
The correlation between self-Hounsfield units and adjacent vertebral fracture after percutaneous vertebral augmentation: a retrospective cohort study
Introduction
In 2019, there were 8.6 million cases of vertebral fractures worldwide, representing a 38% increase compared to 1990. Among these cases, 1.4 million were attributed to osteoporosis vertebral compressive fractures (OVCF) (1). As the global population continues to grow and age, this issue is becoming increasingly significant, resulting in low back pain that affects the quality of life and can even pose life-threatening risks (2,3). Percutaneous vertebral augmentation (PVA), encompassing procedures such as percutaneous vertebroplasty (PVP) and percutaneous kyphoplasty (PKP), is commonly employed to treat OVCF. It is well-established that PVA procedures offer immediate pain relief, early ambulation, and the correction of local kyphosis. However, a notable complication following PVA is an adjacent vertebral fracture (AVF), which has a reported incidence rate of 3–52%, according to the literature (4-7). AVF can lead to recurrent pain and reduced mobility and often necessitates a second surgical intervention. The most critical risk factors associated with AVF after PVA are decreased bone mineral density (BMD) and intervertebral leakage (5). Evaluating BMD and implementing anti-osteoporosis treatment, as well as preventing intervertebral leakage, are essential strategies for mitigating the risk of AVF (3,6,7). Dual-energy X-ray absorptiometry (DXA) measurement of the lumbar spine and hip is considered the gold standard for assessing BMD (8). However, DXA may overestimate BMD due to its planar projection techniques, which measure the density of all mineral components along the X-ray projection paths, including vertebral bodies, anterior/posterior structures, and other structures susceptible to interference, such as vertebral osteophytes, calcified vessel walls, and patient-specific body shapes (9). Additionally, DXA’s limited adoption in clinical practice is influenced by the need for additional examinations, increased costs, and radiation exposure. A study by Leslie et al. (10) revealed that only 8.9% of patients who experienced fragility fractures in 2007/2008 underwent DXA examinations.
In 2011, Pickhardt et al. (11) introduced the use of computed tomography (CT) trabecular attenuation, expressed in Hounsfield units (HU), to assess bone quality. Their work included diagnosing osteoporosis by measuring the first lumbar vertebra (L1) HU (≤110 HU) with high sensitivity and specificity. Subsequent studies have substantiated a significant correlation between vertebral HU and BMD and T values measured by DXA, establishing HU as an effective tool for assessing bone quality and diagnosing osteoporosis (12-14).
Zhang et al. (15) reported a substantial increase in the risk of AVF following PVA when CT attenuation of L1 was ≤95 HU. Given that different parts of the vertebral body endure varying stresses and ranges of motion, there may be variations in vertebral HU (16,17). In theory, utilizing HU measurements of the adjacent vertebrae themselves may offer greater accuracy in predicting AVF compared to focusing solely on the L1 vertebra. Yamaura et al. (18) discovered that the adjacent vertebral HU of the fractured vertebra could predict the occurrence of AVF after conservative treatment. Notably, the mean HU of the adjacent vertebra with a fracture was 57.3 HU, which was significantly lower than that of the control group. Nevertheless, there is limited research on the relationship between the HU of the fractured adjacent vertebra (referred to as self-HU) and AVF following PVA, especially in cases involving intervertebral leakage. This study sought to explore the risk factors associated with AVF and analyze the relationship between AVF and the Hounsfield units of adjacent vertebrae (self-HU) following PVA. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1233/rc).
Methods
This study adhered to the ethical principles of the Declaration of Helsinki (as revised in 2013) and received approval from the Ethics Committee of Xuzhou Central Hospital (No. XZXY-LK-20200301-041) and the requirement for individual consent for this retrospective analysis was waived. This retrospective cohort study included consecutively enrolled OVCF patients who sought treatment for PVA at Xuzhou Central Hospital between 1 January 2016 and 31 December 2019.
The inclusion criteria were as follows: (I) patients aged 55 years or older; (II) patients with a confirmed single-level osteoporotic vertebral compression fracture, as verified by medical history, physical examination, and magnetic resonance imaging (MRI); (III) patients treated with PVP or PKP; (IV) patients who underwent regular follow-up for at least 36 months.
The exclusion criteria were as follows: (I) patients with a history of prior surgery on the affected vertebra or adjacent vertebrae; (II) patients with 2 or more consecutive vertebral compression fractures; (III) pathological fractures caused by tumors, tuberculosis, and so on; (IV) patients lacking preoperative CT examinations or those for whom MRI examinations were unavailable when pain recurred during follow-up.
Data collection
Clinical and imaging data were documented, encompassing sex, age, body mass index (BMI), comorbidities, prior fractures, fracture location (categorized into thoracolumbar segment: T11–L2, and non-thoracolumbar segment: T5–T10 and L3–L5), surgical procedures (PVP or PKP), presence of intervertebral leakage, self-HU values, and the follow-up duration.
Grouping
Patients were categorized into the AVF group and the no-AVF group based on the presence or absence of AVF during follow-up. Additionally, patients in the AVF group were further divided into the leakage group and the no-leakage group according to the incidence of intervertebral leakage.
Definition of AVF
In patients experiencing new-onset low back pain post-PVA during follow-up or displaying a substantial decrease in the height of adjacent vertebrae on X-rays, AVF was confirmed by MRI findings showing T2 hyperintensity and short tau inversion recovery (STIR) fat-suppression image hyperintensity in the adjacent vertebra. This indicated a fresh compression fracture, excluding remote vertebral fractures and re-collapse of cemented vertebrae.
CT value measurement
All patients underwent preoperative thoracic or lumbar CT scans with sagittal reconstruction (Light Speed VCT; GE HealthCare, Chicago, IL, USA). The scanning parameters included a tube voltage of 120 kV, tube current of 355 mA, slice thickness of 5 mm, interslice distance of 5 mm, bone window width of 2,000 HU, and window level of 350 HU. CT trabecular attenuation of the vertebral body was measured in HU using the Picture Archiving and Communication System (PACS system). A middle cross-sectional plane of the vertebral body was selected along with the sagittal plane, avoiding heterogeneous structures such as cortical bone, posterior plexus, and bone islands. An oval region of interest (ROI) was placed as large as possible, and the PACS software automatically measured the CT trabecular attenuation of the vertebral cancellous bone, defined as the HU value in the ROI (11).
Statistical methods
Statistical analysis was carried out using SPSS 26.0 (IBM Corp., Armonk, NY, USA). Continuous variables were presented as means and standard deviations, with comparisons between groups analyzed using 2 independent samples t-tests. Categorical variables were expressed as numbers and frequencies, with chi-square or Fisher’s exact tests employed as appropriate. A 2-sided P value <0.05 was considered statistically significant.
Age, BMI, fracture location, prior fractures, self-HU values, and intervertebral leakage were considered variables and subjected to logistic regression analysis. Variables with a P value of less than 0.1 were included in a multivariate logistic regression analysis to identify the risk factors for AVF.
Fracture-free time was calculated from the start of surgery to the occurrence of AVF during follow-up. Subgroup analysis was conducted within the AVF group, comparing the leakage group and the no-leakage group. Kaplan-Meier curves were plotted to assess the impact of intervertebral leakage on AVF using a log-rank test.
Results
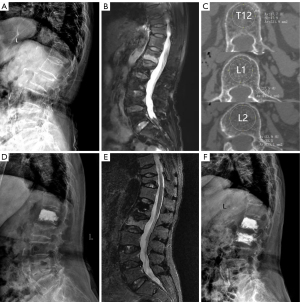
A total of 741 OVCF patients were admitted to the hospital and underwent PVA between 1 January 2016 and 31 December 2019. Out of these patients, 460 met the inclusion and exclusion criteria and were included in this study, as depicted in Figure 1 (flowchart of patient selection). All cases included in this study were Han Chinese. After an average follow-up period of 50.9 months (ranging from 37 to 83 months), 82 patients (17.83%) developed AVF and were categorized into the AVF group. Among them, there were 31 males and 51 females, with a mean age of 71.90±8.01 years (range, 55 to 88 years). The onset of AVF was divided into 4 time periods: 0 to 12 months (period 1), 13 to 24 months (period 2), 25 to 36 months (period 3), and over 36 months (period 4). During period 1, 15 cases were diagnosed with AVF (18.29%); period 2 had 28 cases (34.15%); period 3 had 17 cases (20.73%); and period 4 had 22 cases (26.83%). The no-AVF group comprised 378 patients who did not develop AVF. This group consisted of 138 males and 240 females, with a mean age of 72.50±8.95 years (range, 55 to 95 years). The baseline data for patients in both groups are presented in Table 1. A typical case in the AVF group is illustrated in Figure 2.
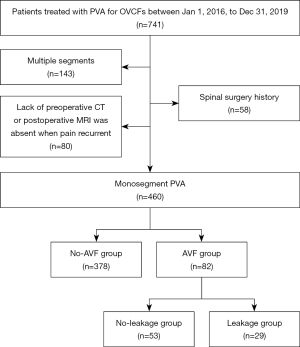
Table 1
| Characteristic | No-AVF group (n=378) | AVF group (n=82) | P value |
|---|---|---|---|
| Age (years) | 72.50±8.95 | 71.90±8.01 | 0.572 |
| Sex (female) | 240 (63.5) | 51 (62.2) | 0.825 |
| Body mass index (kg/m2) | 22.71±3.23 | 23.0±2.74 | 0.439 |
| Comorbidity | 0.418 | ||
| None | 175 (46.3) | 42 (51.2) | |
| ≥1 | 203 (53.7) | 40 (48.8) | |
| Prior fracture | 77 (20.4) | 21 (25.6) | 0.294 |
| Adjacent vertebra HU | 77.76±19.95 | 67.0±19.55 | <0.001 |
| Fractured location | 0.003 | ||
| Thoracolumbar segment (T11–L2) | 204 (54.0) | 59 (72.0) | |
| Non-thoracolumbar segment (T5–T10, L3–L5) | 174 (46.0) | 23 (28.0) | |
| Surgical procedure | 0.096 | ||
| PVP | 118 (31.2) | 18 (22.0) | |
| PKP | 260 (68.8) | 64 (78.0) | |
| Intervertebral leakage | 57 (15.08) | 29 (35.37) | <0.001 |
| Follow-up duration (months) | 51.17±9.85 | 49.38±9.98 | 0.138 |
Data are presented as mean ± standard deviation or n (%). OVCF, osteoporosis vertebral compressive fracture; PVA, percutaneous vertebral augmentation; AVF, adjacent vertebral fracture; HU, Hounsfield units; PVP, percutaneous vertebroplasty; PKP, percutaneous kyphoplasty.
Univariate logistic regression analysis revealed that lower adjacent vertebral HU [odds ratio (OR) =0.972, 95% confidence interval (CI): 0.959–0.985, P<0.001], thoracolumbar segment (OR =0.457, 95% CI: 0.271–0.771, P=0.003), and intervertebral leakage (OR =3.081, 95% CI: 1.808–5.252, P<0.001) were associated with AVF after PVA. Subsequently, variables with a P value less than 0.1 were incorporated in the multivariate logistic regression analysis, which showed that lower adjacent vertebral HU (OR =0.972, 95% CI: 0.959–0.985, P<0.001) and intervertebral leakage (OR =2.618, 95% CI: 1.415–4.844, P=0.002) were risk factors for AVF following PVA (Table 2).
Table 2
| Variable | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | ||
| Age (years) | 0.992 | 0.965–1.020 | 0.571 | – | – | – | |
| Sex (female) | 1.003 | 0.611–1.647 | 0.989 | – | – | – | |
| Body mass index | 1.030 | 0.955–1.111 | 0.438 | – | – | – | |
| Adjacent vertebral HU | 0.972 | 0.960–0.985 | <0.001* | 0.972 | 0.959–0.985 | <0.001 | |
| Thoracolumbar segment | 0.457 | 0.271–0.771 | 0.003* | 0.416 | 0.152–1.137 | 0.087 | |
| Kyphoplasty | 0.620 | 0.352–1.092 | 0.098* | 1.903 | 0.664–5.455 | 0.231 | |
| Intervertebral leakage | 3.081 | 1.808–5.252 | <0.001* | 2.618 | 1.415–4.844 | 0.002 | |
*, variables with P<0.1 were included in multivariate analysis. AVF, adjacent vertebral fracture; OR, odd ratio; CI, confidence interval; HU, Hounsfield units.
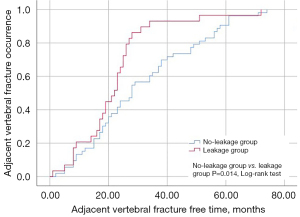
Within the AVF group, 29 patients (35.37%) presented with intervertebral leakage and were classified into the leakage group, whereas 53 patients (64.63%) without intervertebral leakage were placed in the no-leakage group. The leakage group exhibited a shorter time to AVF (22.07±13.83 vs. 31.42±18.73, P=0.021) and higher self-HU values (78.05±16.41 vs. 64.23±20.49, P=0.002) compared to the no-leakage group (Table 3). Kaplan-Meier curves revealed that the fracture-free time was shorter in the leakage group compared to the no-leakage group (log-rank test, P=0.014) (Figure 3).
Table 3
| Variable | Leakage group | No-leakage group | 95% CI | P value |
|---|---|---|---|---|
| AVF occurrence time (months) | 22.07±13.83 | 31.42±18.73 | 1.45–17.24 | 0.021 |
| Self-HU | 78.05±16.41 | 64.23±20.49 | −22.09 to −5.01 | 0.002 |
Data are presented as mean ± standard deviation. PVA, percutaneous vertebral augmentation; AVF, adjacent vertebral fracture; HU, Hounsfield units; CI, confidence interval.
Discussion
The results of the present study revealed an incidence rate of 17.83% (82/460) for AVF after PVA in patients with OVCF. Notably, lower self-HU and the presence of intervertebral leakage were identified as risk factors for the development of AVF following PVA. Moreover, in the presence of intervertebral leakage, higher self-HU values were associated with a shorter time to AVF, consistent with the literature (3,6,19,20).
The mechanisms underlying AVF are multifaceted, with some researchers proposing it as a natural progression of osteoporosis (21). Nevertheless, most studies suggest a strong association with osteoporosis and alterations in the local mechanical environment following the introduction of bone cement into the vertebra (22-25). Liu et al. (22) and Movrin and Komadina (23) conducted analyses of AVF risk factors and discovered that lower vertebral BMD, intervertebral leakage, steroid use, excessive vertebral body restoration, and fractures located in the thoracolumbar region were all contributors to AVF.
Since Pickhardt et al. (11) introduced the use of vertebral HU for assessing bone mass in 2011, research on vertebral HU in spinal degenerative diseases and osteoporosis has steadily expanded (12-18,26-30). Schreiber et al. (29) found that HU values obtained from opportunistic CT scans correlated with T-scores and compressive strengths based on osseous models. Their findings indicated that the mean lumbar HU value for normal bone density, osteopenia, and osteoporosis was 133.0, 100.8, and 78.5, respectively, consistent with the results of our study. Kurra et al. (26) predicted proximal junctional kyphosis following adult deformity surgery using local vertebral HU and observed that the HU of the upper instrumented vertebrae (UIV) and 1 level proximally (UIV + 1) was significantly lower in cases with proximal junctional fractures compared to the control group. They concluded that lower HU in UIV and UIV + 1 might contribute to proximal junctional fractures, recommending hooks at proximal vertebrae for patients with other fracture risk factors to reduce the incidence of proximal junctional fractures. St Jeor et al. (27) investigated risk factors for osteoporosis-related complications after lumbar fusion and found that both lower DXA-T scores and lower vertebral HU were associated with complications, with lower vertebral HU being an independent risk factor for osteoporosis-related complications.
Hiwatashi et al. (28) observed that most AVFs occurred within 1 year after surgery, particularly within the first 6 months. In contrast, most remote fractures occurred more than 1 year after surgery. Zhang et al. (15) reported that lower L1 HU was associated with reduced subsequent vertebral fracture-free survival, with a significant increase in the risk of subsequent vertebral fractures when L1 HU was lower than 95, suggesting that PVA is a risk factor for AVF, and BMD plays a crucial role in determining the occurrence and timing of AVF. Furthermore, standardized use of anti-osteoporosis drugs after PVA can increase vertebral BMD and reduce the risk of AVF, underlining the role of bone quality in AVF prevention (31).
Considering that the stress experienced varies with vertebral position, the BMD at different levels may exhibit significant heterogeneity (32). Consequently, the HU value of the L1 vertebra can only provide insight into the bone quality of the vertebrae adjacent to the cemented vertebra. Zou et al. (30) employed S1 vertebral HU to assess osteoporosis occurrence in patients with lumbar degenerative diseases, comparing it with L1 vertebral HU measurements and DXA. Their findings indicated that the HU thresholds of S1 for osteoporosis diagnosis were 220 HU (axial HU) and 185 HU (sagittal HU), whereas the threshold for L1 was 110 HU. Using adjacent vertebra HU itself may, therefore, provide a more accurate assessment of bone quality and the risk of AVF following PVA. In the present study, the adjacent vertebral HU was significantly lower in the AVF group compared to the no-AVF group, and multivariate analysis confirmed that lower adjacent vertebral HU and the presence of intervertebral leakage were indeed risk factors for AVF, consistent with the literature (3,6,7).
Intervertebral leakage is a common complication following PVA procedures (33). Cement leakage into the intervertebral space through endplate defects or fracture fissures creates a pillar effect on the adjacent vertebrae, making them susceptible to AVF. In this study, a total of 82 cases of AVF occurred, of which 29 (35.37%) developed intervertebral leakage, mirroring findings in prior literature (34). Subgroup analysis based on the presence of intervertebral leakage was conducted within the AVF group. The results demonstrated that self-HU was higher and AVF occurred earlier in the leakage group compared to the no-leakage group, a pattern further supported by the Kaplan-Meier curves. It is highly conceivable that when intervertebral leakage is present, higher HU values in the adjacent vertebrae may contribute to AVF development. Nonetheless, further research is required to confirm this observation. Consequently, we recommend that patients with OVCF scheduled for PVA undergo CT examinations to measure adjacent vertebral HU while carefully assessing the anatomical basis for intervertebral leakage (e.g., endplate fractures and fissures). Efforts should be made to minimize intervertebral leakage in cases with lower HU values or in combination with other risk factors. In cases where intervertebral leakage does occur, all necessary precautions should be taken to minimize the risk of AVF, including anti-osteoporosis medications, trunk extension exercises, wearing a waist brace, and fall prevention. Furthermore, this study established that prophylactic vertebroplasty is an effective method for reducing the occurrence of AVF (35).
Limitations
- This study was a single-center retrospective case-control study with a relatively small sample size, necessitating larger multi-center studies to further establish the relationship between self-HU and AVF.
- The patients included in this study were all Han Chinese, so race was not analyzed as a risk factor in the article.
- The influence of anti-osteoporosis treatment and patient compliance, which are crucial factors in AVF development, were not standardized in this study, potentially affecting the results.
- In this study, only patients who experienced significant pain or a notable reduction in adjacent vertebral height received MRI examinations for AVF confirmation. As a result, AVF cases without significant pain or those with normal vertebral height may have been missed.
Conclusions
Intervertebral leakage and lower self-HU are risk factors for AVF. When intervertebral leakage is present, higher self-HU may lead to AVF.
Acknowledgments
Funding: This study was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-23-1233/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1233/coif). All authors report that this study was supported by the Innovation Project of Science and Technology of Xuzhou (No. KC21206). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Xuzhou Central Hospital (No. XZXY-LK-20200301-041) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dong Y, Peng R, Kang H, Song K, Guo Q, Zhao H, Zhu M, Zhang Y, Guan H, Li F. Global incidence, prevalence, and disability of vertebral fractures: a systematic analysis of the global burden of disease study 2019. Spine J 2022;22:857-68. [Crossref] [PubMed]
- Choi HG, Lee JK, Sim S, Kim M. Mortality and Cause of Death in Patients With Vertebral Fractures: A Longitudinal Follow-Up Study Using a National Sample Cohort. Spine (Phila Pa 1976) 2020;45:E280-7. [Crossref] [PubMed]
- Wáng YXJ. Fragility fracture prevalence among elderly Chinese is no more than half of that of elderly Caucasians. Quant Imaging Med Surg 2022;12:874-81. [Crossref] [PubMed]
- Morozumi M, Matsubara Y, Muramoto A, Morita Y, Ando K, Kobayashi K, Machino M, Ota K, Tanaka S, Kanbara S, Ito S, Ishiguro N, Imagama S. A Study of Risk Factors for Early-Onset Adjacent Vertebral Fractures After Kyphoplasty. Global Spine J 2020;10:13-20. [Crossref] [PubMed]
- Polikeit A, Nolte LP, Ferguson SJ. The effect of cement augmentation on the load transfer in an osteoporotic functional spinal unit: finite-element analysis. Spine (Phila Pa 1976) 2003;28:991-6. [Crossref] [PubMed]
- Ahn Y, Lee JH, Lee HY, Lee SH, Keem SH. Predictive factors for subsequent vertebral fracture after percutaneous vertebroplasty. J Neurosurg Spine 2008;9:129-36. [Crossref] [PubMed]
- Zhong BY, Wu CG, He SC, Zhu HD, Fang W, Chen L, Guo JH, Deng G, Zhu GY, Teng GJ. ANVCFV Score System: Assessment for Probability of New Vertebral Compression Fractures after Percutaneous Vertebroplasty in Patients with Vertebral Compression Fractures. Pain Physician 2015;18:E1047-57.
- Lewiecki EM, Gordon CM, Baim S, Leonard MB, Bishop NJ, Bianchi ML, Kalkwarf HJ, Langman CB, Plotkin H, Rauch F, Zemel BS, Binkley N, Bilezikian JP, Kendler DL, Hans DB, Silverman S. International Society for Clinical Densitometry 2007 Adult and Pediatric Official Positions. Bone 2008;43:1115-21. [Crossref] [PubMed]
- Kaiser J, Allaire B, Fein PM, Lu D, Jarraya M, Guermazi A, Demissie S, Samelson EJ, Bouxsein ML, Morgan EF. Correspondence between bone mineral density and intervertebral disc degeneration across age and sex. Arch Osteoporos 2018;13:123. [Crossref] [PubMed]
- Leslie WD, Giangregorio LM, Yogendran M, Azimaee M, Morin S, Metge C, Caetano P, Lix LM. A population-based analysis of the post-fracture care gap 1996-2008: the situation is not improving. Osteoporos Int 2012;23:1623-9. [Crossref] [PubMed]
- Pickhardt PJ, Lee LJ, del Rio AM, Lauder T, Bruce RJ, Summers RM, Pooler BD, Binkley N. Simultaneous screening for osteoporosis at CT colonography: bone mineral density assessment using MDCT attenuation techniques compared with the DXA reference standard. J Bone Miner Res 2011;26:2194-203. [Crossref] [PubMed]
- Meredith DS, Schreiber JJ, Taher F, Cammisa FP Jr, Girardi FP. Lower preoperative Hounsfield unit measurements are associated with adjacent segment fracture after spinal fusion. Spine (Phila Pa 1976) 2013;38:415-8. [Crossref] [PubMed]
- Li D, Sun C, Jiang J, Lu F, Xia X, Wang H, Zou F, Ma X. A study of screw placement to obtain the optimal pull-out resistance of lumbar pedicle screws-analysis of Hounsfield units measurements based on computed tomography. BMC Musculoskelet Disord 2022;23:124. [Crossref] [PubMed]
- Pinto EM, Neves JR, Teixeira A, Frada R, Atilano P, Oliveira F, Veigas T, Miranda A. Efficacy of Hounsfield Units Measured by Lumbar Computer Tomography on Bone Density Assessment: A Systematic Review. Spine (Phila Pa 1976) 2022;47:702-10. [Crossref] [PubMed]
- Zhang SB, Xu HW, Yi YY, Hu T, Wang SJ, Wu DS. Evaluation of the Use of CT Attenuation for the Prediction of Subsequent Vertebral Fracture in Patients with Osteoporosis. Pain Physician 2021;24:E493-500.
- Driessen JHM, van Dort MJ, Romme EAPM, Wouters EFM, Smeenk FWJM, van Rietbergen B, van den Bergh JPW, Geusens P. Associations between bone attenuation and prevalent vertebral fractures on chest CT scans differ with vertebral fracture locations. Osteoporos Int 2021;32:1869-77. [Crossref] [PubMed]
- Berger-Groch J, Thiesen DM, Ntalos D, Hennes F, Hartel MJ. Assessment of bone quality at the lumbar and sacral spine using CT scans: a retrospective feasibility study in 50 comparing CT and DXA data. Eur Spine J 2020;29:1098-104. [Crossref] [PubMed]
- Yamaura T, Maruo K, Arizumi F, Kishima K, Yoshie N, Kusukawa T, Tachibana T. Adjacent vertebral Hounsfield unit value of acute osteoporotic vertebral fracture is a risk factor for concomitant domino osteoporotic vertebral fractures. J Orthop Sci 2023;28:536-42. [Crossref] [PubMed]
- Mudano AS, Bian J, Cope JU, Curtis JR, Gross TP, Allison JJ, Kim Y, Briggs D, Melton ME, Xi J, Saag KG. Vertebroplasty and kyphoplasty are associated with an increased risk of secondary vertebral compression fractures: a population-based cohort study. Osteoporos Int 2009;20:819-26. [Crossref] [PubMed]
- Tao W, Biao W, Xingmei C, Hu Q, Jinpeng S, Yue G, Jun L. Predictive Factors for Adjacent Vertebral Fractures After Percutaneous Kyphoplasty in Patients With Osteoporotic Vertebral Compression Fracture. Pain Physician 2022;25:E725-32.
- Yang W, Yang J, Liang M. Percutaneous Vertebroplasty Does Not Increase the Incidence of New Fractures in Adjacent and Nonadjacent Vertebral Bodies. Clin Spine Surg 2019;32:E99-E106. [Crossref] [PubMed]
- Liu WG, He SC, Deng G, Guo JH, Fang W, Zhu GY, Teng GJ. Risk factors for new vertebral fractures after percutaneous vertebroplasty in patients with osteoporosis: a prospective study. J Vasc Interv Radiol 2012;23:1143-9. [Crossref] [PubMed]
- Movrin I, Vengust R, Komadina R. Adjacent vertebral fractures after percutaneous vertebral augmentation of osteoporotic vertebral compression fracture: a comparison of balloon kyphoplasty and vertebroplasty. Arch Orthop Trauma Surg 2010;130:1157-66. [Crossref] [PubMed]
- Bae JS, Park JH, Kim KJ, Kim HS, Jang IT. Analysis of Risk Factors for Secondary New Vertebral Compression Fracture Following Percutaneous Vertebroplasty in Patients with Osteoporosis. World Neurosurg 2017;99:387-94. [Crossref] [PubMed]
- Ji C, Rong Y, Wang J, Yu S, Yin G, Fan J, Tang P, Jiang D, Liu W, Gong F, Ge X, Cai W. Risk Factors for Refracture following Primary Osteoporotic Vertebral Compression Fractures. Pain Physician 2021;24:E335-40.
- Kurra S, Farhadi HF, Metkar U, Viswanathan VK, Minnema AJ, Tallarico RA, Lavelle WF. CT based bone mineral density as a predictor of proximal junctional fractures. N Am Spine Soc J 2022;11:100130. [Crossref] [PubMed]
- St Jeor JD, Jackson TJ, Xiong AE, Freedman BA, Sebastian AS, Currier BL, Fogelson JL, Bydon M, Nassr A, Elder BD. Average Lumbar Hounsfield Units Predicts Osteoporosis-Related Complications Following Lumbar Spine Fusion. Global Spine J 2022;12:851-7. [Crossref] [PubMed]
- Hiwatashi A, Yoshiura T, Yamashita K, Kamano H, Dashjamts T, Honda H. Subsequent fracture after percutaneous vertebroplasty can be predicted on preoperative multidetector row CT. AJNR Am J Neuroradiol 2009;30:1830-4. [Crossref] [PubMed]
- Schreiber JJ, Anderson PA, Rosas HG, Buchholz AL, Au AG. Hounsfield units for assessing bone mineral density and strength: a tool for osteoporosis management. J Bone Joint Surg Am 2011;93:1057-63. [Crossref] [PubMed]
- Zou D, Li W, Xu F, Du G. Use of Hounsfield units of S1 body to diagnose osteoporosis in patients with lumbar degenerative diseases. Neurosurg Focus 2019;46:E6. [Crossref] [PubMed]
- Su CH, Tu PH, Yang TC, Tseng YY. Comparison of the therapeutic effect of teriparatide with that of combined vertebroplasty with antiresorptive agents for the treatment of new-onset adjacent vertebral compression fracture after percutaneous vertebroplasty. J Spinal Disord Tech 2013;26:200-6. [Crossref] [PubMed]
- Bruno AG, Burkhart K, Allaire B, Anderson DE, Bouxsein ML. Spinal Loading Patterns From Biomechanical Modeling Explain the High Incidence of Vertebral Fractures in the Thoracolumbar Region. J Bone Miner Res 2017;32:1282-90. [Crossref] [PubMed]
- Baroud G, Nemes J, Heini P, Steffen T. Load shift of the intervertebral disc after a vertebroplasty: a finite-element study. Eur Spine J 2003;12:421-6. [Crossref] [PubMed]
- Zhan Y, Jiang J, Liao H, Tan H, Yang K. Risk Factors for Cement Leakage After Vertebroplasty or Kyphoplasty: A Meta-Analysis of Published Evidence. World Neurosurg 2017;101:633-42. [Crossref] [PubMed]
- Jiang JL, Liu YJ, Xiao B, Zhang GL, Tian W. Prophylactic vertebral augmentation in patients with intra-disc leakage after kyphoplasty. Ann Palliat Med 2021;10:5433-43. [Crossref] [PubMed]


