
The value of the improved percutaneous and intravenous contrast-enhanced ultrasound diagnostic classification in sentinel lymph nodes of breast cancer
Introduction
Breast cancer is one of the most common malignant tumors in women worldwide (1). Axillary lymph node (ALN) status is an important prognostic factor for breast cancer and it contributes to clinical treatment decisions (2,3). Sentinel lymph nodes (SLNs) are the initial metastatic sites, and cancer cells may bypass the SLNs and enter the vascular channels directly to spread to the systemic sites (4). Breast cancer patients following with further radiotherapy and systemic treatment can be exempted from axillary lymph node dissection (ALND) without increasing the risk of locoregional recurrence and decreasing disease-free survival, if sentinel lymph node biopsy (SLNB) shows two or fewer metastatic SLN (5). SLNB has replaced ALND as the preferred therapy for treating early-stage breast cancer with two or less metastatic SLNs, providing adequate axillary node staging information with minimal morbidity, and it has become the treatment standard for breast cancer management (6-10).
SLN detection methods mainly include blue dye-guided method, radioisotope method, indocyanine green fluorescence imaging, and contrast-enhanced ultrasound (CEUS) (11-13). CEUS is performed preoperatively, while other methods are performed intraoperatively. Percutaneous contrast-enhanced ultrasound (P-CEUS) is a non-invasive method used for detecting SLN in breast cancer patients with high predictive efficiency (14,15). The status of SLN could be diagnosed with this technique by dividing the P-CEUS pattern into three types (16-19). Intravenous contrast-enhanced ultrasound (IV-CEUS) is also used to diagnose SLN according to the pattern of homogeneous enhancement and uniform regression, or non-uniform regression (20). However, the diagnostic performance of the previous P-CEUS and IV-CEUS pattern were not satisfied, may be due to the neglect of structural characteristics of lymph nodes. The pattern classification of contrast-enhanced ultrasound usually did not combine the structural characteristics of lymph nodes, and only classified the enhancement patterns of the whole lymph nodes.
In this study, the structure of lymph nodes was considered and a new evaluation system was constructed by subdividing the pattern of heterogeneous enhancement. The diagnostic efficiency of the new system was tested by comparing with the original system. We present this article in accordance with the STARD reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1210/rc).
Methods
Patients
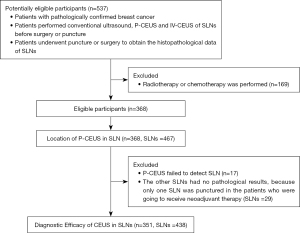
This retrospective study included consecutive 537 female breast cancer patients in the First Affiliated Hospital of Sun Yat-sen University from June 2019 to December 2021. The inclusion criteria were: (I) patients with pathological breast cancer; (II) patients with conventional ultrasound, P-CEUS and IV-CEUS of SLNs before surgery or puncture; (III) patients underwent puncture or surgery to obtain the histopathological data of SLNs. Patients with history of performed radiotherapy or chemotherapy were excluded. Eventually, 368 patients were included (Figure 1). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the First Affiliated Hospital of Sun Yat-sen University [(2020)316] and informed consent was taken from all the patients.
Instruments and methods
The contrast-enhanced ultrasound instruments included Siemens ACUSON (10L4 linear array probe), Mindray Resona 7 (L9-3 linear array probe) and Philips iU22 (L9-3 linear array probe), and the microbubble continuous real-time contrast-enhanced ultrasound imaging mode under low mechanical index (MI <0.15) was adopted. The ultrasound contrast agent was a suspension prepared by SonoVue (Bracco, Italy) lyophilized powder (59 mg sulfur hexafluoride microbubbles) and 5 mL normal saline, which had been shaken for 30 s for standby. The P-CEUS and IV-CEUS for SLN were commonly performed in our hospital. The ultrasound examination was performed by two physicians with more than 10 years of experience in CEUS. After approximately 20 cases, the physician became proficient in P-CEUS and IV-CEUS for SLN, reducing the scanning time from about 30 to 15 minutes. Physicians were unaware of the clinical information of the patients.
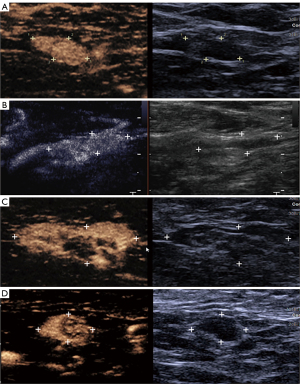
The patients lied in the supine position with the upper extremities abducted to fully expose the breast and axilla. After conventional ultrasound examination of the breast lesions and armpits of the patients, the skin was disinfected and 0.6–0.8 mL ultrasound contrast agent was injected into the upper abdomen around the affected side areola (Figure 2A). If the breast lesion was located in the nipple-areola complex, the contrast agent was injected away from the breast lesion. If the lymphatic vessel and SLN were not detected after a single injection, multi-point injection around the areola area was performed. Immediately after the injection of the contrast agent, the SLN was confirmed by observing the enhanced lymphatic drainage route. The lymphatic drainage route and SLNs on the skin surface were marked (Figure 2B). Then the SLN was observed under conventional ultrasound. After 2.4 mL ultrasound contrast agent was injected into the median elbow vein, the enhancement characteristics of SLN were observed. The number, size, cortical thickness, P-CEUS and IV-CEUS pattern of the SLNs were recorded.
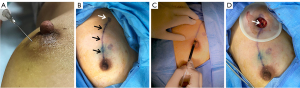
Patients who planned to receive neoadjuvant therapy underwent SLN histological biopsy. For patients undergoing surgical treatment, 1 mL of 1% methylene blue solution was injected subcutaneously into the upper quadrant outside the areola area under general anesthesia during the operation (Figure 2C). After massaging the breast for 5–10 min, the blue stained lymphatics and blue stained SLNs were dissected at the marks on the body surface, and attention was paid to observe whether the blue stained lymphatics and blue stained lymph nodes were consistent with those marked by P-CEUS (Figure 2D). Blue stained lymph nodes were excised and sent to pathological examination. Micrometastases were defined as metastases of less than 2 mm (21).
Statistical methods
Statistical software package SPSS 27.0 and Medcalc 19.5.6 were used in this study. If the localization mark of P-CEUS was consistent with blue dye, the P-CEUS localization mark of SLN and drainage lymphatic vessel was considered as correct. The status of SLN was based on pathological results. The receiver operating characteristic (ROC) curve was used to calculate the area under curve (AUC) of the diagnostic method. The diagnostic efficacy of the examination methods was compared with Delong test. The Cohen’s kappa value was used to evaluate the consistency between observers and diagnostic methods. 0.6< AUC ≤0.8 was classified as good; 0.4< AUC ≤0.6 was classified as moderate. A two-sided P<0.05 was considered as a statistically significant difference.
Results
Participant characteristics
In this study, 368 female breast cancer patients were included, including three patients with bilateral breast cancer. The mean participant age was 51.1±11.2 years (age range was 25–86 years). The clinical characteristics of these patients are shown in Table 1. No patients experienced adverse reactions, such as allergies during the study.
Table 1
| Parameter | Value |
|---|---|
| Age (years) | 51.1±11.2 |
| Histologic type | |
| Carcinoma in situ | 34 (9.2) |
| Invasive breast carcinoma | 336 (90.6) |
| Paget disease of the nipple | 1 (0.3) |
| Pathological acquisition of lymph nodes | |
| Puncture | 68 (18.3) |
| SLNB | 249 (67.1%) |
| ALND | 54 (14.6) |
| ALN status | |
| Positive | 106 (28.6) |
| Negative | 265 (71.4) |
| Lymphatic vessel (tracing by P-CEUS) | |
| 0 | 8 (2.2) |
| 1 | 282 (76.0) |
| 2 | 71 (19.1) |
| 3 | 9 (2.4) |
| 4 | 1 (0.3) |
| SLN (tracing by P-CEUS) | |
| 0 | 17 (4.6) |
| 1 | 272 (73.3) |
| 2 | 58 (15.6) |
| 3 | 19 (5.1) |
| 4 | 3 (0.8) |
| 5 | 2 (0.5) |
| Total | 371 |
Values are n (%) or mean ± standard deviation. P-CEUS, percutaneous contrast-enhanced ultrasound; SLN, sentinel lymph node; SLNB, sentinel lymph node biopsy; ALND, axillary lymph node dissection; ALN, axillary lymph node.
Location of P-CEUS in SLNs
The detection rate was 95.42% (354 of 371) in the P-CEUS examination. Among these cases, four cases underwent suspicious lymph node puncture without blue staining, and the other 13 cases underwent blue staining, only 1 case of whom was not detected with blue-stained SLN. P-CEUS indicated that the average number of lymphatic vessels on each side was 1.23±0.53 (455/371), and the mean number of SLNs on each side was 1.26±0.71 (467/371) (Table 1). Only one SLN was punctured in the patients who were going to receive neoadjuvant therapy, and the other 29 SLNs had no pathological results. Thus, a total of 438 SLNs were included.
P-CEUS and IV-CEUS classification of SLN
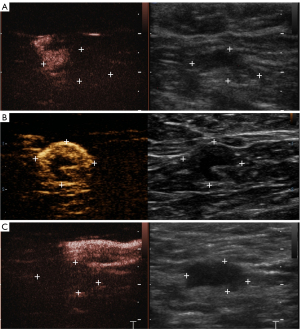
P-CEUS pattern of SLNs was divided into six types (Figures 3,4):
- Type I: only part of the cortex was enhanced, others were non-enhanced, and the lymph node cortex was unevenly thickened;
- Type II: partial cortical filling defect;
- Type III: non-enhancement;
- Type IV: homogeneous high enhancement;
- Type V: diffuse inhomogeneous high enhancement;
- Type VIa: non/low enhancement of lymphatic hilus, homogeneous high enhancement of cortex;
- Type VIb: one half showed Type IV, V or VIa, and the other showed non-enhancement;
- Type VIc: only part of the cortex was enhanced, others were not enhanced, and lymph node cortex was evenly thickened.
- Type I–III were diagnosed as malignant lymph nodes, and Types IV–VI as benign lymph nodes.
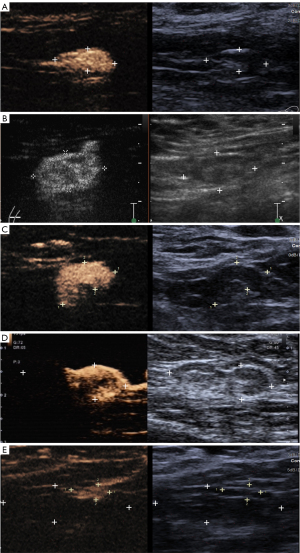
IV-CEUS sequence was divided into three types based on the orders of bubbles entering the lymph nodes: centrifugal enhancement, centripetal enhancement and diffuse enhancement. IV-CEUS mode was divided into four modes (Figure 5): Type I: homogeneous high enhancement; Type II: diffuse inhomogeneous high enhancement; Type III: no/low enhancement of lymphatic hilus, homogeneous high enhancement of cortex; Type IV: part of the cortical filling defect, low enhancement or high enhancement, the rest showed the performance of Types I, II or III. Malignant lymph node was diagnosed as long as one of the following conditions was met: (I) centripetal enhancement; (II) diffuse enhancement; (III) the enhancement mode of IV-CEUS was type IV. SLN was diagnosed as malignant when P-CEUS and/or IV-CEUS diagnosed the SLN as malignant.
Diagnostic efficacy of CEUS in SLNs
Among the 438 SLNs, 105 (23.97%) were malignant and 333 (76.03%) were benign. Besides, skip metastasis was found in this study, four non-SLNs of two patients were malignant, while the SLNs located by P-CEUS and blue dye were non-metastatic.
The pathology and CEUS of 438 SLNs are shown in Table 2. There was excellent inter-observer consistency in P-CEUS, IV-CEUS enhancement sequence, and IV-CEUS pattern. The Kappa values were 0.884, 0.868, and 0.826, respectively (P<0.001).
Table 2
| Diagnostic method | Pathology | Total | |
|---|---|---|---|
| Malignant | Benign | ||
| P-CEUS | |||
| Type I | 17 (89.47%) | 2 (10.53%) | 19 |
| Type II | 39 (88.64%) | 5 (11.36%) | 44 |
| Type III | 23 (95.83%) | 1 (4.17%) | 24 |
| Type IV | 15 (6.88%) | 203 (93.12%) | 218 |
| Type V | 3 (18.75%) | 13 (81.25%) | 16 |
| Type VIa | 5 (7.58%) | 61 (92.42%) | 66 |
| Type VIb | 1 (2.56%) | 38 (97.44%) | 39 |
| Type VIc | 2 (16.67%) | 10 (83.33%) | 12 |
| IV-CEUS enhancement sequence | |||
| Centrifugal | 34 (9.37%) | 329 (90.63%) | 363 |
| Centripetal | 13 (100.00%) | 0 (0.00%) | 13 |
| Diffuse | 58 (93.55%) | 4 (6.45%) | 62 |
| IV-CEUS pattern | |||
| Type I | 68 (20.67%) | 261 (79.33%) | 329 |
| Type II | 9 (47.37%) | 10 (52.63%) | 19 |
| Type III | 14 (18.67%) | 61 (81.33%) | 75 |
| Type IV | 14 (93.33%) | 1 (6.67%) | 15 |
| IV-CEUS diagnosis | |||
| Malignant | 73 (93.59%) | 5 (6.41%) | 78 |
| Benign | 32 (8.89%) | 328 (91.11%) | 360 |
| Combined CEUS | |||
| Malignant | 86 (90.53%) | 9 (9.47%) | 95 |
| Benign | 19 (5.54%) | 324 (94.46%) | 343 |
| Total | 105 | 333 | 438 |
Values are n or n (%). Combined CEUS, combined percutaneous and intravenous contrast-enhanced ultrasound; SLN, sentinel lymph node; P-CEUS, percutaneous contrast-enhanced ultrasound; IV-CEUS, intravenous contrast-enhanced ultrasound.
The correspondence of P-CEUs and IV-CEUS of 438 SLNs are shown in Table 3. The McNemar test results showed that both methods were consistent in diagnosis (P=0.108), Kappa =0.812, P<0.001, indicating that both methods had good consistency in diagnosis results. Out of a total of 371 axillae, 261 axillae were free of lymph node metastases, of which 254 (97.32%) were negative for correctly diagnosed lymph node metastases, indicating that preoperative P-CEUS and IV-CEUS might potentially prevent unnecessary SLNB in patients without ALN metastasis.
Table 3
| P-CEUS | IV-CEUS | Total | |
|---|---|---|---|
| Malignant | Benign | ||
| Malignant | 69 | 17 | 86 |
| Benign | 8 | 344 | 352 |
| Total | 77 | 361 | 438 |
P-CEUS, percutaneous contrast-enhanced ultrasound; IV-CEUS, intravenous contrast-enhanced ultrasound; SLN, sentinel lymph node.
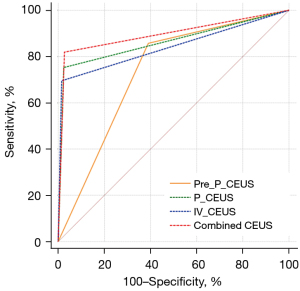
The diagnostic performances of the previous classification of P-CEUS, P-CEUS, IV-CEUS and combined CEUS were compared (Table 4). The ROC curves of different diagnostic methods are shown in Figure 6. DeLong test results showed that the diagnostic performance of P-CEUS, IV-CEUS and combined CEUS was better than the previous classification of P-CEUS, respectively. There was no statistical difference in diagnostic performance between P-CEUS and IV-CEUS. The combined CEUS showed the best diagnostic performance. Surprisingly, the new combined CEUS pattern significantly increased the specificity and the accuracy than the previous pattern, indicating that the structural characteristics of lymph node had significance in diagnosis.
Table 4
| Diagnostic method | Sensitivity | Specificity | Positive predictive value | Negative predictive value | Accuracy | AUC (95% CI) |
|---|---|---|---|---|---|---|
| Previous classification of P-CEUS | 85.71% | 60.96% | 40.91% | 93.12% | 66.89% | 0.733 (0.689-0.774) |
| P-CEUS | 75.24% | 97.60% | 90.80% | 92.59% | 92.24% | 0.864 (0.828-0.895) |
| IV-CEUS | 69.52% | 98.50% | 93.59% | 91.11% | 91.55% | 0.840 (0.802-0.873) |
| Combined CEUS | 81.90% | 97.30% | 90.53% | 94.46% | 93.61% | 0.896 (0.864-0.923) |
SLN, sentinel lymph node; AUC, area under curve; CI, confidence interval; P-CEUS, percutaneous contrast-enhanced ultrasound; IV-CEUS, intravenous contrast-enhanced ultrasound; Combined CEUS, combined percutaneous and intravenous contrast-enhanced ultrasound.
Discussion
Accurate localization and assessment of SLN properties and ALN burden are critical for treatment decision-making and prognostic assessment in breast cancer patients. Failure of intraoperative SLN mapping indicates a significantly increased risk of breast cancer metastasis to the axillary lymphatic system (22,23). Different blue agent, injection volume, injection sites (injecting peri-tumorally, subareolarly, intradermally, subdermally) and waiting time might result in variations in the SLN detection rate of the blue dye method (11). When P-CEUS was performed, the injection points of contrast agent were mostly at 3, 6, 9, and 12 of the areola with SLN detection rate of 95.7–98.2% (16,17,19,20), or in the upper outer quadrant of the areola with SLN detection rate of 100% (24). In this study, only a single-point injection of contrast agent in the upper quadrant outside the areola region obtained a SLN detection rate of 95.42% with 17 cases failed to track SLNs. The lymphatic drainage pathway could be visualized with only one injection, which could reduce the patients’ pain and simplify the operation process. Discontinuous lymphatic channel and non-enhanced SLN on CEUS might be a sign of SLN metastasis, in which case SLNB is not recommended (25). The failure to trace SLN in 17 cases might be related to stenosis and obstruction of the lymphatic channel. In this study, more SLNs were traced by blue staining than CEUS, which might be due to smaller tracer molecules and longer waiting time after injection. It is also difficult to distinguish SLNs from closely adjacent non-SLNs which could take up the blue dye, resulting in unnecessary resection of extensive lymph nodes (26,27). In this study, two patients had metastasis to four non-SLNs and non-metastatic SLNs (located by P-CEUS and blue staining). It was considered as skip metastasis or SLN connected to another lymphatic channel which was ignored in P-CEUS. This was one of the reasons for the false negative diagnosis of SLNs by P-CEUS.
P-CEUS could help diagnose the status of SLNs while locating SLNs (16-19). In previous studies, P-CEUS enhancement modes of SLNs were divided into three types, and there were great differences in diagnostic efficiency among them (16-19). In this study, the P-CEUS enhancement pattern of SLN was combined with corresponding structural characteristics and a total of six P-CEUS types was classified based on the variety of histological and pathological manifestations of SLNs. In previous studies, the P-CEUS patterns of SLN were often divided into three types: homogeneous enhancement, heterogeneous enhancement, and no enhancement (16-19). Where, homogeneous enhancement SLN was deemed as benign, and the latter two were diagnosed as malignant. In this study, it was also found that 93.12% of homogeneously enhanced SLNs were benign lymph nodes, and 95.83% of non-enhanced SLNs were malignant lymph nodes. However, in this study, the heterogeneously enhanced SLNs were divided into types I–II and types V–VI. Where, the P-CEUS manifestations of Type I–II were diagnosed as malignant SLN, and the diagnosis of Type V–VI was diagnosed as benign SLN. The results in this study showed that 88.24% of Type I and 85.71% of Type II SLN were malignant by pathology; 88.89% of Type V, 91.07% of Type VIa, 95.83% of Type VIb and 100.00% of Type VIc SLN were benign by pathology. Therefore, subdivision of SLNs showing heterogeneous enhancement by P-CEUS could help to more accurately diagnose the nature of SLNs. Compared with the previous three-type classification, the large gap in diagnostic performance might be related to differences between different observers and different populations included in the study.
Benign lymph nodes with heterogeneous enhancement on P-CEUS might be associated with intralymphatic fibrofatty tissues or might be due to relatively little contrast media access to sentinel nodes. Human lymph nodes begin to degenerate after puberty, with signs of adipocyte accumulation and fibrosis in degenerative lymph nodes (28). In conventional ultrasound, the involutional lymph nodes are slightly hyperechoic fatty, fibrous connective tissue near the lymphatic hilum, and hypoechoic lymphoid tissue near the cortex. For lymph nodes with only partial cortical enhancement, i.e., Type I and Type VIc on P-CEUS, it is necessary to distinguish whether the lymph node cortex is unevenly thickened. Malignant SLN manifested as P-CEUS Type I–III might be related to cancer cells invading lymph nodes; the tumor tissue continues to grow, and gradually occupies the SLN. Tumor cells can enter the lymphatics using a chemokine gradient as a guide, either through the intercellular space between endothelial cell junctions, or possibly by inducing larger discontinuities in the endothelial cell layer, into the lymphatics, and through the afferent lymphatics lymph nodes (29,30). Cancer cells first seed in the medulla and/or marginal sinus of lymph nodes through the afferent lymphatic vessels to form micrometastases with a diameter of less than 2 mm (31). Micrometastases are more difficult to be identified than macrometastases when performing P-CEUS (32). This is one of the reasons for false negatives. Due to the invasive procedure performed on the patient, reactive hyperplasia of axillary lymph nodes might be resulted, resulting in thickening of the nodal cortex. P-CEUS at this time could help differentiate cortical thickening from tumor growth in the malignant SLNs.
P-CEUS could reflect the lymphatic drainage of lymph nodes, while IV-CEUS could reflect the blood supply of SLN. When lymph node metastasis occurs, the metastatic tumor tissue destroys the normal lymphatic drainage pathway, and its blood supply might be different from that of normal lymphoid tissue according to the pathological type and progression of the disease. The blood supply of early metastatic lymph nodes dose not change significantly, and the diagnostic sensitivity is poor. Different from previous study (20), this study presented a new classification of IV-CEUS enhancement order and patterns for SLNs. For malignant lymph nodes, the IV-CEUS enhancement sequence of lymph nodes was non-centrifugal due to the presence of blood supply to the metastatic tumor tissue. In Type IV SLNs, the enhancement degree of part of the cortex was inconsistent with the surrounding cortex, and the blood supply of the metastatic tumor tissue was considered as being inconsistent with the surrounding normal lymph node tissue. Combining P-CEUS and IV-CEUS, while maintaining high specificity, positive predictive value, negative predictive value and accuracy, the sensitivity could reach 81.90%. Both types of angiography could provide effective information for accurate diagnosis of SLN properties from the two aspects of lymphatic drainage and blood supply characteristics.
This study was a single-center study, which might have selection bias. When the SLN is too small, it is more difficult to accurately judge the CEUS classification, but the classification criteria for CEUS enhancement patterns of SLNs of different sizes were consistent. In addition to the possibility of skip metastasis, the reasons for false negatives in the study included the very small size of metastases in malignant lymph nodes and overlapping enhancement patterns of benign and malignant lymph nodes. Therefore, how to more effectively diagnose breast cancer SLN remains to be further studied.
Conclusions
The new classification of the P-CEUS and IV-CEUS features of SLNs was performed based on structural characteristics of lymph nodes. Compared with the previous classification method of P-CEUS, the new classification method has higher diagnostic performance. The combination of P-CEUS and IV-CEUS is helpful to further improve the diagnostic performance of SLN.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-23-1210/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1210/coif). All authors report that this work was supported by the Major Research Plan of the National Natural Science Foundation of China (grant number: 92059201) and postdoctoral research foundation of China (grant number: 2022M723619). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the First Affiliated Hospital of Sun Yat-sen University [(2020)316] and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Chang JM, Leung JWT, Moy L, Ha SM, Moon WK. Axillary Nodal Evaluation in Breast Cancer: State of the Art. Radiology 2020;295:500-15. [Crossref] [PubMed]
- Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Costantino JP, Ashikaga T, Weaver DL, Mamounas EP, Jalovec LM, Frazier TG, Noyes RD, Robidoux A, Scarth HM, Wolmark N. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol 2010;11:927-33. [Crossref] [PubMed]
- Leong SP, Zager JS. Future perspectives: cancer metastases. Clin Exp Metastasis 2018;35:559-61. [Crossref] [PubMed]
- Giuliano AE, Ballman KV, McCall L, Beitsch PD, Brennan MB, Kelemen PR, Ollila DW, Hansen NM, Whitworth PW, Blumencranz PW, Leitch AM, Saha S, Hunt KK, Morrow M. Effect of Axillary Dissection vs No Axillary Dissection on 10-Year Overall Survival Among Women With Invasive Breast Cancer and Sentinel Node Metastasis: The ACOSOG Z0011 (Alliance) Randomized Clinical Trial. JAMA 2017;318:918-26. [Crossref] [PubMed]
- Galimberti V, Cole BF, Viale G, Veronesi P, Vicini E, Intra M, Mazzarol G, Massarut S, Zgajnar J, Taffurelli M, Littlejohn D, Knauer M, Tondini C, Di Leo A, Colleoni M, Regan MM, Coates AS, Gelber RD, Goldhirsch A. International Breast Cancer Study Group Trial 23-01. Axillary dissection versus no axillary dissection in patients with breast cancer and sentinel-node micrometastases (IBCSG 23-01): 10-year follow-up of a randomised, controlled phase 3 trial. Lancet Oncol 2018;19:1385-93.
- Gradishar WJ, Anderson BO, Balassanian R, Blair SL, Burstein HJ, Cyr A, et al. Breast Cancer, Version 4.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2018;16:310-20. [Crossref] [PubMed]
- Lucci A, McCall LM, Beitsch PD, Whitworth PW, Reintgen DS, Blumencranz PW, Leitch AM, Saha S, Hunt KK, Giuliano AEAmerican College of Surgeons Oncology Group. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group Trial Z0011. J Clin Oncol 2007;25:3657-63. [Crossref] [PubMed]
- Veronesi U, Paganelli G, Viale G, Luini A, Zurrida S, Galimberti V, Intra M, Veronesi P, Robertson C, Maisonneuve P, Renne G, De Cicco C, De Lucia F, Gennari R. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med 2003;349:546-53. [Crossref] [PubMed]
- Kuehn T, Klauss W, Darsow M, Regele S, Flock F, Maiterth C, Dahlbender R, Wendt I, Kreienberg R. Long-term morbidity following axillary dissection in breast cancer patients--clinical assessment, significance for life quality and the impact of demographic, oncologic and therapeutic factors. Breast Cancer Res Treat 2000;64:275-86. [Crossref] [PubMed]
- Peek MC, Charalampoudis P, Anninga B, Baker R, Douek M. Blue dye for identification of sentinel nodes in breast cancer and malignant melanoma: a systematic review and meta-analysis. Future Oncol 2017;13:455-67. [Crossref] [PubMed]
- Ahmed M, Purushotham AD, Douek M. Novel techniques for sentinel lymph node biopsy in breast cancer: a systematic review. Lancet Oncol 2014;15:e351-62. [Crossref] [PubMed]
- Fleissig A, Fallowfield LJ, Langridge CI, Johnson L, Newcombe RG, Dixon JM, Kissin M, Mansel RE. Post-operative arm morbidity and quality of life. Results of the ALMANAC randomised trial comparing sentinel node biopsy with standard axillary treatment in the management of patients with early breast cancer. Breast Cancer Res Treat 2006;95:279-93. [Crossref] [PubMed]
- Cui Q, Dai L, Li J, Xue J. Accuracy of CEUS-guided sentinel lymph node biopsy in early-stage breast cancer: a study review and meta-analysis. World J Surg Oncol 2020;18:112. [Crossref] [PubMed]
- Niu Z, Xiao M, Ma L, Qin J, Li W, Zhang J, Zhu Q, Jiang Y. The value of contrast-enhanced ultrasound enhancement patterns for the diagnosis of sentinel lymph node status in breast cancer: systematic review and meta-analysis. Quant Imaging Med Surg 2022;12:936-48. [Crossref] [PubMed]
- Li J, Lu M, Cheng X, Hu Z, Li H, Wang H, Jiang J, Li T, Zhang Z, Zhao C, Ma Y, Tan B, Liu J, Yu Y. How Pre-operative Sentinel Lymph Node Contrast-Enhanced Ultrasound Helps Intra-operative Sentinel Lymph Node Biopsy in Breast Cancer: Initial Experience. Ultrasound Med Biol 2019;45:1865-73. [Crossref] [PubMed]
- Hu Z, Cheng X, Li J, Jiang J, Jiang Z, Li H, Li T, Zhang Z, Tan B, Lu M. Preliminary study of real-time three-dimensional contrast-enhanced ultrasound of sentinel lymph nodes in breast cancer. Eur Radiol 2020;30:1426-35. [Crossref] [PubMed]
- Zhao J, Zhang J, Zhu QL, Jiang YX, Sun Q, Zhou YD, Wang MQ, Meng ZL, Mao XX. The value of contrast-enhanced ultrasound for sentinel lymph node identification and characterisation in pre-operative breast cancer patients: A prospective study. Eur Radiol 2018;28:1654-61. [Crossref] [PubMed]
- Zhu Y, Jia Y, Pang W, Duan Y, Chen K, Nie F. Ultrasound contrast-enhanced patterns of sentinel lymph nodes: predictive value for nodal status and metastatic burden in early breast cancer. Quant Imaging Med Surg 2023;13:160-70. [Crossref] [PubMed]
- Zhuang L, Ming X, Liu J, Jia C, Jin Y, Wang J, Shi Q, Wu R, Jin L, Du L. Comparison of lymphatic contrast-enhanced ultrasound and intravenous contrast-enhanced ultrasound in the preoperative diagnosis of axillary sentinel lymph node metastasis in patients with breast cancer. Br J Radiol 2022;95:20210897. [Crossref] [PubMed]
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471-4.
- Nowikiewicz T, Głowacka-Mrotek I, Tarkowska M, Nowikiewicz M, Zegarski W. Failure of sentinel lymph node mapping in breast cancer patients qualified for treatment sparing axillary lymph nodes-Clinical importance and management strategy-One-center analysis. Breast J 2020;26:873-81. [Crossref] [PubMed]
- Jin L, Wang R, Zhuang L, Jin Y, Sun X, Jia C, Lin L, Shi Q, Zhang W, Du L. Evaluation of whole axillary status with lymphatic contrast-enhanced ultrasound in patients with breast cancer. Eur Radiol 2022;32:630-8. [Crossref] [PubMed]
- Zhong J, Sun DS, Wei W, Liu X, Liu J, Wu X, Zhang Y, Luo H, Li Y. Contrast-Enhanced Ultrasound-Guided Fine-Needle Aspiration for Sentinel Lymph Node Biopsy in Early-Stage Breast Cancer. Ultrasound Med Biol 2018;44:1371-8. [Crossref] [PubMed]
- Wang Y, Zhou W, Li C, Gong H, Li C, Yang N, Zha X, Chen L, Xia T, Liu X, Wang M, Ding Q. Variation of sentinel lymphatic channels (SLCs) and sentinel lymph nodes (SLNs) assessed by contrast-enhanced ultrasound (CEUS) in breast cancer patients. World J Surg Oncol 2017;15:127. [Crossref] [PubMed]
- Scherer K, Studer W, Figueiredo V, Bircher AJ. Anaphylaxis to isosulfan blue and cross-reactivity to patent blue V: case report and review of the nomenclature of vital blue dyes. Ann Allergy Asthma Immunol 2006;96:497-500. [Crossref] [PubMed]
- Sever AR, Mills P, Jones SE, Mali W, Jones PA. Sentinel node identification using microbubbles and contrast-enhanced ultrasonography. Clin Radiol 2012;67:687-94. [Crossref] [PubMed]
- Luscieti P, Hubschmid T, Cottier H, Hess MW, Sobin LH. Human lymph node morphology as a function of age and site. J Clin Pathol 1980;33:454-61. [Crossref] [PubMed]
- Alitalo A, Detmar M. Interaction of tumor cells and lymphatic vessels in cancer progression. Oncogene 2012;31:4499-508. [Crossref] [PubMed]
- Park Y, Kitahara T, Takagi R, Kato R. Does surgery for breast cancer induce angiogenesis and thus promote metastasis? Oncology 2011;81:199-205. [Crossref] [PubMed]
- Wei Y, Yu MA, Niu Y, Hao Y, Di JX, Zhao ZL, Cao XJ, Peng LL, Li Y. Combination of Lymphatic and Intravenous Contrast-Enhanced Ultrasound for Evaluation of Cervical Lymph Node Metastasis from Papillary Thyroid Carcinoma: A Preliminary Study. Ultrasound Med Biol 2021;47:252-60. [Crossref] [PubMed]
- Shimazu K, Ito T, Uji K, Miyake T, Aono T, Motomura K, Naoi Y, Shimomura A, Shimoda M, Kagara N, Kim SJ, Noguchi S. Identification of sentinel lymph nodes by contrast-enhanced ultrasonography with Sonazoid in patients with breast cancer: a feasibility study in three hospitals. Cancer Med 2017;6:1915-22. [Crossref] [PubMed]


