Current status of carotid ultrasound in atherosclerosis
Atherosclerosis is a leading cause for cardiovascular disease (CVD) (1), of which including stroke remains a major cause of death and disability in developed countries (2). Current guidelines and primary prevention of asymptomatic CVD are mainly based on the use of calculated risk scores e.g., Framingham risk score that utilize a few standard risk factors to stratify future risk and guide treatment. Although intensive control of traditional CVD risk factors based on these guidelines has led to marked reduction in the mortality rate of atherosclerotic CVD (3), there are well-documented limitations (4). Firstly, the guidelines based on the estimated scores are primarily used to predict population risk. They may not be applicable in some individuals. Secondly, some relevant risk factors such as family history of premature coronary heart disease, previous risk factor treatment and the variability in blood pressure are not included in the estimation. Thirdly, the use of ‘one-time’ measures of traditional modifiable risk scores may significantly underestimate risks in some individuals leading to under-treatment (4).
The advantage of imaging technique is its ability to monitor the disease process. Ultrasound as a cheap, non-invasive and non-ionizing imaging technique, is an ideal tool for long-term CVD risk assessment for the asymptomatic patients, helping refine the risk score stratification and guiding intervention to the patients (5-7). With recent advances in ultrasound technology, enhanced assessment of carotid atherosclerotic lesions is possible in subclinical stages (8-10). This article reviews the literature on the current status of carotid ultrasound in the risk assessment and primary prevention of CVD.
Atherosclerosis
Atherosclerosis is a chronic inflammatory disease (1). The earliest atherosclerotic lesion (the fatty streak) may occur in infancy or childhood because maternal hypercholesterolemia predisposes fatty streak formation in a fetus (11). In the presence of an unopposed and excessive amount of fatty streak formation, the artery undergoes progressive atherosclerotic changes from endothelial dysfunction, remodeling of the artery with wall thickening and arterial dilatation to vessel wall damage resulting in complicated stenotic lesion (12). Nonetheless, in the initial stages of atherosclerosis, the lesions are usually asymptomatic. Neurological symptoms are usually associated with advanced lesions with plaque rupture and thrombosis as complications (1).
Imaging of carotid intima-media thickness (CIMT)
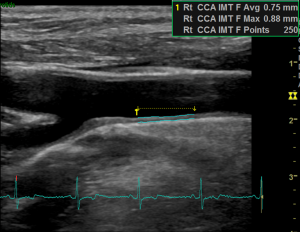
CIMT is widely studied as a surrogate marker for detecting subclinical atherosclerosis for risk assessment and monitoring the progress of atherosclerosis for medical intervention (13-16). On ultrasound, CIMT is the distance between two echogenic lines representing the lumen-intima interface and the media-adventitia interface of the carotid arterial wall (Figure 1). This finding has been histologically validated with better correlation of CIMT at the far wall than at the near wall (17).

Although a number of clinical studies have demonstrated that thickened CIMT independently predicts CVD risk or events (13,14,16,18), conflicting results are obtained by others (19-21) (Table 1). The inconsistent predictive value of CIMT is likely attributed to the variability of CIMT measurement methodology such as image acquisition, CIMT reading and processing methods, apart from differences in study end-points, cardiovascular risk and factor profiles of the studied population and operator errors (25,26).

Full table
CIMT image acquisition
CIMT image acquisition differs in the number of segments, sides and angles between studies. Some studies examined CIMT at one segment predominantly the distal common carotid artery (CCA) because it is easily accessible (18,20,21,23). The success rate of acquiring IMT at the near and far wall of CCA had been reported to be >98% and 100% respectively while those at the bulb were >98% and >99% and those at the internal carotid artery (ICA) were >86% and >98% (27). Other studies examined CIMT at two segments (CCA and ICA) (19,24) or three segments (CCA, carotid bulb and ICA) (22). Imaging of IMT varies between unilateral and bilateral capturing in different studies (13,20,23). If it is imaged unilaterally, the right CCA is usually captured (21,23).
Number of imaging angles is another aspect of discrepancy in CIMT acquisition. Some studies captured the CIMT antero-obliquely at an insonation angle around 45 degrees (22) while others acquired the CIMT from three angles in anterior, lateral and posterior approaches (28) or five angles at an increment of 30 degrees bilaterally (29). These approaches have been used in epidemiological and interventional studies (16,21,30).
CIMT reading and processing methods
Varying CIMT reading methods exist between studies. Some studies read the CIMT measurement at the far wall of the CCA because the far wall measures reflect the true wall thickness and are more accurate than the near-wall measures (13,17,31). Others took CIMT measurements from both near and far walls (20) because a combination of the near and far wall measures is more reproducible than the far-wall measures alone presumably due to reduced random error after averaging the measures (32). However, there is no evidence showing that the combined measures are superior to the far wall measures for CVD prediction (33).
Quantification of the CIMT measures is inconsistent in the studies. The mean or maximum of a single segment (16,23); mean of the mean or mean of the maximum of two or more segments; or composite measures from both sides and different arterial sites have been reported (13,19,21). Among different types of CIMT measures, mean of mean values averaged multiple points along the traced segment is more reproducible, but is less sensitive to change. Mean of maximum values is more sensitive to change, but less reproducible because it is derived from a single point measurement along the 1-cm region (34). However, composite scores including both plaque and IMT are not recommended (35).
Two tracing methods are commonly used in the CIMT studies, viz manual tracing and automated edge-detection. Some studies traced CIMT manually by electronic calipers while others used automated/semi-automated edge-detection system (23,27). Although it is generally agreed that automated edge detection method is more reproducible than the manual tracing, recent evidences show that both methods result in high reproducibility and similar associations with CVD risk factors, outcomes, rate of change and treatment effects (30,36,37). Therefore, choices between automated/semi-automated and manual reading software for CIMT studies should be based on logistical considerations and cost implication rather than differences in expected data quality (38).
Recording IMT values at different phases of cardiac cycle is also a cause of discrepancy (13,21,22). Of note, the CIMT values vary in different phases of cardiac cycle with the peak-systolic IMT slightly thinner than the end-diastolic IMT by an average of 0.041 mm (39). Despite both types of IMT values are similarly associated with CVD risk factors; the end-diastolic IMT is preferred in most studies. It is because peak-systolic IMT tends to give a higher CVD risk for the asymptomatic subjects than would be expected for diastolic IMT (39).
Standardization of CIMT measurement methods
In spite of the extensive use of CIMT measures, there is no widely accepted ultrasound protocol for CIMT measurement in epidemiological and interventional studies. Two consensus reports, the Mannheim CIMT Consensus Report (35) and the American Society of Echocardiography (ASE) Consensus Statement (34) were released attempting to address the issues of standardization.
The Mannheim CIMT Consensus Report was first published in 2004 and updated in 2006 and 2011. This report emphasizes the importance of standardizing CIMT measurement method and distinguishing IMT thickening from early plaque formation. Only when these aspects have been addressed, consistent data collection/analysis, improved power of randomized clinical trials and matching of large databases for meta-analyses can be possible (35). Standardized CIMT measurement is suggested to be performed on the far wall of distal 1-cm segment of CCA at least 5 mm away from its bifurcation within a region free of plaque (Figure 2). Plaque-free IMT should be measured at the carotid bulb and proximal ICA and on a shorter length in case of vessel tortuosity, but these values must be recorded separately. It is mentioned that imaging of distal CCA segment has the advantages of increased accuracy and reproducibility of the measures; allowing automated-edge detection for IMT measurement; and producing data that could be compared to a majority of reference data within large epidemiological studies (35). Plaques should be distinguished from thickened IMT because they are distinct phenotypes with different localization, natural history, risk factors and predictive value for CVD events. A plaque is defined as a focal structure encroaching into the arterial lumen of at least 0.5 mm or 50% of the surrounding IMT value, or demonstrating an IMT thicker than 1.5 mm. Furthermore, CIMT and plaque presence are recommended for the initial investigation of CVD risk in asymptomatic patients (35).
Similarly, a detailed CIMT measurement method is proposed in the ASE Consensus Statement based on large epidemiologic studies (34). In this Statement, the CIMT measurement is limited to the far wall of the distal 1-cm CCA and supplemented by documenting the carotid plaque in the near and far walls of CCA, bulb and ICA segments so as to increase sensitivity for detecting subclinical CVD. The CIMT should be obtained in the longitudinal planes from three imaging angles (anterior, lateral and posterior) with clear depiction of double lines on near and far walls. The imaging depth is adjusted at a depth of 4 cm to avoid slice thickness artifacts. Use of zoomed images is not recommended. The CIMT measurement should be made at end-diastole on an R-wave gated still frame with inclusion of the plaque if detected (Figure 2). A semi-automated edge detection program with validated accuracy is preferred to manual tracing using electronic calipers for the former tends to improve reproducibility and reduce reading time. Simple point-to-point measurements of CIMT are not accepted. Plaque defined by the ASE task force is basically same as that defined in the Mannheim report, except with the omission of a focal structure encroaching into the arterial lumen of at least 0.5 mm” (34). Lastly, the Consensus Statement stresses on the importance of measuring CIMT by appropriately trained sonographers and readers who could carefully adhere to predefined scanning protocol so that measurement error can be minimized.
Extensive or restrictive ultrasound protocols
Debate continues for adoption of extensive (multiple angles, walls and segments) or restrictive (one single-angle far wall CIMT) protocols for CIMT measurement in clinical studies. Proponents for extensive ultrasound protocols maintain that although extensive protocols increase examination time, length of training of sonographers and cost of the procedure, it enhances the reproducibility, magnitude and precision of progression of CIMT over time and treatment effect (32). The best protocols suggested are mean common CIMT protocols in which both the near and far walls are measured at multiple angles (32,40) because these protocols could produce data of the highest precision to observe a treatment effect and to fully reflect the asymmetric nature of atherosclerotic burden (30). The opponents to the extensive protocols argue that extensive protocols using multiple angles and multiple projections have been shown to produce similar variability for determining IMT change as those derived from restriction of analyses to only one segment and to only one projection (41). In addition, the long examination time of around three hours for an extensive three-segment, five-angle protocol is clinically impracticable as compared to a half-an-hour restrictive CIMT protocol. More importantly, since IMT progression rates vary with the carotid artery segment, a global measurement of IMT progression as done in extensive protocols might underrate the association between specific segment IMT progression and outcome (41).
Recommendations for appropriate use of CIMT screening
With the inconsistent results of the predictive power of CIMT measures for CVD risk, it is not surprising to find the recommendations for use of CIMT are variable. European Guidelines on CVD prevention in clinical practice (version 2012) support CIMT screening in asymptomatic individuals at moderate risk (5). Likewise, Canadian Cardiovascular Society guidelines for the prevention of CVD recommend CIMT measurements as a means to enhance risk assessment provided the test is restricted to centers with specific expertise (6). On the contrary, the Mannheim report does not recommend serial monitoring of CIMT in individual patients (35). While the ASE Consensus Statement affirms the value of CIMT measures including plaque presence for re-stratifying CVD risk in patients at intermediate risk, CIMT testing is not warranted unless the results would be expected to alter therapy and serial IMT studies are not recommended for use in clinical practice. Similarly, the 2013 American College of Cardiology/American Heart Association guidelines on cardiovascular risk assessment and cholesterol treatment do not advocate routine measurement of CIMT in clinical practice because of the concerns about the quality and standardization of CIMT measurement (7). It is apparent that the use of CIMT as a marker for risk assessment and the use of CIMT progression to guide intervention are controversial. Only when consistent results are yielded in the studies with improved quality through standardization of the measurement methodology, the sonographic CIMT measurement as a screening marker for CVD risk assessment and a guide for intervention will be generally accepted in clinical practice.
Imaging of carotid plaque
Screening of plaque presence
Even though CIMT measures have been widely used for risk prediction in the past decades, increasing evidences show that IMT adds little value to risk prediction and progression of IMT does not predict CVD events (42-44). Nonetheless, inclusion of plaque to CIMT measurement has been consistently shown to improve the predictive power for CVD and coronary events (42,45-47). Compared with traditional risk factors, carotid plaque presence also improves prediction of stroke/transient ischemic attacks (28).
The improved predictive power of plaque presence can be accounted for by the potential pathological differences between CIMT and plaque, and the geometrical configuration and flow properties of the carotid bifurcation. It should be noted that increased sonographic CIMT is not necessarily indicative of atherosclerosis and can occur in patients without atherosclerosis. It is because atherosclerosis exclusively involves the intimal layer whereas thickened CIMT may be due to medial hypertrophy as a result of adaptive arterial wall remodeling (48) or aging with thickening of both the intimal and medial layers (8). In contrast, carotid plaque is characteristic of advanced atherosclerosis (1) with prevalence at the region where the wall shear stress is low such as the outer wall of proximal ICA and carotid bulb (49). However, these segments are usually excluded from the standard CIMT measurements explaining why the measures are less sensitive to predict CVD risks or events. In ASE Consensus Statement, screening of plaque for its presence in CIMT measurement should cover bilateral CCA, carotid bulb and ICA (34).
Measurement of plaque burden
To enhance the predictive power of plaque screening for CVD events beyond plaque presence, quantifying the carotid plaque burden has been developed as a promising alternative to CIMT measurement. Carotid plaque burden can be measured either as total plaque area (TPA) by 2-dimensional (2D) ultrasound (Figure 3) or total plaque volume (TPV) by 3-dimensional (3D) ultrasound (Figure 4). TPA is the total cross-sectional area of all detected plaques on longitudinal views. When comparing the usefulness of TPA and TPV, progression of TPV strongly predicts CVD events while that of TPA only does it weakly (50).
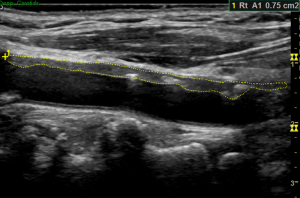
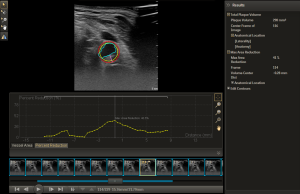
Quantification of plaque burden is superior to CIMT because the plaque burden measures and their progression have been shown to strongly predict CVD events and can identify high risk patients (50,51). In contrast, CIMT without plaque thickness is a weak predictor of CVD risk (42) and its change over time neither predicts CVD events (52) nor enables monitoring of medical therapeutic effects in clinically meaningful timeframes because the CIMT change is subtle around 0.15 mm/year (53). For TPA and TPV, the changes are more noticeable around 10 mm2/year and 50 to 100 mm3/year respectively (54). It is thus easier to monitor the changes in these parameters for treatment effect with higher sensitivity for TPV than TPA (50). In addition, the easily measurable changes in plaque burden allows smaller sample size and shorter duration of follow-up required to study effects of new therapies (55). More importantly, plaque burden measures can be used in managing patients with asymptomatic carotid stenosis by a strategy called “treating arteries instead of risk factors”. With this strategy, more intensive medical therapy can be given to the patients based on plaque measurement resulting in marked reduction in CVD risks among patients (56,57).
Measurement of plaque burden is also useful in genetic research because it helps genotyping the sampled individuals. Of note, carotid ultrasound phenotypes are biologically distinct implying that genetic factors affecting IMT, plaque burden, stenosis and plaque rupture are different. These distinct ultrasound phenotypes are important with regard to studies of new therapies (54). Making use of plaque burden measures, targeted genotyping of individuals with specific phenotypes and known risk factors is feasible. In this way, the sample size needed for genome-wide association studies can be reduced (58).
Characterization of plaque
Sonographic plaque characteristics are found to be predictive of subsequent cerebral ischemic events (59). Although current guidelines have established degree of carotid stenosis as the primary surrogate for stroke risk and indication of intervention, there is increasing evidence showing that vulnerable carotid plaques are more prone to cerebroembolic events regardless of degree of stenosis (60) and a high proportion of these strokes are likely due to rupture or erosion of non-stenotic, unstable plaques (61). Carotid ultrasound, by means of plaque echogenicity and morphology, provides a clue to differentiate “vulnerable” from “non-vulnerable” plaques and may help in risk stratification and therapy. A recent meta-analysis of the literature demonstrated that several sonographic features of the complex plaques such as intraplaque echolucency, neovascularization and ulceration were found to associate with cerebral ischemic symptoms (59).
Intraplaque echolucency
Plaque echolucency is a strong marker for risk of ischemic stroke (62) and is histologically proven to represent lipid-rich necrotic core (LRNC) or intraplaque hemorrhage (IPH) (63). Ultrasound is sensitive in detecting plaque echolucency with a detection rate up to 90% (64). Detection of the size and site of “juxtaluminal echolucency” which represents either a LRNC or IPH is important because a large LRNC near the lumen is associated with increased risk of stroke and clinical ischemic events (65,66).
Neovascularization
Neovascularization as a source of IPH is associated with plaque progression and vulnerability (67). Contrast-enhanced ultrasound (CEUS) can detect neovascularization and allow quantification of the neovessels in the entire plaque (68) or on the plaque shoulder (69). The latter technique is suggested to be more reliable to predict the risk of plaque rupture and IPH compared to studies evaluating the contrast effects of the entire plaque (Saito K, 2014).
Plaque ulceration
Ultrasound lacks sensitivity for the detection of plaque ulceration with wide variation ranging from 33–75% in sensitivity and 33–92% in specificity (70). The conventional criteria defines plaque ulceration as a recess of the plaque surface measuring at least 2 mm deep and 2 mm long, with a well-defined wall at its base and an area of reversed color Doppler flow within the recess (71). However, a simplified criteria adopted by Muraki et al. who simply considered plaque ulceration as a clearly depicted concavity with a less intense border echo at its base resulted in significant improvement in the diagnostic accuracy of plaque ulceration compared to the use of conventional criteria (72). Both CEUS and 3D ultrasound have been suggested to reliably characterize the surface morphology of atherosclerotic carotid plaques and is superior to 2D in detecting ulcers in the plaques (73,74).
Plaque motion
Abnormal plaque motions have been observed with high-resolution ultrasound by Muraki et al. on 49 symptomatic carotid stenoses, which were histologically proven to represent high-risk carotid lesions from plaque rupture to ulcer (75). Two types of abnormal plaque motions were detected: a fine trembling motion within the plaque and a systolic retractive motion of the plaque surface. The former motion was reported to have a high sensitivity of 95% of predicting plaque rupture/ulcer while the latter motion was highly associated with soft content within the plaque. Further studies may be required to evaluate the diagnostic accuracy of these observations.
From degree of stenosis to plaque vulnerability
Quantification of carotid artery stenosis is universally adopted for stratifying patients for therapeutic intervention over the past two decades. With current state-of-the-art advances in carotid plaque imaging, patient stratification can be made by identification of vulnerable plaques. Currently, MR angiography and CT angiography with improved resolution are highly sensitive to detect vulnerable plaques. MRI is the gold standard in carotid plaque imaging for identifying IPH, ulceration, LRNC, neovascularization and inflammation but is limited by its long examination time while CT is reliable to detect ulceration and calcifications but is insensitive to differentiate IPH from LRNC (60). Compared with MRI and CT, ultrasound is inferior to detect ulceration (76,77) but is relatively sensitive for detecting LRNC and IPH which appear as plaque echolucency (78). With the aid of contrast imaging, the sensitivity and specificity of ultrasound for detecting ulceration and neovascularization can be increased (68,74). Therefore carotid ultrasound may be used for risk assessment for ischemic stroke beyond measurement of luminal stenosis.
Conclusions
Despite the extensive use of CIMT measures in recent decades for risk prediction of CVD, CIMT measurement methods are variable in clinical studies leading to conflicting results. It is noteworthy that CIMT measurement alone without plaque presence or plaque burden measures has limited value in risk prediction. Only when consistent results are yielded in the clinical studies with improved quality through standardization of the measurement methodology, CIMT measurement as a screening marker for CVD risk assessment and a guide for medical intervention will be widely accepted in clinical practice. With advances in ultrasound technology, enhanced assessment of carotid plaques is feasible to detect high-risk/vulnerable plaques, and provide risk assessment for ischemic stroke beyond measurement of luminal stenosis.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med 1999;340:115-26. [Crossref] [PubMed]
- Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation 2015;131:e29-322. [Crossref] [PubMed]
- Writing Group Members, Mozaffarian D, Benjamin EJ, et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation 2016;133:e38-60. [Crossref] [PubMed]
- Weber LA, Cheezum MK, Reese JM, Lane AB, Haley RD, Lutz MW, Villines TC. Cardiovascular Imaging for the Primary Prevention of Atherosclerotic Cardiovascular Disease Events. Curr Cardiovasc Imaging Rep 2015;8:36. [Crossref] [PubMed]
- Perk J, De Backer G, Gohlke H, et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012): The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Atherosclerosis 2012;223:1-68. [Crossref] [PubMed]
- Anderson TJ, Grégoire J, Hegele RA, Couture P, Mancini GB, McPherson R, Francis GA, Poirier P, Lau DC, Grover S, Genest J Jr, Carpentier AC, Dufour R, Gupta M, Ward R, Leiter LA, Lonn E, Ng DS, Pearson GJ, Yates GM, Stone JA, Ur E. 2012 update of the Canadian Cardiovascular Society guidelines for the diagnosis and treatment of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol 2013;29:151-67. [Crossref] [PubMed]
- Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129:S49-73. [Crossref] [PubMed]
- Nagai Y, Metter EJ, Earley CJ, Kemper MK, Becker LC, Lakatta EG, Fleg JL. Increased carotid artery intimal-medial thickness in asymptomatic older subjects with exercise-induced myocardial ischemia. Circulation 1998;98:1504-9. [Crossref] [PubMed]
- Fenster A, Blake C, Gyacskov I, Landry A, Spence JD. 3D ultrasound analysis of carotid plaque volume and surface morphology. Ultrasonics 2006;44 Suppl 1:e153-7. [Crossref] [PubMed]
- Picano E, Paterni M. Ultrasound tissue characterization of vulnerable atherosclerotic plaque. Int J Mol Sci 2015;16:10121-33. [Crossref] [PubMed]
- Napoli C, D'Armiento FP, Mancini FP, Postiglione A, Witztum JL, Palumbo G, Palinski W. Fatty streak formation occurs in human fetal aortas and is greatly enhanced by maternal hypercholesterolemia. Intimal accumulation of low density lipoprotein and its oxidation precede monocyte recruitment into early atherosclerotic lesions. J Clin Invest 1997;100:2680-90. [Crossref] [PubMed]
- Stary HC, Chandler AB, Glagov S, Guyton JR, Insull W Jr, Rosenfeld ME, Schaffer SA, Schwartz CJ, Wagner WD, Wissler RW. A definition of initial, fatty streak, and intermediate lesions of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 1994;89:2462-78. [Crossref] [PubMed]
- Chambless LE, Heiss G, Folsom AR, Rosamond W, Szklo M, Sharrett AR, Clegg LX. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the Atherosclerosis Risk in Communities (ARIC) Study, 1987-1993. Am J Epidemiol 1997;146:483-94. [Crossref] [PubMed]
- O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK Jr. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med 1999;340:14-22. [Crossref] [PubMed]
- Crouse JR 3rd, Raichlen JS, Riley WA, Evans GW, Palmer MK, O'Leary DH, Grobbee DE, Bots ML; METEOR Study Group. Effect of rosuvastatin on progression of carotid intima-media thickness in low-risk individuals with subclinical atherosclerosis: the METEOR Trial. JAMA 2007;297:1344-53. [Crossref] [PubMed]
- Polak JF, Pencina MJ, Pencina KM, O'Donnell CJ, Wolf PA, D'Agostino RB Sr. Carotid-wall intima-media thickness and cardiovascular events. N Engl J Med 2011;365:213-21. [Crossref] [PubMed]
- Pignoli P, Tremoli E, Poli A, Oreste P, Paoletti R. Intimal plus medial thickness of the arterial wall: a direct measurement with ultrasound imaging. Circulation 1986;74:1399-406. [Crossref] [PubMed]
- van der Meer IM, Bots ML, Hofman A, del Sol AI, van der Kuip DA, Witteman JC. Predictive value of noninvasive measures of atherosclerosis for incident myocardial infarction: the Rotterdam Study. Circulation 2004;109:1089-94. [Crossref] [PubMed]
- Cao JJ, Arnold AM, Manolio TA, Polak JF, Psaty BM, Hirsch CH, Kuller LH, Cushman M. Association of carotid artery intima-media thickness, plaques, and C-reactive protein with future cardiovascular disease and all-cause mortality: the Cardiovascular Health Study. Circulation 2007;116:32-8. [Crossref] [PubMed]
- Plichart M, Celermajer DS, Zureik M, Helmer C, Jouven X, Ritchie K, Tzourio C, Ducimetière P, Empana JP. Carotid intima-media thickness in plaque-free site, carotid plaques and coronary heart disease risk prediction in older adults. The Three-City Study. Atherosclerosis 2011;219:917-24. [Crossref] [PubMed]
- Yeboah J, McClelland RL, Polonsky TS, Burke GL, Sibley CT, O'Leary D, Carr JJ, Goff DC, Greenland P, Herrington DM. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA 2012;308:788-95. [Crossref] [PubMed]
- Lorenz MW, von Kegler S, Steinmetz H, Markus HS, Sitzer M. Carotid intima-media thickening indicates a higher vascular risk across a wide age range: prospective data from the Carotid Atherosclerosis Progression Study (CAPS). Stroke 2006;37:87-92. [Crossref] [PubMed]
- Polak JF, Pencina MJ, O'Leary DH, D'Agostino RB. Common carotid artery intima-media thickness progression as a predictor of stroke in multi-ethnic study of atherosclerosis. Stroke 2011;42:3017-21. [Crossref] [PubMed]
- Johnsen SH, Mathiesen EB, Joakimsen O, Stensland E, Wilsgaard T, Løchen ML, Njølstad I, Arnesen E. Carotid atherosclerosis is a stronger predictor of myocardial infarction in women than in men: a 6-year follow-up study of 6226 persons: the Tromsø Study. Stroke 2007;38:2873-80. [Crossref] [PubMed]
- Touboul PJ, Grobbee DE, den Ruijter H. Assessment of subclinical atherosclerosis by carotid intima media thickness: technical issues. Eur J Prev Cardiol 2012;19:18-24. [Crossref] [PubMed]
- Naqvi TZ, Lee MS. Carotid intima-media thickness and plaque in cardiovascular risk assessment. JACC Cardiovasc Imaging 2014;7:1025-38. [Crossref] [PubMed]
- Dogan S, Duivenvoorden R, Grobbee DE, Kastelein JJ, Shear CL, Evans GW, Visseren FL, Bots ML. Radiance 1 and 2 Study Groups. Completeness of carotid intima media thickness measurements depends on body composition: the RADIANCE 1 and 2 trials. J Atheroscler Thromb 2010;17:526-35. [Crossref] [PubMed]
- Gepner AD, Young R, Delaney JA, Tattersall MC, Blaha MJ, Post WS, Gottesman RF, Kronmal R, Budoff MJ, Burke GL, Folsom AR, Liu K, Kaufman J, Stein JH. Comparison of coronary artery calcium presence, carotid plaque presence, and carotid intima-media thickness for cardiovascular disease prediction in the Multi-Ethnic Study of Atherosclerosis. Circ Cardiovasc Imaging 2015.8. [PubMed]
- Kitamura A, Iso H, Imano H, Ohira T, Okada T, Sato S, Kiyama M, Tanigawa T, Yamagishi K, Shimamoto T. Carotid intima-media thickness and plaque characteristics as a risk factor for stroke in Japanese elderly men. Stroke 2004;35:2788-94. [Crossref] [PubMed]
- Peters SA, den Ruijter HM, Palmer MK, Grobbee DE, Crouse JR 3rd, O'Leary DH, Evans GW, Raichlen JS, Bots ML; METEOR Study Investigators. Extensive or restricted ultrasound protocols to measure carotid intima-media thickness: analysis of completeness rates and impact on observed rates of change over time. J Am Soc Echocardiogr 2012;25:91-100. [Crossref] [PubMed]
- Wong M, Edelstein J, Wollman J, Bond MG. Ultrasonic-pathological comparison of the human arterial wall. Verification of intima-media thickness. Arterioscler Thromb 1993;13:482-6. [Crossref] [PubMed]
- Dogan S, Duivenvoorden R, Grobbee DE, Kastelein JJ, Shear CL, Evans GW, Visseren FL, Bots ML. Radiance 1 and Radiance 2 Study Groups. Ultrasound protocols to measure carotid intima-media thickness in trials; comparison of reproducibility, rate of progression, and effect of intervention in subjects with familial hypercholesterolemia and subjects with mixed dyslipidemia. Ann Med 2010;42:447-64. [Crossref] [PubMed]
- Peters SA, den Ruijter HM, Bots ML. Ultrasound protocols to measure carotid intima-media thickness: one size does not fit all. J Am Soc Echocardiogr 2012;25:1135-7. [Crossref] [PubMed]
- Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, Najjar SS, Rembold CM, Post WS; American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr 2008;21:93-111; quiz 189-90. [Crossref] [PubMed]
- Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, Csiba L, Desvarieux M, Ebrahim S, Hernandez Hernandez R, Jaff M, Kownator S, Naqvi T, Prati P, Rundek T, Sitzer M, Schminke U, Tardif JC, Taylor A, Vicaut E, Woo KS. Mannheim carotid intima-media thickness and plaque consensus (2004-2006-2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc Dis 2012;34:290-6. [Crossref] [PubMed]
- Polak JF, Pencina MJ, Herrington D, O'Leary DH. Associations of edge-detected and manual-traced common carotid intima-media thickness measurements with Framingham risk factors: the multi-ethnic study of atherosclerosis. Stroke 2011;42:1912-6. [Crossref] [PubMed]
- Polak JF, O'Leary DH. Edge-detected common carotid artery intima-media thickness and incident coronary heart disease in the multi-ethnic study of atherosclerosis. J Am Heart Assoc 2015;4:e001492. [Crossref] [PubMed]
- Peters SA, den Ruijter HM, Palmer MK, Grobbee DE, Crouse JR 3rd, O'Leary DH, Evans GW, Raichlen JS, Lind L, Bots ML; METEOR Study Group. Manual or semi-automated edge detection of the maximal far wall common carotid intima-media thickness: a direct comparison. J Intern Med 2012;271:247-56. [Crossref] [PubMed]
- Polak JF, Johnson C, Harrington A, Wong Q, O'Leary DH, Burke G, Yanez ND. Changes in carotid intima-media thickness during the cardiac cycle: the multi-ethnic study of atherosclerosis. J Am Heart Assoc 2012;1:e001420. [Crossref] [PubMed]
- Peters SA, Dogan S, Meijer R, Palmer MK, Grobbee DE, Crouse JR 3rd, O'Leary DH, Evans GW, Raichlen JS, Bots ML. The use of plaque score measurements to assess changes in atherosclerotic plaque burden induced by lipid-lowering therapy over time: the METEOR study. J Atheroscler Thromb 2011;18:784-95. [Crossref] [PubMed]
- Polak JF. Sancta simplicitas! J Am Soc Echocardiogr 2012;25:1137-9. [Crossref] [PubMed]
- Den Ruijter HM, Peters SA, Anderson TJ, et al. Common carotid intima-media thickness measurements in cardiovascular risk prediction: a meta-analysis. JAMA 2012;308:796-803. [Crossref] [PubMed]
- Bots ML, Groenewegen KA, Anderson TJ, et al. Common carotid intima-media thickness measurements do not improve cardiovascular risk prediction in individuals with elevated blood pressure: the USE-IMT collaboration. Hypertension 2014;63:1173-81. [Crossref] [PubMed]
- Lorenz MW, Price JF, Robertson C, et al. Carotid intima-media thickness progression and risk of vascular events in people with diabetes: results from the PROG-IMT collaboration. Diabetes Care 2015;38:1921-9. [Crossref] [PubMed]
- Nambi V, Chambless L, Folsom AR, He M, Hu Y, Mosley T, Volcik K, Boerwinkle E, Ballantyne CM. Carotid intima-media thickness and presence or absence of plaque improves prediction of coronary heart disease risk: the ARIC (Atherosclerosis Risk In Communities) study. J Am Coll Cardiol 2010;55:1600-7. [Crossref] [PubMed]
- Inaba Y, Chen JA, Bergmann SR. Carotid plaque, compared with carotid intima-media thickness, more accurately predicts coronary artery disease events: a meta-analysis. Atherosclerosis 2012;220:128-33. [Crossref] [PubMed]
- Polak JF, Szklo M, Kronmal RA, Burke GL, Shea S, Zavodni AE, O'Leary DH. The value of carotid artery plaque and intima-media thickness for incident cardiovascular disease: the multi-ethnic study of atherosclerosis. J Am Heart Assoc 2013;2:e000087. [Crossref] [PubMed]
- Bots ML, Hofman A, Grobbee DE. Increased common carotid intima-media thickness. Adaptive response or a reflection of atherosclerosis? Findings from the Rotterdam Study. Stroke 1997;28:2442-7. [Crossref] [PubMed]
- Glagov S, Zarins C, Giddens DP, Ku DN. Hemodynamics and atherosclerosis. Insights and perspectives gained from studies of human arteries. Arch Pathol Lab Med 1988;112:1018-31. [PubMed]
- Wannarong T, Parraga G, Buchanan D, Fenster A, House AA, Hackam DG, Spence JD. Progression of carotid plaque volume predicts cardiovascular events. Stroke 2013;44:1859-65. [Crossref] [PubMed]
- Spence JD, Eliasziw M, DiCicco M, Hackam DG, Galil R, Lohmann T. Carotid plaque area: a tool for targeting and evaluating vascular preventive therapy. Stroke 2002;33:2916-22. [Crossref] [PubMed]
- Lorenz MW, Polak JF, Kavousi M, Mathiesen EB, Völzke H, Tuomainen TP, Sander D, Plichart M, Catapano AL, Robertson CM, Kiechl S, Rundek T, Desvarieux M, Lind L, Schmid C, DasMahapatra P, Gao L, Ziegelbauer K, Bots ML, Thompson SG; PROG-IMT Study Group. Carotid intima-media thickness progression to predict cardiovascular events in the general population (the PROG-IMT collaborative project): a meta-analysis of individual participant data. Lancet 2012;379:2053-62. [Crossref] [PubMed]
- Spence JD. Measurement of carotid plaque burden. JAMA Neurol 2015;72:383-4. [Crossref] [PubMed]
- Spence JD. Carotid Ultrasound Phenotypes Are Biologically Distinct. Arterioscler Thromb Vasc Biol 2015;35:1910-3. [Crossref] [PubMed]
- Ainsworth CD, Blake CC, Tamayo A, Beletsky V, Fenster A, Spence JD. 3D ultrasound measurement of change in carotid plaque volume: a tool for rapid evaluation of new therapies. Stroke 2005;36:1904-9. [Crossref] [PubMed]
- Spence JD, Hackam DG. Treating arteries instead of risk factors: a paradigm change in management of atherosclerosis. Stroke 2010;41:1193-9. [Crossref] [PubMed]
- Spence JD, Parraga G. Three-Dimensional Ultrasound of Carotid Plaque. Neuroimaging Clin N Am 2016;26:69-80. [Crossref] [PubMed]
- Lanktree MB, Hegele RA, Schork NJ, Spence JD. Extremes of unexplained variation as a phenotype: an efficient approach for genome-wide association studies of cardiovascular disease. Circ Cardiovasc Genet 2010;3:215-21. [Crossref] [PubMed]
- Brinjikji W, Rabinstein AA, Lanzino G, Murad MH, Williamson EE, DeMarco JK, Huston J 3rd. Ultrasound Characteristics of Symptomatic Carotid Plaques: A Systematic Review and Meta-Analysis. Cerebrovasc Dis 2015;40:165-74. [Crossref] [PubMed]
- Brinjikji W, Huston J 3rd, Rabinstein AA, Kim GM, Lerman A, Lanzino G. Contemporary carotid imaging: from degree of stenosis to plaque vulnerability. J Neurosurg 2016;124:27-42. [Crossref] [PubMed]
- Freilinger TM, Schindler A, Schmidt C, Grimm J, Cyran C, Schwarz F, Bamberg F, Linn J, Reiser M, Yuan C, Nikolaou K, Dichgans M, Saam T. Prevalence of nonstenosing, complicated atherosclerotic plaques in cryptogenic stroke. JACC Cardiovasc Imaging 2012;5:397-405. [Crossref] [PubMed]
- Huibers A, de Borst GJ, Bulbulia R, Pan H, Halliday A; ACST-1 collaborative group. Plaque Echolucency and the Risk of Ischaemic Stroke in Patients with Asymptomatic Carotid Stenosis Within the First Asymptomatic Carotid Surgery Trial (ACST-1). Eur J Vasc Endovasc Surg 2016;51:616-21. [Crossref] [PubMed]
- Hatsukami TS, Thackray BD, Primozich JF, Ferguson MS, Burns DH, Beach KW, Detmer PR, Alpers C, Gordon D, Strandness DE Jr. Echolucent regions in carotid plaque: preliminary analysis comparing three-dimensional histologic reconstructions to sonographic findings. Ultrasound Med Biol 1994;20:743-9. [Crossref] [PubMed]
- Noritomi T, Sigel B, Gahtan V, Swami V, Justin J, Feleppa E, Shirouzu K. In vivo detection of carotid plaque thrombus by ultrasonic tissue characterization. J Ultrasound Med 1997;16:107-11. [PubMed]
- Bassiouny HS, Sakaguchi Y, Mikucki SA, McKinsey JF, Piano G, Gewertz BL, Glagov S. Juxtalumenal location of plaque necrosis and neoformation in symptomatic carotid stenosis. J Vasc Surg 1997;26:585-94. [Crossref] [PubMed]
- Kakkos SK, Griffin MB, Nicolaides AN, Kyriacou E, Sabetai MM, Tegos T, Makris GC, Thomas DJ, Geroulakos G; Asymptomatic Carotid Stenosis and Risk of Stroke (ACSRS) Study Group. The size of juxtaluminal hypoechoic area in ultrasound images of asymptomatic carotid plaques predicts the occurrence of stroke. J Vasc Surg 2013;57:609-618.e1; discussion 617-8.
- Virmani R, Kolodgie FD, Burke AP, Finn AV, Gold HK, Tulenko TN, Wrenn SP, Narula J. Atherosclerotic plaque progression and vulnerability to rupture: angiogenesis as a source of intraplaque hemorrhage. Arterioscler Thromb Vasc Biol 2005;25:2054-61. [Crossref] [PubMed]
- Xiong L, Deng YB, Zhu Y, Liu YN, Bi XJ. Correlation of carotid plaque neovascularization detected by using contrast-enhanced US with clinical symptoms. Radiology 2009;251:583-9. [Crossref] [PubMed]
- Saito K, Nagatsuka K, Ishibashi-Ueda H, Watanabe A, Kannki H, Iihara K. Contrast-enhanced ultrasound for the evaluation of neovascularization in atherosclerotic carotid artery plaques. Stroke 2014;45:3073-5. [Crossref] [PubMed]
- Huibers A, de Borst GJ, Wan S, Kennedy F, Giannopoulos A, Moll FL, Richards T. Non-invasive Carotid Artery Imaging to Identify the Vulnerable Plaque: Current Status and Future Goals. Eur J Vasc Endovasc Surg 2015;50:563-72. [Crossref] [PubMed]
- de Bray JM, Baud JM, Delanoy P, Camuzat JP, Dehans V, Descamp-Le Chevoir J, Launay JR, Luizy F, Sentou Y, Cales P. Reproducibility in ultrasonic characterization of carotid plaques. Cerebrovasc Dis 1998;8:273-7. [Crossref] [PubMed]
- Muraki M, Mikami T, Yoshimoto T, Fujimoto S, Tokuda K, Kaneko S, Kashiwaba T. New criteria for the sonographic diagnosis of a plaque ulcer in the extracranial carotid artery. AJR Am J Roentgenol 2012;198:1161-6. [Crossref] [PubMed]
- Heliopoulos J, Vadikolias K, Piperidou C, Mitsias P. Detection of carotid artery plaque ulceration using 3-dimensional ultrasound. J Neuroimaging 2011;21:126-31. [Crossref] [PubMed]
- ten Kate GL, van Dijk AC, van den Oord SC, Hussain B, Verhagen HJ, Sijbrands EJ, van der Steen AF, van der Lugt A, Schinkel AF. Usefulness of contrast-enhanced ultrasound for detection of carotid plaque ulceration in patients with symptomatic carotid atherosclerosis. Am J Cardiol 2013;112:292-8. [Crossref] [PubMed]
- Muraki M, Mikami T, Yoshimoto T, Fujimoto S, Kitaguchi M, Kaga S, Sugawara T, Tokuda K, Kaneko S, Kashiwaba T. Sonographic Detection of Abnormal Plaque Motion of the Carotid Artery: Its Usefulness in Diagnosing High-Risk Lesions Ranging from Plaque Rupture to Ulcer Formation. Ultrasound Med Biol 2016;42:358-64. [Crossref] [PubMed]
- Saba L, Caddeo G, Sanfilippo R, Montisci R, Mallarini G. CT and ultrasound in the study of ulcerated carotid plaque compared with surgical results: potentialities and advantages of multidetector row CT angiography. AJNR Am J Neuroradiol 2007;28:1061-6. [Crossref] [PubMed]
- Watanabe Y, Nagayama M, Suga T, Yoshida K, Yamagata S, Okumura A, Amoh Y, Nakashita S, Van Cauteren M, Dodo Y. Characterization of atherosclerotic plaque of carotid arteries with histopathological correlation: vascular wall MR imaging vs. color Doppler ultrasonography (US). J Magn Reson Imaging 2008;28:478-85. [Crossref] [PubMed]
- Arai D, Yamaguchi S, Murakami M, Nakakuki T, Fukuda S, Satoh-Asahara N, Tsukahara T. Characteristics of carotid plaque findings on ultrasonography and black blood magnetic resonance imaging in comparison with pathological findings. Acta Neurochir Suppl 2011;112:15-9. [Crossref] [PubMed]


