Endovascular stent placement for chronic post-thrombotic symptomatic ilio-femoral venous obstructive lesions: a single-center study of safety, efficacy and quality-of-life improvement
Introduction
Deep vein thrombosis (DVT) is a significant health care and socio-economical problem, with an incidence of 1 per 1,000 per year in Caucasian population (1,2). Chronic venous conditions are mostly related to intrinsic venous obstructions and/or valvular reflux (secondary to DVT) but also to extrinsic ones (3-5). Extrinsic obstructions are secondary to malignancy or rare anatomic variants (May-Thurner syndrome) (6). Despite universal usage of anticoagulants, post-thrombotic syndrome (PTS) is the most frequent long-term complication of lower limb DVT, affecting about 23% to 60% of patients within 2 years even with an adequate treatment, major symptoms being chronic pain and oedema of the lower limb. PTS is severe in 5–10% of cases degrading the quality of life and inducing an economic burden (6-13). Proximal thrombosis, involving iliac veins, is more at risk of PTS (14-16). The frequency of this complication led to its definition as clinical entity by the European Venous Forum (17,18). So far, there is no specific medical treatment for PTS (19). The outcomes of surgical procedures remain poor leading to the first venous angioplasties in the late 90s in case of chronic obstruction or PTS (14,20). Since then, percutaneous endovascular venous recanalization techniques have shown encouraging results in terms of safety and effectiveness in the literature (2,4,6,7,16,21-28). Still, few reports analysed the impact of angioplasty on quality-of-life scores in PTS patients. In the present study, we evaluated the safety and clinical efficacy of endovascular treatment for PTS patients with chronic symptomatic ilio-femoral venous obstructive lesions and its impact on quality-of-life scores.
Methods
Study population
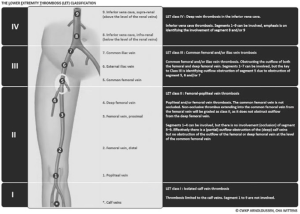
From March 2012 to April 2016, 21 PTS patients (11 females, 10 males; median age, 41 years; range, 32–60) with chronic symptomatic ilio-femoral venous obstructive lesions were included in our single-center prospective observational cohort. Occluded venous segment was evaluated according to the lower extremity thrombosis (LET) classification (29,30). Patients with chronic thrombosis (at least 28 days) (31), which were LET class II–III, III, III–IV ones (Figure 1), were referred to our Interventional Radiology Department for endovascular recanalization and venous angioplasty. PTS was diagnosed by their angiologist on the basis of clinical and duplex ultrasound (US) features. Age <18 years, non thrombotic obstructive condition, acute thrombosis, and a Villalta score ≤4 were exclusion criteria.
Villalta score and CIVIQ-20 scale assessment
Severity of PTS was evaluated before the procedure (at baseline) and at 3 months by the Villalta scale being relevant in this pathology according to the literature (17,18,31). This scale is based on clinical examination of both legs, including five symptoms (heaviness, pain, cramps, pruritus and paraesthesia) and six signs (edema, induration, hyperpigmentation, new venous ecstasies as varicose veins, redness and pain during calf compression) scored from 0 to 3. A total score of 0–4= no PTS, 5–9= mild PTS, 10–14= moderate PTS, and 15 or more = severe PTS (32). Pain and edema was also analyzed separately.
The Chronic Venous Insufficiency Questionnaire in 20 questions (CIVIQ-20) was used to evaluate the quality of life of each patient, at baseline and after the procedure at 3 months. Patients were asked to complete the CIVIQ-20 consisting in 20 questions designed to assess four quality-of-life dimensions: “pain” (four items), “physical” (four items), “psychological” (nine items) and “social” (three items). Items on the CIVIQ-20 scale were scored from 1 to 5. A lower score corresponded to greater patient comfort. A score of 20 corresponded to a normal quality of life and a score of 100 corresponded to a maximal quality-of-life alteration. Absolute scores could be converted into an index ranging from 0 to 100, the global index score (GIS), so as to obtain a score directly proportional to the quality of life. Therefore, an improvement of quality of life from baseline to 3 months after the procedure was represented by a decrease in CIVIQ-20 score or an increase in GIS. Questionnaires with 3 or more missing items were excluded from the analysis. CIVIQ-20 was considered the most adapted score for our study due to its specificity in venous disease and its wide validation (31,33,34).
Peri-procedural imaging
US
Patients had a US (Diamond Select iU22, Philips Healthcare, Amsterdam, the Netherland) with a 5 MHz convex array transducer from the superior vena cava to the groin and with a high frequency linear array transducer (8 MHz) from the groin to the foot of both legs with their angiologist before the procedure, at 1 day, 1, 3, 6, 12 months after the procedure, and every 6 months until 2 years, and then annually. Iliac, femoral, popliteal, deep calf veins and vena cava were evaluated in the longitudinal and axial planes. The following criteria indicated post-thrombotic disease: wall thickening, irregularities, occluded or reduced lumen (incompressibility), prominent collateral veins. It also allowed the clinical examination and assessed the stent patency.
Computed tomography (CT)
Every patient had an angio-CT scan (Somatom® Definition Flash, Siemens AG, Germany) before the procedure to make an accurate assessment of topography and extension of thrombosis and to evaluate presence of synechiae and morphology of collateral veins. Angio-CT scan at 2 months was also systematically performed to evaluate the stents’ integrity, patency and morphology. Protocol was standardized: patients received iodinated contrast agent 400 mg/mL (Iomeron 400, Bracco, Milano, Italy) through the forearm (0.6 gI/kg at a rate of 4 mL/s) and the acquisition ranged from the diaphragm to the knees at 100 seconds after intravenous injection. Reading and interpretation in frontal, axial and sagittal planes and three-dimensional (3D) reconstructions were carried on.
Endovascular procedure
All interventions were performed by the study investigator (RL, with 15 years of experience in endovascular therapeutics) in an interventional radiology platform, using a Philips Allura Xper FD20 angio room (Philips, Amsterdam, the Netherland). The procedure was usually performed under local anaesthesia, using 5 to 10 mL of lidocaine hydrochloride 20 mg/mL (Xylocaïne® 2%, AstraZeneca, London, UK). Only three procedures required general anaesthesia, 1 by requirement of the patient, 1 for an extension of the stenting and 1 for a recanalization attempt after early in-stent thrombosis. All patients received 5,000 IU of heparin intravenously at the beginning of the procedure to avoid in situ thrombosis during or immediately after angioplasty.
Then contralateral transjugular or transfemoral, or ipsilateral popliteal cannulation permitted a selective venography of the cavo-ilio-femoral veins through a long 45 or 65 cm length 6-Fr “Destination” sheath (Terumo Interventional Systems, Japan). Double approach could be required if the recanalization was difficult. Venous puncture and catheterization was always carried out under ultrasonography control. Selective venographies were performed, in frontal and sagittal incidences. Ilio-femoral thrombosis was crossed using a 0.0035’ “Terumo stiff” hydrophilic guidewire (Terumo Interventional Systems, Japan). Then, a 4-Fr or 5-Fr catheter (Vertebral catheter, Cook Europe, Bjaeverskov, Denmark) was used and interchanged on “Amplatz Super stiff” guidewire (Boston Scientific, Natick, MA, USA) in case of synechiae. Recanalization was cautious because of the risk of venous wall break. Retrograde recanalization needed more time because of the risk of going into collaterals. We performed first delicate angioplasty with balloons from 5 to 12 mm in diameter. High-pressure angioplasty could be achieved if the stenosis was very fibrous (until 30 bar). In a second time, extensive stenting was performed: stents were placed from the vena cava or common iliac vein to a landing below in a stenosis-free location which was often the common femoral vein. Procedure needed from 1 to 7 stents, whom diameter ranged from 8 to 18 mm and with a 40, 60 or 80 mm in length. Nitinol stents were used (Nickel Titanium alloy, Protege®GPS™ Self-Expanding Peripheral System, Covidien, Plymouth, USA) with high radial force to remain stable in a vein due to axial and longitudinal flexibility (35) (Figures 2 and 3). If diameter superior to 14 mm necessary, Wallstent™ (Boston Scientific, Natick, MA, USA) was used. One case needed a 22 mm stent (Optima™ 22 mm × 60 mm, Bard Medical, Covington, Georgia) in the vena cava. In case of bilateral angioplasty of iliac veins, we performed a kissing procedure. A final angioplasty with a 9 to 12 mm balloon was achieved to restore a good stent caliber (15 mm for the Wallstent™). In case of attempt of the proximal common iliac vein, the stent ran over inferior vena cava with low risks of complication, less than 1% (16). When several stents were used a 1 cm overlap was recommended. A final venogram ensured the final positioning and stent patency. The whole procedure used a maximum of 260 cc of contrast agent (Visipaque 270 mgI/mL, GE Healthcare, Chicago). The sheath was removed and pressure was applied for 5 to 10 minutes at puncture site.
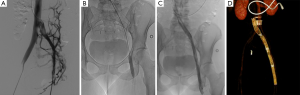
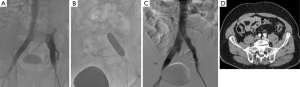
All patients were hospitalized over night and returned to their normal daily activities after 24 hours. Heavy physical activities were contraindicated during 15 days. Class II compression socks were recommended for all patients.
In absence of consensus, each patient received 3 months of dual-therapy: antivitamin K (with a target INR of 2.0–3.0) and antiplatelet drug (Aspegic® per os 100 mg per day). In the literature, antiplatelet drug for life is necessary for some (22,36), useless for others (23,25,37). Antivitamin K was prescribed in case of bad haemodynamic or thrombophilia (22,25,38).
Follow-up
Patency data were based on the results of imaging studies (venography, US and/or CT scan) as described above.
Immediate technical success was defined as recanalization restoring patency to the target vessel with anterograde flow and <50% residual stenosis after stent placement at the perioperative phlebography and confirmed by US at day 1 (39).
Complications were classified as minor and major, immediate (within 48 hours of treatment) and secondary (at 30 days and overall), based on the Society of Interventional Radiology (SIR) standards: infectious complication, allergic reaction, pain >5 on visual analogue scale for pain (VAS), minor or major bleeding, renal failure, thrombosis extension and pulmonary embolism, or even death were evaluated (40).
Early in-stent thrombosis was defined as occlusion or >50% stenosis on CT scan or US exam during the first 2 months.
Clinical improvement was evaluated according to Villalta and CIVIQ-20 scores.
Statistical analyses
Statistical analyses were performed using STATA software (version 12.0, STATA corp., College Station, Texas, USA). Continuous variables were described using Chi2 (χ2) as means (with standard deviations) (SDs) and medians (with interquartile range) (IQR). Qualitative variables and data, Villalta and CIVIQ-20 scores from baseline to postoperative scores were analyzed using Wilcoxon paired rank test, a non-parametric test. A P value of ≤0.05 was considered significant. Values were described as median (IQR, p25–p75). Multivariate analyses were performed. Robust variance estimator by multiple linear regressions was chosen to reveal the internal validity. Finally, a model was developed to consider repeated measures in patients operated twice to consider time.
Results
Patients characteristics
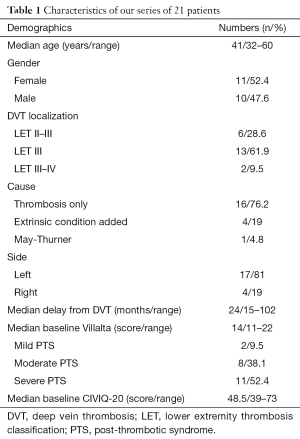
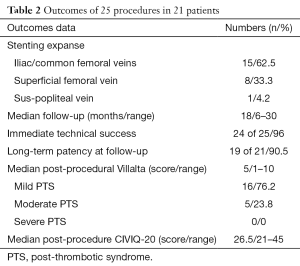
Between March 2012 and April 2016, 25 angioplasties were performed in 21 PTS patients, two patients had a further procedure for stenting extension and two for recanalization attempt after in-stent thrombosis. Median age at baseline was 41 years (range, 32–60 years) and female/male ratio was 1.1:1. Left/right symptomatic limb ratio was 4.25:1, and two patients had a bilateral iliac stenting for contralateral extension. Sixteen (76.2%) of 21 patients had PTS because of idiopathic thrombosis, 4 (19%) of 21 had an added compressive component and 1 (4.8%) of 21 had an iliac venous compression syndrome (Cockett syndrome) proven by phlebography and venous pressure measures. Thirteen (61.9%) of 21 patients were LET class III, 6 (28.6%) of 21 were LET class II–III and 2 (9.5%) of 21 were LET class III–IV. Two (9.5%) of 21 patients presented mild PTS, 8 (38.1%) of 21 had moderate PTS and 11 (52.4%) of 21 presented a severe one. Median time from thrombosis diagnosis to the endovascular procedure was 24 months (range, 15–102 months). Median follow-up was 18 months (range, 6–30 months). Demographic data are summarized in Table 1.
Full table
Technical success and stent patency
Three (12%) of 25 procedures were performed under general anaesthesia. Median number of stents for each procedure was 3 (range, 2–4) and the median total number of stents per patient was 3.5 (range, 2–4). Overall, 68 stents were placed: 66 Protege® GPS™ stents, 1 Wallstent™ and 1 Optima™ stent. Fifteen (62.5%) of 24 stenting procedures involved very proximal segments (vena cava, iliac veins and/or common femoral vein), 8 (33.3%) of 24 also included the superficial femoral vein and 1 (4.2%) of 24 also included the sus-popliteal vein. In 7 (33.3%) of 21 cases, one stent ran over inferior vena cava. Stenting was successfully accomplished in all patients (21/21) for prime procedure (100%). For 25 procedures, 24 (96%) were an immediate technical success, 1 (4%) immediate failure of an attempt of early in-stent thrombosis recanalization was noted. No minor or major complications immediately occur. No venous wall break was reported during any procedure. All US exams after prime procedure at day 1 showed permeable stents. At 1 month, 20 (95.2%) of 21 stents were patent on US, and 1 (4.8%) of 21 was not, which required endovascular thrombectomy and extensive stenting. Stent patency was observed along the follow-up.
Three (14.3%) of 21 patients had an early in-stent thrombosis at 2 months on CT scan and confirmed on US. For one of them, secondary recanalization attempt failed, one benefitted from a successful thrombectomy and extensive stenting. Stent patency was obtained along the study follow-up. The third patient could so far not benefit from a secondary procedure due to complications of anticoagulants. At 3, 6, and 12 months, no other in-stent thrombosis was detected for the rest of the cohort on US exams.
Despite stent patency, one patient underwent a secondary extensive stenting due to the presence of synechiae on CT scan and moderate residual PTS symptoms which were subsequently alleviated. Nineteen (90.5%) of the 21 patients had stent patency at the end of the median follow-up. Outcomes data are summarized in Table 2.
Full table
Villalta and CIVIQ-20 scores improvement
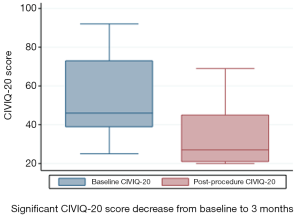
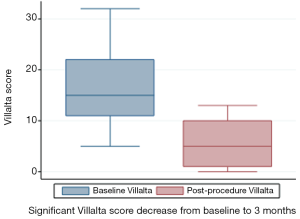
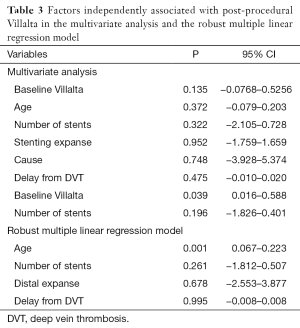
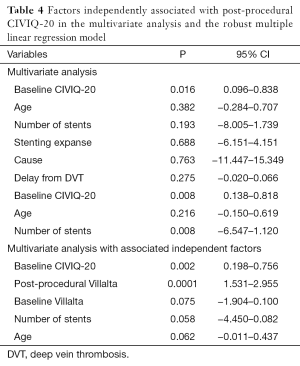
CIVIQ-20 score was significantly decreased from baseline compared with 3 months after stenting [48.5 (range, 39–73) and 26.5 (range, 21–45), respectively, P<0.0001] (Figure 4). It could be expressed in median GIS [64.5 (range, 34–76) and 93.5 (range, 73–99), respectively, P<0.0001]. Thus, showing a relevant improvement in the quality of life. Villalta scale score showed a significant decrease in the severity of PTS symptoms and signs after the procedure. Sixteen (76.2%) of 21 patients had mild PTS and 5 (23.8%) of 21 had moderate PTS after the procedure. No more severe PTS was observed. Villalta score was significantly decreased from baseline compared with 3 months after stenting [14 (range, 11–22) and 5 (range, 1–10), respectively, P<0.0001] (Figure 5). Particular items (pain and edema) of the Villalta score were specifically studied. Pain median score was significantly decreased from baseline compared with 3 months [2 (range, 2–3) and 1 (range, 0–1), respectively, P<0.0001]. Complete pain relief concerned 9 (42.9%) of the 21 patients. Edema median score was significantly decreased compared with 3 months after stenting [3 (range, 3–3) and 1 (range, 0–1), respectively, P<0.0001]. Seven (33.3%) of the 21 patients had complete edema relief. Different variables were analyzed in a multiple linear regression model, then progressively only the most significant factors were kept in a manual backward procedure to take into account medical and therapeutic constraints. In this model, baseline Villalta score was the only independent factor significantly related to post-procedural Villalta score (95% CI, 0.0168655–0.5884017, P=0.039). Age, delay from DVT, and number of stents or stenting expanse were not significantly associated with Villalta score at 3 months. Robust model showed that age (95% CI, 0.0657294–0.2186861; P=0.001) could be an independent risk factor of post-procedural Villalta score. Baseline CIVIQ-20 score (95% CI, 0.1981609–0.7562401; P<0.002) and post-procedural Villalta score (severity of the residual PTS) (95% CI, 1.531922–2.955227; P<0.0001) were independent factors related to post-procedural CIVIQ-20 score. Analysis with multiple linear regressions with robust variance estimator (to test the model stability) showed that Villalta gain (95% CI, 1.56813–2.565814; P<0.0001) was an independent factor related to CIVIQ-20 score. Statistical data are summarized in Tables 3 and 4.
Full table
Full table
Discussion
Our study confirms the high clinical success rate and safety of endovascular PTS treatment and highlights the significant impact of stenting on the quality of life of patients with chronic symptomatic ilio-femoral venous obstructive lesions. Immediate technical success rate was 96% considering 25 procedures, performed without any complications. Median follow-up was 18 months (range, 6–30) with a 90.5% stent patency rate. Villalta score was significantly decreased from baseline compared with 3 months after the procedure [14 (range, 11–22) and 5 (range, 1–10), respectively, P<0.0001), showing a significant decrease in the severity of PTS. CIVIQ-20 score was significantly decreased from baseline compared with 3 months after stenting [48.5 (range, 39–73) and 26.5 (range, 21–45), respectively, P<0.0001) thus showing a significant improvement of quality-of-life.
Despite optimal anticoagulation which is the gold standard DVT treatment, PTS remains a frequent complication of DVT and leads to significant morbidity, suffering and high health costs (21). Endovascular approach with venous angioplasty and stenting has become a widely accepted treatment option in chronic venous obstruction with high technical success rates, minimal complications and very short hospitalization as compared with conventional surgical treatments (16,41,42). Surgery showed lower patency rates, ranging from 44% to 85% (43). Our results are in accordance with a meta-analysis including major series gathering 1,118 patients (3,6,16,26,41,44-46). In our study, 21 PTS patients underwent endovascular treatment with angioplasty and venous stenting with an immediate technical success rate of 96% corroborating that of the meta-analysis (94.1%) (6). Moreover, in our series we reported a 90.5% patency rate with a median follow-up of 18 months comparable with that of meta-analysis (94%) with a median follow-up of 15 months (6). Comparison of data between studies remains difficult because of imprecise definitions of short-, mid- and long-term patency. In our series, no in-stent thrombosis and no recurrent DVT was observed after 2 months of follow-up.
Most studies used Wallstent™ (Boston Scientific, Marlborough, MA, USA), whereas others used various types of stents. We preferentially implanted a single type of nitinol stent (Nickel Titanium alloy, Protege® GPS™ Self-Expanding Peripheral System, Covidien, Plymouth, USA) because of their axial and longitudinal flexibility and high radial force, except for two cases requiring sizes unavailable with these stents (35). Therefore, our series fully displays the results of venous stenting with nitinol stents being comparable to those of series using Wallstent™.
Very few studies combined Villalta score grading PTS severity and CIVIQ-20 score being relevant for quality of life grading in chronic venous disease (26,28). So far, no study has analyzed factors associated with quality of life in such a setting. Ye et al. (28) reported a significant decrease of Villalta score from 22 at baseline to 9.3 after the endovascular procedure in accordance with our results [14 (range, 11–22) and 5 (range, 1–10), respectively]. Still, this study of life quality may be considered as irrelevant due to a partial analysis of CIVIQ-20. In the study from Sarici et al. (26), Villalta score decreased from 18 (range, 7–30) at baseline to 8 (range, 4–19) at 3 months and CIVIQ-20 (expressed in GIS) increased from 64 (range, 50–75) to 81 (range, 63–90), corroborating our data.
To our knowledge, our study is the first one showing post-procedural Villalta and baseline CIVIQ-20 as independent risk factors of final CIVIQ-20. Moreover, our model developed by multiple linear regressions showed that only Villalta gain could be an independent risk factor of final CIVIQ-20, but not the number of stents or length of stenting as we could expect. Post-procedural Villalta was only related to baseline Villalta. This model also showed that it could be related to age but not to delay from DVT.
In the literature, stent integrity was not generally assessed unlike into arterial system (23,41). By CT scan at 2 months, we showed no fracture and no stent migration, especially in the common femoral vein across the hip joint. Moreover, 2 patients were high level mountain bike riders and one patient had two normal pregnancies during follow-up without stent damage. According to studies with longer follow-up (>5 years), no further anticoagulation was prescribed after 3 months of dual-therapy if no thrombophilia or other conditions. This is still a debate of matter. However, the ability to stop anticoagulants shortly after venous endovascular procedures, is helpful because of their long-term risks (37,47). No late in-stent thrombosis was observed in our series, suggesting a better mid-term stent patency in veins than in arteries.
Our study has some limitations. First, it reports a small number of patients, in part because lot of angiologists in Burgundy did not know the feasibility of such procedure in our institution at the beginning of the study. Recruitment is currently increasing. A lack of power due to the small number of patients may also explain that delay from symptoms onset to the endovascular procedure does not appear associated with the effectiveness of treatment. Second, calf and thigh circumferences were not considered, it could have strengthened the objectivity of edema decrease.
Conclusions
Our study confirms the high technical and clinical success rates and the safety of ilio-femoral venous angioplasty/stenting in PTS patients with symptomatic chronic venous obstructive lesions and highlights the significant impact on the quality of life of patients at mid-term. Such a procedure shows good long-term stent patency. Our study demonstrates that angioplasty can treat mild or severe PTS and avoid complications, and lead to a decrease of the severity of PTS with quality-of-life improvement. A further randomized controlled trial may determine whether treating PTS patients earlier provides better results.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Ethics Committee Approval of the institution was waived because the endovascular procedures were routinely performed as part of clinical practice. Written informed consent was obtained from all patients.
References
- Spencer FA, Emery C, Joffe SW, Pacifico L, Lessard D, Reed G, Gore JM, Goldberg RJ. Incidence rates, clinical profile, and outcomes of patients with venous thromboembolism. The Worcester VTE study. J Thromb Thrombolysis 2009;28:401-9. [Crossref] [PubMed]
- Enden T, Haig Y, Kløw NE, Slagsvold CE, Sandvik L, Ghanima W, Hafsahl G, Holme PA, Holmen LO, Njaastad AM, Sandbæk G, Sandset PM. CaVenT Study Group. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet 2012;379:31-8. [Crossref] [PubMed]
- O'Sullivan GJ. The role of interventional radiology in the management of deep venous thrombosis: advanced therapy. Cardiovasc Intervent Radiol 2011;34:445-61. [Crossref] [PubMed]
- O'Sullivan GJ, Semba CP, Bittner CA, Kee ST, Razavi MK, Sze DY, Dake MD. Endovascular management of iliac vein compression (May-Thurner) syndrome. J Vasc Interv Radiol 2000;11:823-36. [Crossref] [PubMed]
- O'Sullivan GJ, Waldron D, Mannion E, Keane M, Donnellan PP. Thrombolysis and iliofemoral vein stent placement in cancer patients with lower extremity swelling attributed to lymphedema. J Vasc Interv Radiol 2015;26:39-45. [Crossref] [PubMed]
- Razavi MK, Jaff MR, Miller LE. Safety and effectiveness of stent placement for iliofemoral venous outflow obstruction: systematic review and meta-analysis. Circ Cardiovasc Interv 2015;8:e002772. [Crossref] [PubMed]
- Prandoni P, Kahn SR. Post-thrombotic syndrome: prevalence, prognostication and need for progress. Br J Haematol 2009;145:286-95. [Crossref] [PubMed]
- Stain M, Schönauer V, Minar E, Bialonczyk C, Hirschl M, Weltermann A, Kyrle PA, Eichinger S. The post-thrombotic syndrome: risk factors and impact on the course of thrombotic disease. J Thromb Haemost 2005;3:2671-6. [Crossref] [PubMed]
- Wakefield TW, Myers DD, Henke PK. Mechanisms of venous thrombosis and resolution. Arterioscler Thromb Vasc Biol 2008;28:387-91. [Crossref] [PubMed]
- Kahn SR, Shrier I, Julian JA, Ducruet T, Arsenault L, Miron MJ, Roussin A, Desmarais S, Joyal F, Kassis J, Solymoss S, Desjardins L, Lamping DL, Johri M, Ginsberg JS. Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann Intern Med 2008;149:698-707. [Crossref] [PubMed]
- Prandoni P, Lensing AW, Prins MH, Frulla M, Marchiori A, Bernardi E, Tormene D, Mosena L, Pagnan A, Girolami A. Below-knee elastic compression stockings to prevent the post-thrombotic syndrome: a randomized, controlled trial. Ann Intern Med 2004;141:249-56. [Crossref] [PubMed]
- Kahn SR, M'Lan CE, Lamping DL, Kurz X, Bérard A, Abenhaim L. Veines Study Group. The influence of venous thromboembolism on quality of life and severity of chronic venous disease. J Thromb Haemost 2004;2:2146-51. [Crossref] [PubMed]
- Yin M, Shi H, Ye K, Lu X, Li W, Huang X, Lu M, Jiang M. Clinical assessment of endovascular stenting compared with compression therapy alone in post-thrombotic patients with iliofemoral obstruction. Eur J Vasc Endovasc Surg 2015;50:101-7. [Crossref] [PubMed]
- Akesson H, Brudin L, Dahlström JA, Eklöf B, Ohlin P, Plate G. Venous function assessed during a 5 year period after acute ilio-femoral venous thrombosis treated with anticoagulation. Eur J Vasc Surg 1990;4:43-8. [Crossref] [PubMed]
- Bergan JJ. A major step forward in the treatment of venous occlusion. J Endovasc Ther 2000;7:92-3. [Crossref] [PubMed]
- Neglén P, Hollis KC, Olivier J, Raju S. Stenting of the venous outflow in chronic venous disease: long-term stent-related outcome, clinical, and hemodynamic result. J Vasc Surg 2007;46:979-90. [Crossref] [PubMed]
- Eklof B, Partsch H, Vedantham S, Prandoni P, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Updated terminology of chronic venous disorders: the VEIN-TERM transatlantic interdisciplinary consensus document. J Vasc Surg 2009;49:498-501. [Crossref] [PubMed]
- Kahn SR, Partsch H, Vedantham S, Prandoni P, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of post-thrombotic syndrome of the leg for use in clinical investigations: a recommendation for standardization. J Thromb Haemost 2009;7:879-83. [Crossref] [PubMed]
- Kolbach DN, Sandbrink MW, Hamulyak K, Neumann HA, Prins MH. Non-pharmaceutical measures for prevention of post-thrombotic syndrome. Cochrane Database Syst Rev 2004.CD004174. [PubMed]
- Zollikofer CL, Largiader I, Bruhlmann WF, Uhlschmid GK, Marty AH. Endovascular stenting of veins and grafts: preliminary clinical experience. Radiology 1988;167:707-12. [Crossref] [PubMed]
- Ashrani AA, Heit JA. Incidence and cost burden of post-thrombotic syndrome. J Thromb Thrombolysis 2009;28:465-76. [Crossref] [PubMed]
- Delis KT, Bjarnason H, Wennberg PW, Rooke TW, Gloviczki P. Successful iliac vein and inferior vena cava stenting ameliorates venous claudication and improves venous outflow, calf muscle pump function, and clinical status in post-thrombotic syndrome. Ann Surg 2007;245:130-9. [Crossref] [PubMed]
- Kölbel T, Gottsäter A, Kühme T, Lindh M, Ivancev K. Endovascular treatment of venous occlusive disease. Ann Vasc Dis 2008;1:91-101. [Crossref] [PubMed]
- Kurklinsky AK, Bjarnason H, Friese JL, Wysokinski WE, McBane RD, Misselt A, Moller SM, Gloviczki P. Outcomes of venoplasty with stent placement for chronic thrombosis of the iliac and femoral veins: single-center experience. J Vasc Interv Radiol 2012;23:1009-15. [Crossref] [PubMed]
- Rosales A, Sandbaek G, Jorgensen JJ. Stenting for chronic post-thrombotic vena cava and iliofemoral venous occlusions: mid-term patency and clinical outcome. Eur J Vasc Endovasc Surg 2010;40:234-40. [Crossref] [PubMed]
- Sarici IS, Yanar F, Agcaoglu O, Ucar A, Poyanli A, Cakir S, Aksoy SM, Kurtoglu M. Our early experience with iliofemoral vein stenting in patients with post-thrombotic syndrome. Phlebology 2014;29:298-303. [PubMed]
- Titus JM, Moise MA, Bena J, Lyden SP, Clair DG. Iliofemoral stenting for venous occlusive disease. J Vasc Surg 2011;53:706-12. [Crossref] [PubMed]
- Ye K, Lu X, Jiang M, Yang X, Li W, Huang Y, Huang X, Lu M. Technical details and clinical outcomes of transpopliteal venous stent placement for postthrombotic chronic total occlusion of the iliofemoral vein. J Vasc Interv Radiol 2014;25:925-32. [Crossref] [PubMed]
- Arnoldussen CW, Wittens CH. An imaging approach to deep vein thrombosis and the lower extremity thrombosis classification. Phlebology 2012;27:143-8. [Crossref] [PubMed]
- Strijkers RH, Arnoldussen CW, Wittens CH. Validation of the LET classification. Phlebology 2015;30:14-9. [Crossref] [PubMed]
- Vedantham S, Grassi CJ, Ferral H, Patel NH, Thorpe PE, Antonacci VP, Janne d'Othée BM, Hofmann LV, Cardella JF, Kundu S, Lewis CA, Schwartzberg MS, Min RJ, Sacks D; Technology Assessment Committe of the Society of Interventional Radiology. Reporting standards for endovascular treatment of lower extremity deep vein thrombosis. J Vasc Interv Radiol 2006;17:417-34. [Crossref] [PubMed]
- Strijkers RH, Wittens CH, Kahn SR. Villalta scale: goals and limitations. Phlebology 2012;27:130-5. [Crossref] [PubMed]
- Launois R, Mansilha A, Jantet G. International psychometric validation of the Chronic Venous Disease quality of life Questionnaire (CIVIQ-20). Eur J Vasc Endovasc Surg 2010;40:783-9. [Crossref] [PubMed]
- Launois R, Mansilha A, Lozano F. Linguistic validation of the 20 item-chronic venous disease quality-of-life questionnaire (CIVIQ-20). Phlebology 2014;29:484-7. [Crossref] [PubMed]
- Duda SH, Wiskirchen J, Tepe G, Bitzer M, Kaulich TW, Stoeckel D, Claussen CD. Physical properties of endovascular stents: an experimental comparison. J Vasc Interv Radiol 2000;11:645-54. [Crossref] [PubMed]
- Neglén P, Raju S. Balloon dilation and stenting of chronic iliac vein obstruction: technical aspects and early clinical outcome. J Endovasc Ther 2000;7:79-91. [Crossref] [PubMed]
- Gutzeit A, Zollikofer ChL, Dettling-Pizzolato M, Graf N, Largiadèr J, Binkert CA. Endovascular stent treatment for symptomatic benign iliofemoral venous occlusive disease: long-term results 1987-2009. Cardiovasc Intervent Radiol 2011;34:542-9. [Crossref] [PubMed]
- Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ; American College of Chest Physicians. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008;133:454S-545S.
- Vedantham S, Grassi CJ, Ferral H, Patel NH, Thorpe PE, Antonacci VP, Janne d'Othée BM, Hofmann LV, Cardella JF, Kundu S, Lewis CA, Schwartzberg MS, Min RJ, Sacks D; Technology Assessment Committee of the Society of Interventional Radiology. Reporting standards for endovascular treatment of lower extremity deep vein thrombosis. J Vasc Interv Radiol 2009;20:S391-408. [Crossref] [PubMed]
- Patel N, Sacks D, Patel RI, Moresco KP, Ouriel K, Gray R, Ambrosius WT, Lewis CA; Society of Interventional Radiology Technology Assessment Committee. SIR reporting standards for the treatment of acute limb ischemia with use of transluminal removal of arterial thrombus. J Vasc Interv Radiol 2003;14:S453-65. [Crossref] [PubMed]
- Hartung O, Otero A, Boufi M, De Caridi G, Barthelemy P, Juhan C, Alimi YS. Mid-term results of endovascular treatment for symptomatic chronic nonmalignant iliocaval venous occlusive disease. J Vasc Surg 2005;42:1138-44. [Crossref] [PubMed]
- Schwarzbach MH, Schumacher H, Böckler D, Fürstenberger S, Thomas F, Seelos R, Richter GM, Allenberg JR. Surgical thrombectomy followed by intraoperative endovascular reconstruction for symptomatic ilio-femoral venous thrombosis. Eur J Vasc Endovasc Surg 2005;29:58-66. [Crossref] [PubMed]
- Nicolaides AN, Allegra C, Bergan J, Bradbury A, Cairols M, Carpentier P, Comerota A, Delis C, Eklof B, Fassiadis N, Georgiou N, Geroulakos G, Hoffmann U, Jantet G, Jawien A, Kakkos S, Kalodiki E, Labropoulos N, Neglen P, Pappas P, Partsch H, Perrin M, Rabe E, Ramelet AA, Vayssaira M, Ioannidou E, Taft A. Management of chronic venous disorders of the lower limbs: guidelines according to scientific evidence. Int Angiol 2008;27:1-59. [PubMed]
- Neglén P. Stenting is the "Method-of-Choice" to treat iliofemoral venous outflow obstruction. J Endovasc Ther 2009;16:492-3. [Crossref] [PubMed]
- O'Sullivan GJ, Sheehan J, Lohan D, McCann-Brown JA. Iliofemoral venous stenting extending into the femoral region: initial clinical experience with the purpose-designed Zilver Vena stent. J Cardiovasc Surg (Torino) 2013;54:255-61. [PubMed]
- Raju S, Darcey R, Neglen P. Unexpected major role for venous stenting in deep reflux disease. J Vasc Surg 2010;51:401-8. [Crossref] [PubMed]
- Wells PS, Forgie MA, Simms M, Greene A, Touchie D, Lewis G, Anderson J, Rodger MA. The outpatient bleeding risk index: validation of a tool for predicting bleeding rates in patients treated for deep venous thrombosis and pulmonary embolism. Arch Intern Med 2003;163:917-20. [Crossref] [PubMed]