
Cardiac computed tomography angiography-derived pulmonary vein volumetry as a predictor for atrial fibrillation recurrence after catheter ablation
Introduction
Atrial fibrillation (AF), the most common cardiac arrhythmia in clinical practice, is associated with a higher chance of experiencing ischemic stroke, heart failure, and cardiovascular death (1). Catheter ablation (CA) has emerged as a firmly established choice for patients with symptomatic and drug-refractory AF (2,3). Cardiac computed tomographic angiography (CCTA) can be used to accurately visualize the left atrium (LA) and pulmonary vein (PV) structure, and this information can in turn be used to tailor CA procedures for AF patients (4). However, despite the use of invasive therapies, AF is a progressive condition with a prolonged tendency for recurrence.
The size of the LA has been widely acknowledged as a factor contributing to the recurrence of AF after CA (5); however, the exact cause of this failure remains unknown. The significant role of PVs, especially their muscular sleeves, in the formation of AF has been firmly established through their substantial involvement in re-entry and focal ectopic activity (6). Previous studies have suggested that the occurrence of AF is linked to the structural attributes of PVs (6,7). Unlike LA enlargement, the outcomes of AF ablation are still uncertain in terms of the prognostic implications of PV volumetry.
This study sought to examine whether PV volumetry derived from CCTA could serve as a predictive indicator for the success of the CA procedure. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1302/rc).
Methods
Study population
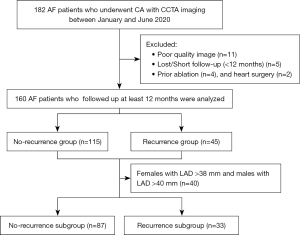
We retrospectively recruited 160 patients with AF who had previously received CCTA before undergoing AF ablation at the Department of Medical Imaging of the Second Hospital of Hebei Medical University, Shijiazhuang, China, between January and June 2020. Paroxysmal AF terminates spontaneously within a week of onset, while persistent AF persists for more than a week or requires cardioversion. Patients were eligible for inclusion in the study if they had documented paroxysmal or persistent AF. Patients were excluded from the study if they had poor quality images, had previously undergone ablation and heart surgery, and/or were lost during the follow-up period or had a short follow-up period. The flowchart for patient enrollment is shown in Figure 1. Patients’ demographic, clinical, cardiac computed tomographic (CT) imaging, and echocardiographic data were retrospectively collected and analyzed. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the Second Hospital of Hebei Medical University (No. 2019-R065). Written informed consent was obtained from the patients.
CCTA image acquisition and analysis
All the images were generated using a CT scanner with 256 slices (Brilliance iCT, Philips Healthcare, Cleveland, OH, USA) and retrospective electrocardiography (ECG) gated spiral data acquisition. Patients with a minimum heart rate of 65 beats/min were given Metoprolol (50–100 mg) approximately 30–60 minutes before the CT scan. The scan parameters were as follows: tube voltage: 100–120 kV, depending on body mass index (BMI) (threshold 25 kg/m2); tube current: 280–350 mA; detector collimation: 128×0.625 mm; gantry rotation time: 330 ms; and beam pitch: 0.18. The BMI-adapted tube voltage and tube current worked synergistically to reduce the radiation dose. A contrast material of iohexol (Omnipaque 350; 0.8–1.0 mL/kg) was injected into the ulnar vein at the rate of 4–5 mL/s to the total dosage of 60–100 mL. An automatic bolus-tracking method was used in the scan. The region of interest was selected in the ascending aorta, and the scan was automatically triggered at a threshold of 130 HU.
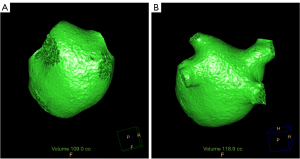
CCTA images were reconstructed for each set of 10 phases, with a 10% interval, covering an R-R interval from 5% to 95%. The images were reconstructed with a slice thickness of 0.9 mm and an interval of 0.45 mm. The raw image data were sent to the post-processing workstation (Extended Brilliance Workstation 4.6, Philips Healthcare). The left atrial volume was automatically segmented by referencing a reconstructed three-dimensional (3D) image. The location at which the PV connects with the LA was recognized as the PV ostium. The PV distal border was defined as the major first branch (8). The PV volume was segmented in a semi-automated manner by tracking the contours from the PV ostium to the major first branch of the PV. Next, the PV volume was determined by subtracting the volume of the LA from the total LA and PV volume (Figure 2). In cases of anatomical variation, the common left PV trunk portion was included in the PV volume, while the accessory vein was not included in the PV volume. Throughout the cardiac cycle, we obtained measurements for the maximum and minimum volumes of the LA, along with the total maximum and minimum volumes of the LA and PVs. The PV and LA volume index indicated the volume divided by the body surface area (BSA).
Echocardiographic examination
All the patients underwent a transthoracic echocardiogram using a standard two-dimensional echocardiogram (iE33, Philips Medical Systems, Bothell, Washington). During the examination, the systolic function of the left ventricle and the left atrial diameters (LADs) were evaluated using M-mode and Doppler echocardiography, with continuous monitoring of the ECG. According to the guidelines of the American Society of Echocardiography (9), LA enlargement is characterized by a LAD >40 mm for males and >38 mm for females.
Ablation procedure
The CA procedure was guided by a CARTO-3 navigation system (Biosense Webster, Diamond Bar, CA, USA), which integrated the CT images and used a 3D mapping technique. Pulmonary vein isolation (PVI) was achieved with the bidirectional conduction block from the atrium to the PVs confirmed by a Lasso catheter (Biosense Webster, Diamond Bar, CA, USA). In cases in which AF did not end following PVI, additional linear lesions were carried out on the mitral isthmus and roof. The procedure was performed by the same surgical team, which had performed >1,000 cases of AF ablation.
Post-ablation follow-up
The patients visited the outpatient clinic at 3, 6 and 12 months post-surgery. AF recurrence was defined as any occurrence lasting more than 30 seconds as detected by a 12-lead ECG or during repeated Holter monitoring following a three-month blank period after CA.
Statistical analyses
The statistical analyses were conducted using SPSS 21.0 (IBM Corp., Armonk, NY, USA). Continuous variables with normally distributed data are expressed as the mean ± standard deviation, while those with non-normally distributed data are expressed as the median (interquartile range). Categorical variables are expressed as the frequency and percentage. Differences between groups were examined using the chi-square test for the categorical variables, and either the Student’s t-test or Mann-Whitney-U test (as appropriate) for the continuous variables. The correlation between any two continuous variables was calculated using Spearman’s test.
To assess the factors linked to the recurrence of AF, both univariable and multivariable regression analyses were conducted. Variables that had a P value <0.1 during the univariable analysis were deemed suitable for inclusion and maintenance in the model. Statistical methods were employed to compute the odds ratios (ORs) and 95% confidence intervals (CIs). To assess collinearity, the model included computations of the variance inflation factor. Two multivariable models were established because the LA maximum volume index and LA minimum volume index had multicollinearity issues. An additional subgroup regression analysis was performed stratified by the LAD (females: LAD ≤38 mm; males: LAD ≤40 mm). Receiver operating characteristic (ROC) curves were used to assess the best cut-off values with the highest sensitivity and specificity for the PV and LA parameters for predicting AF recurrence. A (two-tailed) P value <0.05 was considered statistically significant.
Results
Patient characteristics
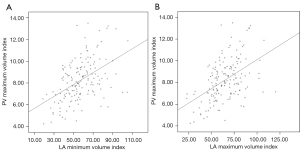
In total, 160 patients [55.6% male, 62.00 (55.25–68.00) years, 23.1% with persistent AF] who underwent ablation were enrolled in this study. The baseline characteristics of the cohort are shown in Table 1. The AF recurrence group had an elevated CHADS2 score (P=0.020). There were no differences between the recurrence and no-recurrence groups in terms of sex, age, AF type, comorbidities, such as hypertension, diabetes mellitus, and congestive heart failure, and the use of anti-arrhythmic drugs (P>0.05). In the cardiac imaging analysis, the typical PV did not different significantly between the two groups (P=0.637). The AF recurrence group showed a significant increase in the LA maximum volume (P<0.001), LA maximum volume index (P=0.002), PV maximum volume (P=0.008), PV maximum volume index (P=0.012), LA minimum volume (P=0.005), LA minimum volume index (P=0.008), and PV minimum volume (P=0.027). The PV maximum index showed a relatively low linear correlation with both the LA maximum volume index and the LA minimum volume index (r=0.473 and 0.480, respectively; P<0.01) (Figure 3).
Table 1
| Variables | All (N=160) | No recurrence (N=115) | Recurrence (N=45) | P |
|---|---|---|---|---|
| Age (y) | 62.00 [55.25, 68.00] | 62.00 [54.00, 67.00] | 61.00 [57.00, 69.00] | 0.243 |
| Male [n, (%)] | 89 (55.6) | 61 (53.0) | 28 (62.2) | 0.293 |
| Persistent AF [n, (%)] | 37 (23.1) | 24 (20.9) | 13 (28.9) | 0.279 |
| AF duration (m) | 24 (6, 48) | 12 (6, 48) | 24 (6, 54) | 0.098 |
| BMI (kg/m2) | 25.89 [23.61, 28.75] | 26.10 [23.7, 28.76] | 25.10 [23.4, 28.90] | 0.672 |
| BSA (m2) | 1.79±0.19 | 1.78±0.19 | 1.81±0.18 | 0.286 |
| Risk factors | ||||
| Hypertension [n, (%)] | 83 (51.9) | 55 (47.8) | 28 (62.2) | 0.101 |
| Diabetes mellitus [n, (%)] | 29 (18.1) | 19 (16.5) | 10 (22.2) | 0.400 |
| Coronary heart failure [n, (%)] | 18 (11.3) | 14 (12.2) | 4 (8.9) | 0.554 |
| Hyperlipidemia [n, (%)] | 25 (15.6) | 19 (16.5) | 6 (13.3) | 0.617 |
| Previous stroke/TIA [n, (%)] | 25 (15.6) | 15 (13.0) | 10 (22.2) | 0.151 |
| Coronary artery disease [n, (%)] | 19 (11.9) | 15 (13.0) | 4 (8.9) | 0.465 |
| CHA2DS2-VASc score | 3 [1, 4] | 3 [1, 4] | 3 [2, 4] | 0.193 |
| CHADS2 score | 1 [0, 2] | 1 [0, 2] | 1 [1, 2] | 0.020 |
| eGFR (mg/dL) | 92.20±13.30 | 92.50±13.70 | 91.50±12.40 | 0.674 |
| Smoke [n, (%)] | 42 (26.3) | 34 (29.6) | 8 (17.8) | 0.128 |
| Medication | ||||
| ACEI/ARB [n, (%)] | 22 (13.8) | 17 (14. 8) | 5 (11.1) | 0.544 |
| Beta-blocker [n, (%)] | 53 (33.1) | 36 (31.3) | 17 (37.8) | 0.434 |
| Class I/III anti-arrhythmic [n, (%)] | 144 (90.0) | 106 (92.2) | 38 (84.4) | 0.241 |
| Statin [n, (%)] | 106 (66.3) | 80 (69.6) | 26 (57. 8) | 0.156 |
| Echocardiographic data | ||||
| LVEF (%) | 62.50 [60.70, 67.18] | 62.50 [60.60, 67.00] | 62.60 [61.15, 68.60] | 0.199 |
| LA dimension (mm) | 36.00 [33.25, 40.00] | 36.00 [33.00, 40.00] | 37.00 [34.00, 40.50] | 0.155 |
| LV-ED dimension (mm) | 47.00 [44.00, 49.00] | 46.00 [44.00, 49.00] | 47.00 [44.50, 49.50] | 0.663 |
| EDV (mL) | 99.00 [87.25, 115.00] | 97.00 [87.00, 114.00] | 106.00 [86.50, 118.50] | 0.298 |
| IVS thickness (mm) | 10 [9, 10] | 10 [9, 10] | 10 [9, 10] | 0.642 |
| Cardiac CTA data | ||||
| Typical pulmonary vein [n, (%)] | 139 (86.9) | 99 (86.1) | 40 (88. 9) | 0.637 |
| Accessory vein [n, (%)] | 13 (8.1) | 10 (8.7) | 3 (6.7) | 0.920 |
| Common trunk vein [n, (%)] | 8 (5.0) | 6 (5.2) | 2 (4.4) | >0.999 |
| LA maximum volume (mL) | 116.56±31.49 | 111.16±28.67 | 130.36 ± 34.38 | <0.001 |
| PV maximum volume (mL) | 14.20 [12.32, 16.88] | 14.10 [12.10, 16.10] | 15. 00 [13.30, 18.75] | 0.008 |
| LA minimum volume (mL) | 100.40 [79.60, 118.35] | 99.30 [77.00, 115.70] | 108.30 [90.05, 135.45] | 0.005 |
| PV minimum volume (mL) | 14.50 [11.60, 16.37] | 14.20 [11.40, 16.20] | 14.80 [11.90, 16.75] | 0.027 |
| LA maximum volume index (mL/m2) | 65.28±16.97 | 62.71±16.05 | 71.83±17.68 | 0.002 |
| PV maximum volume index (mL/m2) | 8.11 [6.98, 9.39] | 7.91 [6.87, 9.22] | 8.44 [7.30, 9.93] | 0.012 |
| LA minimum volume index (mL/m2) | 55.93 [45.66, 67.56] | 54.17 [43.02, 64.82] | 61.89 [48.84, 76.51] | 0.008 |
| PV minimum volume index (mL/m2) | 7.98±1.95 | 7.85±1.80 | 8.30±2.29 | 0.176 |
Data are presented as the mean ± SD, median [interquartile range], or n (%). AF, atrial fibrillation; CTA, computed tomography angiography; BMI, body mass index; BSA, body surface area; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; eGFR, estimated glomerular filtration rate; TIA, transient ischemic attack; LA, left atrial; PV, pulmonary vein; LVEF, left ventricular ejection fraction; LV-ED, left ventricular end-diastole; EDV, end-diastole volume; IVS, interventricular septum.
The clinical characteristics and CCTA measurements according to the AF classification are shown in Table 2. Patients with persistent AF had a lower left ventricular ejection fraction (P=0.003), and a larger LAD, LA maximum and minimum volume index, and PV minimum volume index (P<0.05) than patients with paroxysmal AF. However, there were no significant differences in the PV maximum volume (P=0.306) and PV maximum volume index (P=0.200) between the patients with paroxysmal AF and those with persistent AF.
Table 2
| Variables | All (N=160) | Paroxysmal AF (N=123) | Persistent AF (N=37) | P |
|---|---|---|---|---|
| Age (y) | 62.00 [55.25, 68.00] | 63.00 [55.25, 69.00] | 61.00 [55.00, 66.00] | 0.875 |
| Male [n, (%)] | 71 (44.4) | 55 (44.7) | 16 (43.2) | 0.874 |
| Recurrence [n, (%)] | 45 (28.1) | 32 (26.0) | 13 (35.1) | 0.279 |
| CHADS2 score | 1 [0, 2] | 1 [0, 2] | 2 [1, 3] | 0.072 |
| CHA2DS2-VASc score | 3 [1, 4] | 3 [1, 4] | 3 [2, 4] | 0.479 |
| Echocardiographic data | ||||
| LVEF (%) | 62.50 [60.70, 67.18] | 62.80 [60.78, 68.00] | 61.50 [56.9, 63.13] | 0.003 |
| LA dimension (mm) | 36.00 [33.25, 40.00] | 35.00 [33.00, 38.00] | 40.00 [38.00, 44.00] | <0.001 |
| LV-ED dimension (mm) | 47.00 [44.00, 49.00] | 47.00 [44.00, 49.00] | 46.00 [44.50, 51.50] | 0.589 |
| EDV (mL) | 99.00 [87.25, 115.00] | 101.00 [85.00, 115.00] | 97.00 [87.50, 115.00] | 0.298 |
| IVS thickness (mm) | 10 [9, 10] | 10 [9, 10] | 10 [9, 10] | 0.642 |
| Cardiac CTA data | ||||
| LA maximum volume (mL) | 117.10 [94.30, 140.55] | 111.80 [91.30, 126.80] | 141.20 [121.15, 161.50] | <0.001 |
| PV maximum volume (mL) | 14.20 [12.33, 16.88] | 14.50 [12.10, 16.80] | 14. 20 [13.40, 18.30] | 0.306 |
| LA minimum volume (mL) | 100.40 [79.60, 118.35] | 94.60 [75.80, 106.00] | 126.70 [120.00, 149.80] | <0.001 |
| PV minimum volume (mL) | 14.26±13.99 | 13.86±3.54 | 15.59±4.13 | 0.014 |
| LA maximum volume index (mL/m2) | 65.28±16.97 | 61.20±14.25 | 71.82±18.41 | <0.001 |
| PV maximum volume index (mL/m2) | 8.25±1.91 | 8.14±1.87 | 8.60±2.04 | 0.200 |
| LA minimum volume index (mL/m2) | 57.11±16.71 | 50.59±11.95 | 78.79±10.99 | <0.001 |
| PV minimum volume index (mL/m2) | 7.98±1.95 | 7.69±1.84 | 8.9±2.06 | <0.001 |
Data are presented as the mean ± SD, median [interquartile range], or n (%). AF, atrial fibrillation; CTA, computed tomography angiography; LA, left atrial; PV, pulmonary vein; LVEF, left ventricular ejection fraction; LV-ED, left ventricular end-diastole; EDV, end-diastole volume; IVS, interventricular septum.
Late recurrent AF and geometric parameters
The results of the univariable and multivariable analyses of the clinical and CCTA parameters for recurrent AF are summarized in Table 3. After at least one year of follow up, 28.1% of the patient experienced recurrent AF. In the univariable analysis, potential factors contributing to the recurrence of AF were identified, such as the CHADS2 score, LA maximum volume index, LA minimum volume index, and PV maximum volume index. Due to collinearity, two separate multivariate models were established. In the multivariable regression analysis, the PV maximum volume index (OR: 1.244, 95% CI: 1.008–1.536, P=0.042) and LA minimum volume index (OR: 1.026, 95% CI: 1.001–1.052, P=0.038) were independently associated with AF recurrence after CA.
Table 3
| Variables | UV | MV1 | MV2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | |||
| Age (y) | 1.021 | 0.984–1.059 | 0.274 | ||||||||
| Male | 1.4586 | 0.720–2.951 | 0.295 | ||||||||
| Persistent AF | 1.540 | 0.702–3.381 | 0.281 | ||||||||
| AF duration | 1.006 | 0.997–1.015 | 0.164 | ||||||||
| CHADS2 | 1.381 | 1.054–1.811 | 0.019 | 1.287 | 0.963–1.718 | 0.088 | |||||
| LA maximum volume index | 1.034 | 1.011–1.057 | 0.003 | 1.021 | 0.996–1.046 | 0.101 | |||||
| LA minimum volume index | 1.037 | 1.014–1.060 | 0.002 | 1.026 | 1.001–1.052 | 0.038 | |||||
| PV maximum volume index | 1.364 | 1.126–1.652 | 0.002 | 1.241 | 1.006–1.532 | 0.044 | 1.244 | 1.008–1.536 | 0.042 | ||
MV model 1 excluded the LA minimum volume index variable, and MV model 2 excluded the LA maximum volume index variable. AF, atrial fibrillation; UV, univariable logistic analysis; MV, multivariable logistic analysis; OR, odds ratio; CI, confidence interval; LA, left atrial; PV, pulmonary vein.
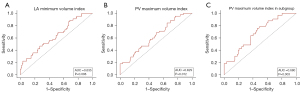
The ROC curves revealed that the PV maximum volume index threshold for predicting AF recurrence was 7.13 mL/m2, with a sensitivity of 84.4% and a specificity of 34.8% [area under the curve (AUC): 0.635, 95% CI: 0.540–0.730, P=0.008], and the LA minimum volume index predictive threshold was 46.16 mL/m2, with a sensitivity of 88.9% and a specificity of 31.3% (AUC: 0.629, 95% CI: 0.534–0.723, P=0.012) (Figure 4A,4B).
Prognostic value of the PV volume in patients without LA enlargement
We also performed a subgroup analysis of 120 patients [56.7% male, 61.50 (54.00–68.00) years, 14.2% with persistent AF, and 27.5% with recurrence] with smaller LAs based on the LAD classification (females with a LAD ≤38 mm and males with a LAD ≤40 mm). The results revealed that the PV maximum volume index predicted AF recurrence (OR: 1.443, 95% CI: 1.145–1.820, P=0.002), while the LA volume index did not (Table 4).
Table 4
| Variables | UV | MV1 | MV2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | |||
| Age (y) | 1.021 | 0.981–1.062 | 0.319 | ||||||||
| Male | 1.783 | 0.772–4.118 | 0.176 | ||||||||
| Persistent AF | 2.073 | 0.716–6.003 | 0.179 | ||||||||
| CHADS2 | 1.406 | 1.038–1.905 | 0.028 | 1.333 | 0.960–1.851 | 0.086 | 1.333 | 0.960–1.851 | 0.086 | ||
| LA maximum volume index | 1.037 | 1.009–1.066 | 0.010 | ||||||||
| LA minimum volume index | 1.040 | 1.011–1.069 | 0.006 | ||||||||
| PV maximum volume index | 1.481 | 1.176–1.867 | 0.001 | 1.443 | 1.145–1.820 | 0.002 | 1.443 | 1.145–1.820 | 0.002 | ||
| PV minimum volume index | 1.182 | 0.959–1.457 | 0.116 | ||||||||
MV model 1 excluded the LA minimum volume index variable, and MV model 2 excluded the LA maximum volume index variable. LAD, left atrial dimension; UV, univariable logistic analysis; MV, multivariable logistic analysis; OR, odds ratio; CI, confidence interval; AF, atrial fibrillation; LA, left atrial; PV, pulmonary vein.
The predictive value was analyzed in the subgroup with smaller LAs. The AUC value for the PV maximum volume index predictive of recurrent AF was 0.680 (95% CI: 0.577–0.781, P=0.003), with a sensitivity of 75.8%, specificity of 54%, and the cut-off value of the maximum AUC was 7.89 mL/m2 (Figure 4C).
Discussion
The core findings of this study are as follows: (I) a higher PV volume index and LA volume index can predict AF recurrence; and (II) the PV volume index was the sole predictor in patients without LA enlargement and showed a significantly better ability to predict the maintenance of sinus rhythm after CA in the subgroup. This study sought to evaluate the prognostic significance of PV volume by CCTA in patients undergoing CA to predict AF recurrence. Our findings suggest that PV volume may serve as an early indicator, preceding evident chamber enlargement, in the anticipation of future AF recurrences.
PV enlargement and AF
The enlargement of LA chambers and the consequent myocardial stretching play critical roles in the development of arrhythmias (10). The myocardial sleeves, which extend directly from the myocardium of the LA, play a role in causing abnormal electrical activation due to ectopic triggering activity and anisotropic conditions (11). Larger PVs with more irregular and scarred atrial myocardium might exhibit a greater occurrence of electrophysiological irregularities and an increased vulnerability to electrical reconnection (7,12). The theoretical framework for the restoration of electrical function following PVI is predicated on electro-conduction guidance. This study presented clinical evidence that ablation failure might be caused by stretch-activated PV arrhythmogenesis (13).
Influence of PV and LA size on AF recurrence after CA
The left atrial volume, measured by CT or magnetic resonance imaging (MRI), is a widely used parameter in studies on AF and is a superior measurement in terms of its accuracy and reproducibility, and prognostic power (5,14-16). The current study is consistent with prior research (5,14,15).
Numerous attempts have been undertaken to establish a connection between PV organization and arrhythmogenicity; however, the results have been inconclusive. Structural abnormalities in PVs, such as the common truck and additional veins, have been detected in approximately 18–30% of AF patients (17-19). Several research studies have reported a greater occurrence of AF in anatomical PV variants compared with normal anatomical PV, and these studies also identified PV anatomical variations as predictors of AF relapse (20,21). However, some studies have been unable to establish a connection between the structure of PVs and ablation results (22,23). In the current study, PV variations did not differ significantly between patients with and without AF recurrence.
It was recently postulated that a larger PV size was linked to a higher incidence of late recurrent AF (7,24). Previous studies have primarily focused on assessing the characteristics of PV orifice diameters and the cross-sectional area. To date, no consensus has been reached as to which PV parameters are the most strongly associated with AF recurrence. Li et al. indicated that the increased major diameter of the right inferior PV might act as a prognostic factor for the recurrence of AF (24). Similarly, Tsyganov et al. reported that a larger left inferior PV orifice was correlated with worse long-term outcomes (25). Conversely, den Uijl et al. found no relation between PV dimensions and AF recurrence (26).
According to recent studies, there is a connection between a large PV volume on CT imaging and the presence of arrhythmogenic PV triggers or AF recurrence after CA (6,8,27). It might be more advantageous to evaluate the PV volume than the PV orifice area. During the initial period of AF, increased pressure in the LA may stretch the PVs at the proximal portion beyond the visceral pericardium, which is composed of collagen fibers that resist expansion, so the contact force over the PV orifices is hardly affected by stretching and dilation than the proximal portion of the PVs (8,28). This study evaluated the efficacy of the PV maximum and minimum volume during the cardiac cycle by CCTA. PV volumes change throughout the cardiac cycle, and generally reach their maximum size at ventricular end-systole (the 45% phase) and their minimum size at end-ventricular diastole (the 75% phase) (29). Our findings revealed a significant correlation between a higher PV maximum volume index and the recurrence of AF following CA. Patients with an increased PV volume were at a greater risk of electrical reconnection and had an elevated risk of AF recurrence following ablation due to insufficient isolation of the PV and a higher occurrence of histological and electrophysiological abnormalities with arrhythmogenic traits.
Compared to the patients with paroxysmal AF, those with persistent AF had larger LA volumes; however, no such significant difference was found between patients in terms of the PV maximum volume. Thus, it appears that progression from paroxysmal to persistent AF is associated with increased LA enlargement, but the PV maximum volume remains relatively stable. In addition, our research suggests that the PV volume was the only factor in AF patients without LA enlargement and had a better predictive ability than severe chamber enlargement. This might be due to chronically elevated LA pressure, and the PV structural remodeling may occur before the enlargement of the LA chamber (30).
Limitations
This research had a number of limitations. First, PV anatomy is variable, and there is no universally accepted standard for determining the distal boundary of the PV volume measurements. A previous study discovered that the muscular sleeve, which possesses arrhythmogenic characteristics, exists in the proximal part of the PVs, about 1–3 cm from the orifice (31). Thus, we identified the PV distal border as the first major branch. Second, this study included a comparatively small group of patients with anatomical alterations of PVs; thus, further research needs to be conducted to evaluate the effects of anatomical variants.
Conclusions
The findings of this research indicate that PV enlargement (as measured by CCTA) develops in the early stage of LA structural remodeling and might have a causative role in predicting the recurrence of AF, and thus could be used to help identify at-risk patients.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-23-1302/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1302/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the Second Hospital of Hebei Medical University (No. 2019-R065). Written informed consent was obtained from the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Friberg L, Tabrizi F, Englund A. Catheter ablation for atrial fibrillation is associated with lower incidence of stroke and death: data from Swedish health registries. Eur Heart J 2016;37:2478-87. [Crossref] [PubMed]
- Nault I, Miyazaki S, Forclaz A, Wright M, Jadidi A, Jaïs P, Hocini M, Haïssaguerre M. Drugs vs. ablation for the treatment of atrial fibrillation: the evidence supporting catheter ablation. Eur Heart J 2010;31:1046-54. [Crossref] [PubMed]
- Zheng S, Zheng Y, Fang C, Xie S, Huang B, Wen K, Geng D, Zhou S. Radiofrequency catheter ablation for improving myocardial work in patients with ventricular pre-excitation. Quant Imaging Med Surg 2023;13:2660-74. [Crossref] [PubMed]
- Ohana M, Bakouboula B, Labani A, Jeung MY, El Ghannudi S, Jesel-Morel L, Roy C. Imaging before and after catheter ablation of atrial fibrillation. Diagn Interv Imaging 2015;96:1113-23. [Crossref] [PubMed]
- Njoku A, Kannabhiran M, Arora R, Reddy P, Gopinathannair R, Lakkireddy D, Dominic P. Left atrial volume predicts atrial fibrillation recurrence after radiofrequency ablation: a meta-analysis. Europace 2018;20:33-42. [Crossref] [PubMed]
- Kim S, Kim YH, Lee SH, Kim JS. Pulmonary Vein Enlargement as an Independent Predictor for New-Onset Atrial Fibrillation. J Clin Med 2020;9:401. [Crossref] [PubMed]
- Hauser TH, Essebag V, Baldessin F, McClennen S, Yeon SB, Manning WJ, Josephson ME. Prognostic value of pulmonary vein size in prediction of atrial fibrillation recurrence after pulmonary vein isolation: a cardiovascular magnetic resonance study. J Cardiovasc Magn Reson 2015;17:49. [Crossref] [PubMed]
- Kurata M, Asano T, Mori H, Mase H, Nagumo S, Wakatsuki D, Shimojima H, Ebato M, Miyazaki A, Suzuki H. Can an increase in the pulmonary vein volume measured by three dimensional computed tomography predict the presence of atrial fibrillation? J Arrhythm 2019;35:230-7. [Crossref] [PubMed]
- Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJChamber Quantification Writing Group. American Society of Echocardiography's Guidelines and Standards Committee; European Association of Echocardiography. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005;18:1440-63. [Crossref] [PubMed]
- Ravens U. Mechano-electric feedback and arrhythmias. Prog Biophys Mol Biol 2003;82:255-66. [Crossref] [PubMed]
- Walters TE, Lee G, Spence S, Larobina M, Atkinson V, Antippa P, Goldblatt J, O'Keefe M, Sanders P, Kistler PM, Kalman JM. Acute atrial stretch results in conduction slowing and complex signals at the pulmonary vein to left atrial junction: insights into the mechanism of pulmonary vein arrhythmogenesis. Circ Arrhythm Electrophysiol 2014;7:1189-97. [Crossref] [PubMed]
- Jæger KH, Edwards AG, Giles WR, Tveito A. Arrhythmogenic influence of mutations in a myocyte-based computational model of the pulmonary vein sleeve. Sci Rep 2022;12:7040. [Crossref] [PubMed]
- Gottlieb LA, Dekker LRC, Coronel R. Arrhythmia mechanism dependent pulmonary vein ablation in paroxysmal atrial fibrillation. Front Physiol 2023;14:1157338. [Crossref] [PubMed]
- Maier J, Blessberger H, Nahler A, Hrncic D, Fellner A, Reiter C, Hönig S, Schmit P, Fellner F, Lambert T, Steinwender C. Cardiac Computed Tomography-Derived Left Atrial Volume Index as a Predictor of Long-Term Success of Cryo-Ablation in Patients With Atrial Fibrillation. Am J Cardiol 2021;140:69-77. [Crossref] [PubMed]
- Nakamori S, Ngo LH, Tugal D, Manning WJ, Nezafat R. Incremental Value of Left Atrial Geometric Remodeling in Predicting Late Atrial Fibrillation Recurrence After Pulmonary Vein Isolation: A Cardiovascular Magnetic Resonance Study. J Am Heart Assoc 2018;7:e009793. [Crossref] [PubMed]
- Tian X, Wang C, Gao D, Gao BL, Li CY. Morphological changes in the orifices of the left atrial appendage and left atrium in patients with atrial fibrillation. Quant Imaging Med Surg 2022;12:5371-82. [Crossref] [PubMed]
- Chen J, Yang ZG, Xu HY, Shi K, Long QH, Guo YK. Assessments of pulmonary vein and left atrial anatomical variants in atrial fibrillation patients for catheter ablation with cardiac CT. Eur Radiol 2017;27:660-70. [Crossref] [PubMed]
- Marom EM, Herndon JE, Kim YH, McAdams HP. Variations in pulmonary venous drainage to the left atrium: implications for radiofrequency ablation. Radiology 2004;230:824-9. [Crossref] [PubMed]
- Scharf C, Sneider M, Case I, Chugh A, Lai SW, Pelosi F Jr, Knight BP, Kazerooni E, Morady F, Oral H. Anatomy of the pulmonary veins in patients with atrial fibrillation and effects of segmental ostial ablation analyzed by computed tomography. J Cardiovasc Electrophysiol 2003;14:150-5. [Crossref] [PubMed]
- Istratoaie S, Roșu R, Cismaru G, Vesa ȘC, Puiu M, Zdrenghea D, Pop D, Buzoianu AD. The Impact of Pulmonary Vein Anatomy on the Outcomes of Catheter Ablation for Atrial Fibrillation. Medicina (Kaunas) 2019;55:727. [Crossref] [PubMed]
- McLellan AJ, Ling LH, Ruggiero D, Wong MC, Walters TE, Nisbet A, Shetty AK, Azzopardi S, Taylor AJ, Morton JB, Kalman JM, Kistler PM. Pulmonary vein isolation: the impact of pulmonary venous anatomy on long-term outcome of catheter ablation for paroxysmal atrial fibrillation. Heart Rhythm 2014;11:549-56. [Crossref] [PubMed]
- Güler E, Güler GB, Demir GG, Kizilirmak F, Güneş HM, Barutçu I, Kiliçaslan F. Effect of Pulmonary Vein Anatomy and Pulmonary Vein Diameters on Outcome of Cryoballoon Catheter Ablation for Atrial Fibrillation. Pacing Clin Electrophysiol 2015;38:989-96. [Crossref] [PubMed]
- Hof I, Chilukuri K, Arbab-Zadeh A, Scherr D, Dalal D, Nazarian S, Henrikson C, Spragg D, Berger R, Marine J, Calkins H. Does left atrial volume and pulmonary venous anatomy predict the outcome of catheter ablation of atrial fibrillation? J Cardiovasc Electrophysiol 2009;20:1005-10. [Crossref] [PubMed]
- Li B, Ma H, Guo H, Liu P, Wu Y, Fan L, Cao Y, Jian Z, Sun C, Li H. Pulmonary vein parameters are similar or better predictors than left atrial diameter for paroxysmal atrial fibrillation after cryoablation. Braz J Med Biol Res 2019;52:e8446. [Crossref] [PubMed]
- Tsyganov A, Petru J, Skoda J, Sediva L, Hala P, Weichet J, Janotka M, Chovanec M, Neuzil P, Reddy VY. Anatomical predictors for successful pulmonary vein isolation using balloon-based technologies in atrial fibrillation. J Interv Card Electrophysiol 2015;44:265-71. [Crossref] [PubMed]
- den Uijl DW, Tops LF, Delgado V, Schuijf JD, Kroft LJ, de Roos A, Boersma E, Trines SA, Zeppenfeld K, Schalij MJ, Bax JJ. Effect of pulmonary vein anatomy and left atrial dimensions on outcome of circumferential radiofrequency catheter ablation for atrial fibrillation. Am J Cardiol 2011;107:243-9. [Crossref] [PubMed]
- Shimamoto K, Miura F, Shimatani Y, Nishioka K, Inoue I. Pulmonary vein volume predicts the outcome of radiofrequency catheter ablation of paroxysmal atrial fibrillation. PLoS One 2018;13:e0201199. [Crossref] [PubMed]
- Chaffanjon P, Brichon PY, Faure C, Favre JJ. Pericardial reflection around the venous aspect of the heart. Surg Radiol Anat 1997;19:17-21. [Crossref] [PubMed]
- Bowman AW, Kovács SJ. Prediction and assessment of the time-varying effective pulmonary vein area via cardiac MRI and Doppler echocardiography. Am J Physiol Heart Circ Physiol 2005;288:H280-6. [Crossref] [PubMed]
- Yagishita A. DE Oliveira S, Cakulev I, Gimbel JR, Sparano D, Manyam H, Manrique-Garcia A, Arredondo M, Mackall J, Arruda M. Correlation of Left Atrial Voltage Distribution Between Sinus Rhythm and Atrial Fibrillation: Identifying Structural Remodeling by 3-D Electroanatomic Mapping Irrespective of the Rhythm. J Cardiovasc Electrophysiol 2016;27:905-12. [Crossref] [PubMed]
- Saito T, Waki K, Becker AE. Left atrial myocardial extension onto pulmonary veins in humans: anatomic observations relevant for atrial arrhythmias. J Cardiovasc Electrophysiol 2000;11:888-94. [Crossref] [PubMed]


