is considered the diagnostic criteria for HCC. The results of our meta-analysis showed that the Sonazoid® modified LR-5 algorithm had better diagnostic sensitivity (0.82 vs. 0.68, P<0.05), lower specificity (0.86 vs. 0.93, P<0.05), and a higher AUC (0.91 vs. 0.86, P<0.05) than SonoVue® LR-5. This indicated that the overall diagnostic performance of the Sonazoid® modified LR-5 algorithm was superior to that of SonoVue® LR-5, especially in detecting lesions. As both CAs provide similar enhancement in the arterial phase (40), we speculate that the difference is mainly related to the distinct performances of the two CAs in the portal/venous phase and KP. The SonoVue® LR-5 algorithm focuses on late and mild washout in the portal/venous phase, which relies on the difference in portal vein blood supply between the liver parenchyma and the tumor (6). Conversely, the Sonazoid® modified LR-5 algorithm uses the KP defect as the primary imaging feature, which is based on the difference in Kupffer cell uptake (7,40). Research has shown that 10–33% of HCCs exhibit defects in the KP phase, without showing washout in the late vascular phase (31,36,38). This may be because the decrease in Kupffer cells occurs earlier than changes in blood supply during hepatocarcinogenesis (41). Therefore, the high sensitivity of Sonazoid® modified LR-5 is beneficial for the early detection of HCC in clinical settings for patients with risk factors. In addition, SonoVue® microbubbles are prone to rupture (38), while Sonazoid® microbubbles have better stability and are more tolerant to high mechanical index and high frame rate scanning conditions (42), which enables comprehensive and long-lasting scanning of the liver to increase the ability to detect lesions (40,43). The high sensitivity of the Sonazoid® modified LI-RADS has significant advantages in clinical practice, particularly in cases in which radical resection or local therapy are more prevalent (44).
Additionally, in some western countries, diagnosis methods with high specificity are required to determine liver transplantation allocations. The LR-5 algorithm was set to specifically diagnose HCC, aiming for a specificity of 100%. Our meta-analysis indicates that SonoVue® has high specificity (0.93), which is consistent with the findings of previous studies (45), and shows the accuracy of SonoVue® LR-5 in diagnosing HCC without pathological evidence. However, the pooled specificity of Sonazoid® modified LR-5 was not desirable. The absence of Kupffer cells is one of the distinctive features of hepatic malignant lesions (46) in both HCC and non-HCC malignancies (33,38). Meanwhile, atypical hemangioma (47) and dysplastic nodules (31) might also be a diagnostic pitfall. Fortunately, as Hwang reported, the integration of gray-scale features could improve the insufficient specificity of Sonazoid® (31). Further research needs to be conducted to verify and establish a sound diagnostic standard for Sonazoid®-enhanced ultrasound.
LR-M was used as the diagnostic criteria for non-HCC malignancies. Our meta-analysis results revealed that the pooled sensitivity, specificity, and AUC of Sonazoid® modified LR-M for non-HCC malignancies were comparable to those of SonoVue® LR-M. Our SonoVue® LR-M results are consistent with the results of previous studies (48). However, the limited number of publications on Sonazoid® modified LR-M may limit its generalized value, and more research needs to be conducted in the future to verify this conclusion.
In the process of evaluating which articles were to be included in the meta-analysis, some studies were specifically included due to the limited number of studies focusing on the Sonazoid® LI-RADS. Two studies in China that included the same 34 patients were included in this meta-analysis, as they included a total of 293 and 171 patients, respectively, and thus only a small number of the patients overlapped (33,34). Another two studies from Korea with a partial time overlap were included; one (32) of which was a single-center study with a patient inclusion period of three years, and the other (31) of which was a three-center study with an inclusion period of eight years with different inclusion conditions. Both of these studies were included in the further analysis, although we failed to obtain the original data. One eligible study from Japan was ultimately removed (39) because of the inclusion overlaps with another two studies (35,36). Another point needs to be made. As there is currently no officially adopted version of the Sonazoid® LI-RADS, different researchers have made slightly different modification suggestions. Two studies (33,34) used modified LR-5 proposed by their institutions, which differed slightly from Sugimoto’s criteria; they rectified the supplementary definition of modified LR-5 to no rim arterial phase hyperenhancement (APHE), early washout, and mild KP defects. The lack of a unified standard is one of the limitations of our study.
Generally, diagnostic efficacy, time efficiency, and safety are all factors influencing the choice of CA in clinical practice. Both SonoVue® and Sonazoid® have shown good safety and tolerance for liver imaging; however, individuals who are allergic to eggs need to consider the pros and cons before choosing Sonazoid® (40,49,50). It should be noted that there was a difference in the time periods during the examination. An examination using SonoVue® that includes all three vascular phases often takes <5 minutes, while an examination using Sonazoid® usually takes >10 minutes due to the presence of the KP (40). If a CA re-injection is necessary, the difference in the examination times increases further.
Our meta-analysis had some limitations. First, there are no unified criteria for the Sonazoid® modified LR-5. Second, substantial heterogeneity was observed. Third, there was a disequilibrium of the involved studies using the two CAs. Further research needs to be conducted to decrease the inclusion bias and verify the results. Fourth, we failed to compare the diagnostic efficiency of the SonoVue modified criteria (the onset time of washout should be revised to 45 seconds), as the research in this area is limited; however, the reported results were encouraging. Finally, it should be noted that of the 26 studies included in this meta-analysis, 22 were from Asia. Thus, the results of this study should be carefully generalized to other populations.
Conclusions
In conclusion, the Sonazoid® modified LR-5 algorithm had better diagnostic sensitivity, lower specificity, and a higher AUC than SonoVue® LR-5. Given the limited number of studies focused on the Sonazoid® modified LI-RADS, these results require further verification.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the PRISMA-DTA reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-23-1616/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1616/coif). W.L. reports that this work was supported by the Sichuan Science and Technology Program (No. 2020YFS0211). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Rumgay H, Ferlay J, de Martel C, Georges D, Ibrahim AS, Zheng R, Wei W, Lemmens VEPP, Soerjomataram I. Global, regional and national burden of primary liver cancer by subtype. Eur J Cancer 2022;161:108-18. [Crossref] [PubMed]
- EASL Clinical Practice Guidelines. Management of hepatocellular carcinoma. J Hepatol 2018;69:182-236. [Crossref] [PubMed]
- Singal AG, Llovet JM, Yarchoan M, Mehta N, Heimbach JK, Dawson LA, Jou JH, Kulik LM, Agopian VG, Marrero JA, Mendiratta-Lala M, Brown DB, Rilling WS, Goyal L, Wei AC, Taddei TH. AASLD Practice Guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology 2023;78:1922-65. [Crossref] [PubMed]
- Greenbaum LD. Foreword to guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the liver--update 2012. Ultraschall Med 2013;34:7. [Crossref] [PubMed]
- Ferraioli G, Meloni MF. Contrast-enhanced ultrasonography of the liver using SonoVue. Ultrasonography 2018;37:25-35. [Crossref] [PubMed]
- Yanagisawa K, Moriyasu F, Miyahara T, Yuki M, Iijima H. Phagocytosis of ultrasound contrast agent microbubbles by Kupffer cells. Ultrasound Med Biol 2007;33:318-25. [Crossref] [PubMed]
- Kang HJ, Lee JM, Yoon JH, Yoo J, Choi Y, Joo I, Han JK. Sonazoid™ versus SonoVue(®) for Diagnosing Hepatocellular Carcinoma Using Contrast-Enhanced Ultrasound in At-Risk Individuals: A Prospective, Single-Center, Intraindividual, Noninferiority Study. Korean J Radiol 2022;23:1067-77. [Crossref] [PubMed]
- Zhai HY, Liang P, Yu J, Cao F, Kuang M, Liu FY, Liu FY, Zhu XY. Comparison of Sonazoid and SonoVue in the Diagnosis of Focal Liver Lesions: A Preliminary Study. J Ultrasound Med 2019;38:2417-25. [Crossref] [PubMed]
- Wu M, Li L, Wang J, Zhang Y, Guo Q, Li X, Zhang X. Contrast-enhanced US for characterization of focal liver lesions: a comprehensive meta-analysis. Eur Radiol 2018;28:2077-88. [Crossref] [PubMed]
- Kono Y, Lyshchik A, Cosgrove D, Dietrich CF, Jang HJ, Kim TK, Piscaglia F, Willmann JK, Wilson SR, Santillan C, Kambadakone A, Mitchell D, Vezeridis A, Sirlin CB. Contrast Enhanced Ultrasound (CEUS) Liver Imaging Reporting and Data System (LI-RADS®): the official version by the American College of Radiology (ACR). Ultraschall Med 2017;38:85-6. [Crossref] [PubMed]
- McInnes MDF, Moher D, Thombs BD, McGrath TA, Bossuyt PM, et al. Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies: The PRISMA-DTA Statement. JAMA 2018;319:388-96. [Crossref] [PubMed]
- Schellhaas B, Görtz RS, Pfeifer L, Kielisch C, Neurath MF, Strobel D. Diagnostic accuracy of contrast-enhanced ultrasound for the differential diagnosis of hepatocellular carcinoma: ESCULAP versus CEUS-LI-RADS. Eur J Gastroenterol Hepatol 2017;29:1036-44. [Crossref] [PubMed]
- Terzi E, Iavarone M, Pompili M, Veronese L, Cabibbo G, Fraquelli M, Riccardi L, De Bonis L, Sangiovanni A, Leoni S, Zocco MA, Rossi S, Alessi N, Wilson SR, Piscaglia FCEUS LI-RADS Italy study group collaborators. Contrast ultrasound LI-RADS LR-5 identifies hepatocellular carcinoma in cirrhosis in a multicenter restropective study of 1,006 nodules. J Hepatol 2018;68:485-92. [Crossref] [PubMed]
- Chen LD, Ruan SM, Lin Y, Liang JY, Shen SL, Hu HT, Huang Y, Li W, Wang Z, Xie XY, Lu MD, Kuang M, Wang W. Comparison between M-score and LR-M in the reporting system of contrast-enhanced ultrasound LI-RADS. Eur Radiol 2019;29:4249-57. [Crossref] [PubMed]
- Li J, Ling W, Chen S, Ma L, Yang L, Lu Q, Luo Y. The interreader agreement and validation of contrast-enhanced ultrasound liver imaging reporting and data system. Eur J Radiol 2019;120:108685. [Crossref] [PubMed]
- Zheng W, Li Q, Zou XB, Wang JW, Han F, Li F, Huang LS, Li AH, Zhou JH. Evaluation of Contrast-enhanced US LI-RADS version 2017: Application on 2020 Liver Nodules in Patients with Hepatitis B Infection. Radiology 2020;294:299-307. [Crossref] [PubMed]
- Wang JY, Feng SY, Xu JW, Li J, Chu L, Cui XW, Dietrich CF. Usefulness of the Contrast-Enhanced Ultrasound Liver Imaging Reporting and Data System in Diagnosing Focal Liver Lesions by Inexperienced Radiologists. J Ultrasound Med 2020;39:1537-46. [Crossref] [PubMed]
- Zhou H, Zhang C, Du L, Jiang J, Zhao Q, Sun J, Li Q, Wan M, Wang X, Hou X, Wen Q, Liu Y, Zhou X, Huang P. Contrast-Enhanced Ultrasound Liver Imaging Reporting and Data System in Diagnosing Hepatocellular Carcinoma: Diagnostic Performance and Interobserver Agreement. Ultraschall Med 2022;43:64-71. [Crossref] [PubMed]
- Li S, Zhou L, Chen R, Chen Y, Niu Z, Qian L, Fang Y, Xu L, Xu H, Zhang L. Diagnostic efficacy of contrast-enhanced ultrasound versus MRI Liver Imaging Reporting and Data System (LI-RADS) for categorising hepatic observations in patients at risk of hepatocellular carcinoma. Clin Radiol 2021;76:161.e1-161.e10. [Crossref] [PubMed]
- Huang Z, Zhou P, Li S, Li K. MR versus CEUS LI-RADS for Distinguishing Hepatocellular Carcinoma from other Hepatic Malignancies in High-Risk Patients. Ultrasound Med Biol 2021;47:1244-52. [Crossref] [PubMed]
- Zuo D, Yang K, Wu S. Diagnostic performance of intravascular perfusion based contrast-enhanced ultrasound LI-RADS in the evaluation of hepatocellular carcinoma. Clin Hemorheol Microcirc 2021;78:429-37. [Crossref] [PubMed]
- Ding J, Qin Z, Zhou Y, Zhou H, Zhang Q, Wang Y, Jing X, Wang F. Impact of Revision of the LR-M Criteria on the Diagnostic Performance of Contrast-Enhanced Ultrasound LI-RADS. Ultrasound Med Biol 2021;47:3403-10. [Crossref] [PubMed]
- Lv K, Cao X, Dong Y, Geng D, Zhang J. CT/MRI LI-RADS version 2018 versus CEUS LI-RADS version 2017 in the diagnosis of primary hepatic nodules in patients with high-risk hepatocellular carcinoma. Ann Transl Med 2021;9:1076. [Crossref] [PubMed]
- Yang D, Hu H, Li R, Tang CL, Ma KS, Guo DY. The diagnostic value of contrast-enhanced ultrasound LI-RADS for hepatocellular carcinoma in patients with cirrhosis and chronic hepatitis B. Abdom Radiol (NY) 2022;47:630-9. [Crossref] [PubMed]
- Vidili G, Arru M, Solinas G, Calvisi DF, Meloni P, Sauchella A, Turilli D, Fabio C, Cossu A, Madeddu G, Babudieri S, Zocco MA, Iannetti G, Di Lembo E, Delitala AP, Manetti R. Contrast-enhanced ultrasound Liver Imaging Reporting and Data System: Lights and shadows in hepatocellular carcinoma and cholangiocellular carcinoma diagnosis. World J Gastroenterol 2022;28:3488-502. [Crossref] [PubMed]
- Huang W, Wen R, Wu Y, Lin P, Guo D, Peng Y, Liu D, Mou M, Chen F, Huang F, Yang H, He Y. Can Modifications of LR-M Criteria Improve the Diagnostic Performance of Contrast-Enhanced Ultrasound LI-RADS for Small Hepatic Lesions up to 3 cm? J Ultrasound Med 2023;42:2403-13. [Crossref] [PubMed]
- Ghiuchici AM, Dănilă M, Popescu A, Șirli R, Moga T, Topan M, Bende F, Sporea I. Contrast-enhanced ultrasound algorithm (ACR CEUS LI-RADSv 2017)- a valuable tool for the noninvasive diagnosis of hepatocellular carcinoma in patients with chronic liver disease. Med Ultrason 2021;23:383-9. [Crossref] [PubMed]
- Pan JM, Chen W, Zheng YL, Cheng MQ, Zeng D, Huang H, Huang Y, Xie XY, Lu MD, Kuang M, Hu HT, Chen LD, Wang W. Tumor size-based validation of contrast-enhanced ultrasound liver imaging reporting and data system (CEUS LI-RADS) 2017 for hepatocellular carcinoma characterizing. Br J Radiol 2021;94:20201359. [Crossref] [PubMed]
- Liao W, Que Q, Wen R, Lin P, Chen Y, Pang J, Guo D, Wen D, Yang H, He Y. Comparison of the Feasibility and Diagnostic Performance of ACR CEUS LI-RADS and a Modified CEUS LI-RADS for HCC in Examinations Using Sonazoid. J Ultrasound Med 2023;42:2501-11. [Crossref] [PubMed]
- Hwang JA, Jeong WK, Kang HJ, Lee ES, Park HJ, Lee JM. Perfluorobutane-enhanced ultrasonography with a Kupffer phase: improved diagnostic sensitivity for hepatocellular carcinoma. Eur Radiol 2022;32:8507-17. [Crossref] [PubMed]
- Hwang JA, Jeong WK, Min JH, Kim YY, Heo NH, Lim HK. Sonazoid-enhanced ultrasonography: comparison with CT/MRI Liver Imaging Reporting and Data System in patients with suspected hepatocellular carcinoma. Ultrasonography 2021;40:486-98. [Crossref] [PubMed]
- Li L, Mao S, Wang J, Zheng W, Shen J, Clevert DA, Zhou J. Intraindividual Comparison of Contrast-Enhanced Ultrasound Using Perfluorobutane With Modified Criteria Versus CT/MRI LI-RADS Version 2018 for Diagnosing HCC in High-Risk Patients. AJR Am J Roentgenol 2023;220:682-91. [Crossref] [PubMed]
- Li L, Zheng W, Wang J, Han J, Guo Z, Hu Y, Li X, Zhou J. Contrast-Enhanced Ultrasound Using Perfluorobutane: Impact of Proposed Modified LI-RADS Criteria on Hepatocellular Carcinoma Detection. AJR Am J Roentgenol 2022;219:434-43. [Crossref] [PubMed]
- Sugimoto K, Kakegawa T, Takahashi H, Tomita Y, Abe M, Yoshimasu Y, Takeuchi H, Kasai Y, Itoi T. Usefulness of Modified CEUS LI-RADS for the Diagnosis of Hepatocellular Carcinoma Using Sonazoid. Diagnostics (Basel) 2020;10:828. [Crossref] [PubMed]
- Takahashi H, Sugimoto K, Kamiyama N, Sakamaki K, Kakegawa T, Wada T, Tomita Y, Abe M, Yoshimasu Y, Takeuchi H, Itoi T. Noninvasive Diagnosis of Hepatocellular Carcinoma on Sonazoid-Enhanced US: Value of the Kupffer Phase. Diagnostics (Basel) 2022;12:141. [Crossref] [PubMed]
- Huang J, Gao L, Li J, Yang R, Jiang Z, Liao M, Luo Y, Lu Q. Head-to-head comparison of Sonazoid and SonoVue in the diagnosis of hepatocellular carcinoma for patients at high risk. Front Oncol 2023;13:1140277. [Crossref] [PubMed]
- Kang HJ, Lee JM, Yoon JH, Lee K, Kim H, Han JK. Contrast-enhanced US with Sulfur Hexafluoride and Perfluorobutane for the Diagnosis of Hepatocellular Carcinoma in Individuals with High Risk. Radiology 2020;297:108-16. [Crossref] [PubMed]
- Sugimoto K, Saito K, Shirota N, Kamiyama N, Sakamaki K, Takahashi H, Wada T, Kakegawa T, Tomita Y, Abe M, Yoshimasu Y, Takeuchi H, Itoi T. Comparison of modified CEUS LI-RADS with sonazoid and CT/MRI LI-RADS for diagnosis of hepatocellular carcinoma. Hepatol Res 2022;52:730-8. [Crossref] [PubMed]
- Barr RG, Huang P, Luo Y, Xie X, Zheng R, Yan K, Jing X, Luo Y, Xu H, Fei X, Lee JM. Contrast-enhanced ultrasound imaging of the liver: a review of the clinical evidence for SonoVue and Sonazoid. Abdom Radiol (NY) 2020;45:3779-88. [Crossref] [PubMed]
- Ohama H, Imai Y, Nakashima O, Kogita S, Takamura M, Hori M, Seki Y, Sawai Y, Igura T, Fukuda K, Makino Y, Morimoto O, Ohsawa M, Sakamoto M, Murakami T. Images of Sonazoid-enhanced ultrasonography in multistep hepatocarcinogenesis: comparison with Gd-EOB-DTPA-enhanced MRI. J Gastroenterol 2014;49:1081-93. [Crossref] [PubMed]
- Fei X, Han P, Jiang B, Zhu L, Tian W, Sang M, Zhang X, Zhu Y, Luo Y. High Frame Rate Contrast-enhanced Ultrasound Helps Differentiate Malignant and Benign Focal Liver Lesions. J Clin Transl Hepatol 2022;10:26-33. [Crossref] [PubMed]
- Li P, Hoppmann S, Du P, Li H, Evans PM, Moestue SA, Yu W, Dong F, Liu H, Liu L. Pharmacokinetics of Perfluorobutane after Intra-Venous Bolus Injection of Sonazoid in Healthy Chinese Volunteers. Ultrasound Med Biol 2017;43:1031-9. [Crossref] [PubMed]
- Chernyak V, Fowler KJ, Kamaya A, Kielar AZ, Elsayes KM, Bashir MR, Kono Y, Do RK, Mitchell DG, Singal AG, Tang A, Sirlin CB. Liver Imaging Reporting and Data System (LI-RADS) Version 2018: Imaging of Hepatocellular Carcinoma in At-Risk Patients. Radiology 2018;289:816-30. [Crossref] [PubMed]
- Jin H, Cai Y, Zhang M, Huang L, Bao W, Hu Q, Chen X, Zhou L, Ling W. LI-RADS LR-5 on contrast-enhanced ultrasonography has satisfactory diagnostic specificity for hepatocellular carcinoma: a systematic review and meta-analysis. Quant Imaging Med Surg 2023;13:957-69. [Crossref] [PubMed]
- Goto E, Masuzaki R, Tateishi R, Kondo Y, Imamura J, Goto T, Ikeda H, Akahane M, Shiina S, Omata M, Yoshida H, Koike K. Value of post-vascular phase (Kupffer imaging) by contrast-enhanced ultrasonography using Sonazoid in the detection of hepatocellular carcinoma. J Gastroenterol 2012;47:477-85. [Crossref] [PubMed]
- Dietrich CF, Mertens JC, Braden B, Schuessler G, Ott M, Ignee A. Contrast-enhanced ultrasound of histologically proven liver hemangiomas. Hepatology 2007;45:1139-45. [Crossref] [PubMed]
- Li L, Hu Y, Han J, Li Q, Peng C, Zhou J. Clinical Application of Liver Imaging Reporting and Data System for Characterizing Liver Neoplasms: A Meta-Analysis. Diagnostics (Basel) 2021;11:323. [Crossref] [PubMed]
- Park JH, Park MS, Lee SJ, Jeong WK, Lee JY, Park MJ, Lee SS, Han K, Nam CM, Park SH, Lee KH. Contrast-enhanced US with Perfluorobutane for Hepatocellular Carcinoma Surveillance: A Multicenter Diagnostic Trial (SCAN). Radiology 2019;292:638-46. [Crossref] [PubMed]
- Tang J, Xi X, Wang S, Li G, Sun M, Zhang B. Prolonged heterogeneous liver enhancement accompanied by abdominal symptoms after sonographic contrast agent injection: a cross-sectional study. Quant Imaging Med Surg 2023;13:3150-60. [Crossref] [PubMed]



