Acute pancreatitis with gradient echo T2*-weighted magnetic resonance imaging
Introduction
Acute pancreatitis (AP) is an acute inflammatory disease in which the inflammatory process may be limited to the pancreas or may spread to surrounding tissues or even the remote organs, resulting in multi-organ failure and occasionally death (1). Although it often has a mild and self-limiting course, it may be severe, resulting in local and systemic complications carrying a significant risk of death (2,3). When local protective responses fail, AP leases local inflammatory mediators into the systemic circulation, resulting in systemic inflammatory response syndrome (SIRS) (4). SIRS may cause multiple organ dysfunction and eventually multi-organ failure, which is associated with very high mortality (5). Early accurate diagnosis and assessment of the severity of AP is crucial to determine an early treatment plan and to improve the prognosis of patients with AP.
Currently, the diagnosis and treatment of AP remain to be standardized. The further use of advanced diagnostic tools will potentially help clinicians manage AP at an appropriate stage (6). In patients with AP, imaging is recommended to confirm the clinical diagnosis, investigate the etiology, and grade the severity of the disease (7). Magnetic resonance imaging (MRI) has an excellent resolution of soft tissue, and it has earned an important role in the diagnosis of AP (7). With the continuous development of MR scanning techniques, multi-echo gradient recalled echo (GRE) T2*-weighted imaging (T2*WI) is a relatively new MRI technique. The sequence of a GRE T2*WI requires high uniformity of the magnetic field and can provide a T2* value, which can indirectly reflect changes in tissue biochemical components and thus be used for the early quantitative diagnosis of some diseases (8,9).
There have been some studies on the GRE T2*WI of pancreas showing T2* was related to iron deposition (10,11). However, until now, there has been no research on the sequence applied in patients with AP. We conducted this study to observe the appearance of the normal pancreas and AP on T2*WI, to measure the T2* value of the pancreas, and to discuss the relationship among T2* values for diagnosing AP and its severity according to the magnetic resonance severity index (MRSI) and the Acute Physiology And Chronic Healthy Evaluation II (APACHE II) scoring system.
Materials and methods
All authors have access to the study data and have reviewed and approved the final manuscript.
Ethics statement
This study has been registered in clinical trials (NCT01911689), and at the beginning of this study, it was also approved by the Institutional Review Board of the Affiliated Hospital of North Sichuan Medical College. Informed consent from all participants involved in our study were obtained, this consent was verbal. For all participants, we only add a multi-echo GRE T2*WI scan after the conventional scan. MRI is radiation-free and the T2*WI has short scan time, which often finish one scan in a minute. So The Ethics Committee concluded the performance of this study would not cause harm or risk to the subjects, and the ethics committees approved and agreed that verbal consent was sufficient for our study.
Patient selection
AP patients admitted to our institution between August 2013 and March 2015 were considered for inclusion in this study. The diagnosis criteria of AP require two of the following three features (12): (I) acute typical abdominal pain; (II) threefold elevation of amylase or lipase serum levels, excluding other causes of elevated enzymes; and (III) abdominal imaging examination. The inclusion criteria for patients in this study were as follows: (I) in-patient; (II) acute history; (III) pancreatitis at first onset; (IV) abdominal MR examination. The exclusion criteria in this study were as follows: (I) inability to cooperate when MR imaging was performed; (II) a history of chronic pancreatitis; (III) AP due to pancreatic carcinoma; (IV) hypoproteinemia; and (V) hypoproteinemia or other peritoneal/retroperitoneal infection diseases; and (VI) iron deposition disorder (e.g., diabetes or blood system diseases). A total of 117 patients with AP were enrolled in this study. There were 45 women and 72 men, with an average age of 50.41±15.55 years (range, 15–84 years). All patients had a clinical assessment and laboratory workup on admission.
Patients without any pancreatic disorders were recruited as controls. They underwent upper abdominal examinations in our hospital as part of physical examinations or assessments for other diseases. The exclusion criteria for this control group were as follows: (I) pancreatic diseases; (II) cirrhosis of the liver; (III) cholecystitis; (IV) biliary tract stones; (V) peritoneal effusion; (VI) abdominal or retroperitoneal tumor; and (VII) iron deposition disorder (e.g., diabetes or blood system diseases). In total, 51 patients comprised the control group. There were 31 men and 20 women, with an average age of 50.90±17.83 years (range, 11–81 years).
MRI techniques
All patients underwent an MRI scan within three days of admission. All MRI examinations were performed with a 3.0T scanner (Discovery MR 750; GE Medical Systems, Milwaukee, Wis) in the supine position. The sequences included two-dimensional coronal and axial single-shot fast spin-echo (SSFSE) T2-weighted; axial fast-recovery fast-spin-echo (FRFSE) T2-weighted with fat suppressed; water-only images generated from axial slab three-dimensional (3D) axial liver acquisition with volume acceleration (LAVA) MR imaging (T1WI); SSFSE radial series slab magnetic resonance cholangiopancreatography (MRCP); and multi-echo GRE T2*WI. GRE T2*WI was obtained in two breath-holds, and the parameters used were as follows: repetition time (TR), 57.3 ms; TE, 1.4, 3.5, 5.7, 7.8, 10.0, 12.2, 14.3, 16.5, 18.6, 20.8, 22.9, 25.1, 27.2, 29.4, 31.5, 33.7 ms; flip angle, 25º; matrix, 256×192; section thickness, 4 mm; overlap, 2.5 mm; and phase field of view (FOV), 0.8 mm. Other parameters of conventional sequences used are in Table 1.
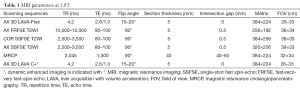
Full table
MRI image interpretation
The original MR imaging data were loaded onto a workstation (advantage workstation 4.4; GE Healthcare) for reviewing. Two observers (with 5 and 8 years of experience in interpreting abdominal MR images) who were blinded to the laboratory data and clinical outcomes independently reviewed the MR images.
On the MR images, AP was defined as edematous and necrotizing pancreatitis. Pancreatic necrosis was defined as a well-marinated area of signal intensity different from the signal intensity of a normal pancreas in a non-enhanced image along with the absence of enhancement in enhanced imaging (13). AP was graded as mild (0–3 points), moderate (4–6 points) or severe (7–10 points) according to the MRSI, which was derived from the CT-severity index (14) (Table 2).

Full table
The features of pancreas in the control group and AP group were observed on GRE T2*WI, and the three segments of the pancreas were evaluated: the head, body, and tail. The regions of interest (ROIs) were placed in a corresponding anatomical position on the GRE-T2*WI. For each segment, the ROI was placed into a central slice as large as possible, avoiding the main pancreatic duct and blood vessels, and the T2* values obtained by copying ROIs in the 3 regions were averaged to provide a regionally independent T2* value. In each subject, the T2* values from the three segments are denominated as T2*-head, T2*-body, and T2*-tail. The average of the T2*-head, T2*-body, and T2*-tail is calculated as the T2* value of the whole pancreas. In acute necrotic pancreatitis, we measured the T2* value in the non-necrotic area in the same manner. In other words, if the necrosis is in the pancreatic tail, the average of the T2*-head and T2*-body values is the T2* value of the whole pancreas. This calculation process for the T2* value was completed using post-processing software in the workstation directly after scanning.
The APACHE-II score
In clinical practice, the physician typically uses the APACHE II to evaluate the severity of AP (15). AP was graded as mild (0–7 points) or severe AP (≥8 points) according to the APACHE II scoring system. An APACHE II score ≥8 points indicates much higher rates of morbidity and mortality (16). The APACHE II score of all 117 patients was calculated according to the laboratory data and clinical outcomes by two physicians, each with 4 years of experience in treating digestive diseases.
Statistical analysis
Data derived from the MR images were expressed as the average of the two observers’ findings, and the T2* value was averaged by multiple measurements. Any discrepancies in the discrete data were discussed by the two observers until a consensus was reached. Kappa statistics were used to assess the inter-rater reliability of the MRSI scoring system. This statistic is generally interpreted as follows: a kappa value equal to or greater than 0.81 indicates very good agreement; a kappa value ranging from 0.80 to 0.61 indicates good agreement; a kappa value ranging from 0.60 to 0.41 indicates moderate agreement; and a kappa value of less than 0.41 indicates poor agreement.
The mean ± SD and range were used to express the continuous variables. A paired-sample t-test and the Spearman rank correlation coefficient were used to analyze the T2* value in the Control group. An independent t-test was used to determine the differences for the T2* value between the Control group and AP group as well as the relationship between the T2* value and the severity of AP according to MRSI and the APACHE II scoring system. The receiver operating characteristic (ROC) curve was used to analyze the usefulness of the T2* value in diagnosing AP and necrotizing AP. Logistic regression analysis was used to test the association of the T2* value with AP.
All statistical tests were performed using the Statistical Package for Social Sciences (SPSS) for Windows (Version 13.0, Chicago, IL, USA). P values <0.05 were considered significant.
Results
Patient sample
In the 117 patients with AP, the etiology of AP included gallstones in 49.57% (58/117) patients, alcohol abuse in 11.11% (13/117) patients, high-fat meals in 8.55% (10/117) patients, postoperative status in 1.71% (2/117) patients, and no specified etiology in 29.06% (34/117) patients.
Of the 51 patients in the control group, 21 patients had no abnormalities in the abdomen, 17 patients had hepatic cysts, 4 patients had hepatic hemangiomas and 9 patients had renal cysts according to MRI.
Findings of normal pancreas on T2*WI
In the 51 patients in the control group, the pancreas showed a well-marinated and consistently homogeneous isointensity on GRE-T2*WI. The corresponding signal decay curve is relatively smooth (Figure 1). The T2*-head, T2*-body and T2*-tail averaged 22.74±3.45 ms, 20.51±3.40 ms and 19.92±3.89 ms, respectively. The average T2* value was 21.06±2.64 ms for the whole pancreas in Control group. The T2* value was significantly different between the head and body (t=3.59, P=0.001) and between the head and the tail (t=4.297, P<0.001), but no significant difference was found between the body and the tail (t=1.30, P=0.20).
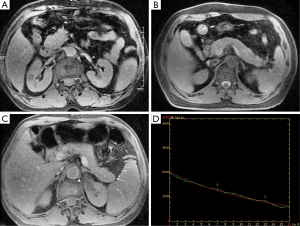
Findings of AP on conventional MRI and T2*WI
In the 117 patients with AP, 79.49% (93/117) of the patients were diagnosed with edematous AP, whereas 20.51% (24/117) of the patients were diagnosed with necrotizing AP on MRI. The agreement between the observers for the MRSI score was very good (κ=0.84). The MRSI score was 3.84±1.75 points (range, 0–10 points). A total of 35.04% (41/117), 58.97% (69/117) and 5.98% (7/117) of the patients had mild, moderate and severe AP according to MRSI, respectively.
On GRE T2*WI, the pancreas in edematous pancreatitis shows an ill-defined and enlarged but homogeneous signal intensity, and the signal decay curve is relatively smooth (Figure 2). However, there were also two patients with clinically confirmed AP (2/117, 1.71%) who had no evidence on the conventional sequence; the T2* value had increased visually with one had T2* values of the head, body and the tail being 20.143, 34.031 and 33.212 ms, respectively.
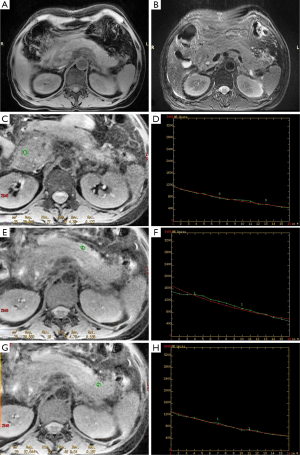
For necrotizing pancreatitis, the non-necrotic area manifests as well as edematous pancreatitis. The signal decay of a necrotic area no longer universally hold, particularly for hemorrhage areas; a local hemorrhage could result in a decreased T2* value and signal loss on the signal decay curve (Figures 3,4). In the cohort, 10.25% (12/117) of patients had elevated signal intensity in the water-only images generated from LAVA-flex, suggesting hemorrhage. Hemorrhage occurred in the pancreatic parenchyma, which manifested as hyperintensity on T1-weighted LAVA-Flex imaging and as corresponding signal loss and a decreased T2* value on T2*WI. The T2* value of the pancreatic hemorrhage lesion was 11.80±2.30 ms, which was significantly lower than that of control group (t=−13.94, P<0.001). In the 12 patients with hemorrhage, there were 5 patients whose hemorrhage occurred in the peripancreatic area, which lacked the characteristic appearance on the GRE T2*WI. The T2* values of the non-necrotic area for the T2*-head, T2*-body and T2*-tail were 26.85±6.66 ms (n=114), 26.02±6.04 ms (n=110) and 24.46±5.57 ms (n=106), respectively, with an average of 25.86±4.90 ms for whole pancreas in AP patients.
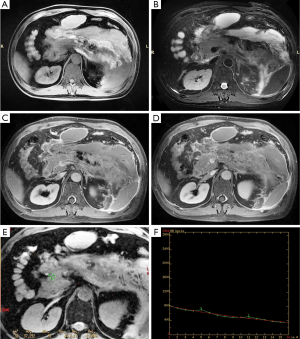
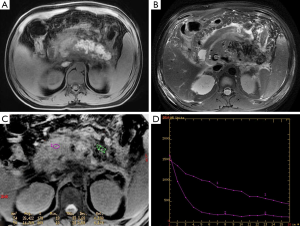
T2* values in the diagnosis and evaluation of the severity of AP according to MRSI
The T2* value of the AP group was significantly higher than that of the control group (t=−8.02, P<0.001). For a diagnosis of AP, the area under the ROC curve of the T2* value is 0.80 (P<0.001) (Figure 5). In regression models, AP was associated with a one standard deviation increment in the T2* value (OR =1.37; 95% CI: 1.22–1.53; P<0.001).
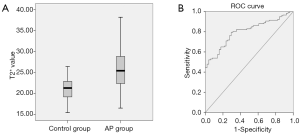
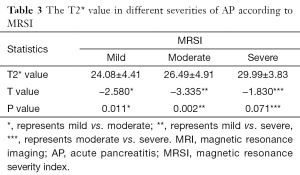
The T2* value shows significant difference between edematous AP and necrotizing AP (25.28±4.56 vs. 28.10±5.59 ms; t=2.57, P=0.01). For diagnosing necrotizing AP, the area under the ROC curve of the T2* value is 0.66 (P=0.02). The T2* values were 24.08±4.41, 26.49±4.91 and 29.99±3.83 ms in mild, moderate and severe AP according to MRSI, respectively. The T2* values increase along with the gradual increase in the MRSI score (Table 3) and are significantly different between mild and moderate AP (P=0.011) as well as mild and severe AP (P=0.002); however, there are no significant differences between moderate and severe AP (P=0.071).
Full table
The relationship between the T2* value and the severity of AP according to APACHE II
Among the 117 patients with AP, the mean APACHE II score was (5.10±3.51) points (range, 0–14 points). A total of 89 patients had mild AP (APACHE II score <8 points), whereas 28 patients had severe AP (APACHE II score ≥8 points). The T2* value was 25.73±4.80 and 26.25±5.26 ms in mild and severe AP according to APACHE II, respectively. There is no significant difference between mild and severe AP (t=−0.49, P=0.63).
Discussion
In this study, we found that GRE-T2*WI of pancreas is feasible; reliable image quality combined with T2* values can contribute to diagnosing AP and determining its severity. The T2* value of the AP group was higher than that of the Control group, both for necrotizing AP and edematous AP, and it had a tendency to increase gradually along with the increasing MRSI scores. Furthermore, the addition of GRE-T2*WI to conventional MR imaging may help in detecting potential hemorrhages.
On GRE-T2*WI, the normal pancreas showed a well-marinated and consistently homogeneous isointensity, and the corresponding signal decay curve was relatively smooth. The T2* values of the head, body and tail of normal pancreas were 22.74±3.45, 20.51±3.40, 19.92±3.90 ms, respectively, and the T2* values were significantly higher for the head than for the body and tail. Our results agree with other related reports (17-19), which use DWI to research the different ADC values of the head, body, and tail of normal pancreas. These different values may be explained by the differences in tissue composition, with an increasing number of islets of Langerhans and a higher density of fatty components toward the tail (18). Another possible reason is the different blood supply to the head, body and tail of normal pancreas (20).
Edematous AP, as well as the non-necrotic area in the necrotizing AP, shows enlarged and ill-defined but homogeneous signal intensity. The T2* value of the AP patient group was 25.86±4.90 ms. AP is an acute inflammatory disease; as in other inflammatory diseases, the pathological changes are mainly edema, hyperemia and inflammatory cell infiltration. Cell edema will lead to an increase in hydrogen protons, which can prolong T2* relaxation and results in an increased T2* value (21,22). In this study, we found that the T2* value in the AP group was higher than that in the control group, and for diagnosing AP, the area under the ROC curve of the T2* value was 0.801. The logistic regression analysis more reliably demonstrated the usefulness of the T2* value in the diagnosis of AP. Our results indicate that T2*WI can reveal the inflammation of pancreas in AP and is helpful for a quantitative diagnosis of AP. In our study, 1.69% (2/117) of the patients showed normal pancreas on the MR image but with an increased T2* value. Thus, we have evidence to speculate that an increased T2* value can help in the diagnosis of AP. In future studies, the results of this work need to be confirmed with a larger population.
AP with hemorrhage in the pancreatic parenchyma but not in the peripancreatic area shows a decrease in the T2* value and a signal loss on the signal decay curve. The addition of these image characteristics to conventional MRI can aid in the qualitative diagnosis of AP and in the detection of hemorrhage. In clinical practice, the basic treatment for AP is conservative medical therapy. Therefore, it is difficult to obtain histopathological evidence of hemorrhage (23). Although CT and angiography can detect hemorrhage, the main disadvantage of these techniques is ionizing radiation (7). Romanova et al. (24) reported that CT is also less sensitive than MRI in detecting hemorrhage. To our knowledge, the hyperintensity on T1-weighted images with fat suppression often refers to the hemorrhage (25,26), but investigators have reported that the T1 hyperintense signal may vary with the combination of blood, high protein content, fat, and calcification (27). In this case, GRE T2*WI was used for the differential diagnosis. Breakdown products of hemorrhage have a large paramagnetic effect or recalled magnetic susceptibility effects, which can change the uniformity of the local magnetic field, as shown in the decrease of the T2* value and the signal loss on the signal decay curve (9). Thus, the addition of GRE-T2*WI to conventional MR imaging can contribute to the detection of hemorrhage. The peripancreatic hemorrhage lacked a characteristic appearance on GRE T2*WI, which may due to peripancreatic inflammatory exudation or even fluid collection.
The T2* value in necrotizing AP is higher than that in edematous AP, and the ROC curve (area =0.66, P=0.02) also suggests the usefulness of the T2* value in the diagnosis of necrotizing AP. Failure in the microcirculation led to a histopathologically significant increase in acinar cell necrosis and edema level in AP (28). We can speculate that the pancreatic cell edema level is higher in necrotizing AP than in edematous AP.
The MRSI was derived from the CT severity score index developed by Balthazar (29), which included the severity scores of pancreatic inflammation and pancreatic necrosis. In this study, we found that the T2* values increased along with the increase in MRSI scores. Although the T2* value had a tendency to increase gradually along with the MRSI scores, there was no significant difference between moderate and severe AP (P=0.071). One possible reason is that the higher the AP severity is according to the MRSI, the more severe the inflammation of the pancreas is. As mentioned above, the T2* value increases in necrotizing AP, and the moderate and severe AP group also contained some patients with necrotizing AP. Another possible reason is that the sample size was not large enough to attain statistical significance in each of its separate arms.
APACHE II has the advantage of reflecting systemic complications. Patients with higher APACHE II scores often have more serious general conditions (30). In this study, we found that there was no statistically significant difference between mild and the severe AP according to the APACHE II score. One possible reason for this result may be that the T2* value reflects only local pancreatic features, which are hardly affected by the APACHE II.
Our study suffers from several limitations: (I) detection bias. We chose a relatively small ROI. Schwenzer et al. (31) studied the iron deposition of pancreas using ROIs ranging from 1.0–1.4 cm2; small ROIs allow flexibility when measuring the T2* value, which is useful to avoid the main pancreatic duct, large vessels, etc. Measurement precision can be improved by averaging the values of repeated measurements; (II) performance bias. The second limitation is that the time interval between the MRI and the onset of AP was variable, which may have affected T2* values and the MRSI scores. We performed MRI examinations within three days after admission to minimize this variability. In addition, the APACHE II score was calculated by several physicians and nurses, which may have led to variations between the different observers. However, this likely does not affect the main results of the study; (III) selection bias. Some diseases, such as chronic anemia, diabetes, etc., may lead to iron deposition in pancreas. Some recruited patients did not have a clear medical history, which can lead to selection bias. In our study, all of the patients met the inclusion and exclusion criteria.
In summary, GRE T2*WI demonstrated some characteristics of the normal pancreas and AP, which was helpful for detecting hemorrhage. The addition of GRE T2*WI to conventional MR can contribute to diagnosing AP and determining its severity. Because it provides a quantitative diagnosis and evaluation of AP, GRE-T2*WI may be a feasible and effective tool for AP.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Beger HG, Rau B, Mayer J, Pralle U. Natural course of acute pancreatitis. World J Surg 1997;21:130-5. [Crossref] [PubMed]
- Hecker M, Mayer K, Askevold I, Collet P, Weigand MA, Krombach GA, Padberg W, Hecker A. Acute pancreatitis. Anaesthesist 2014;63:253-63. [Crossref] [PubMed]
- Novovic S, Malmstrøm ML, Møller Andersen A, Jørgensen LN, Philipsen E, Schmidt PN, Hansen MB. Monitorering and complications by conservative treatment of severe acute pancreatitis. Ugeskr Laeger 2013;175:1478-81. [PubMed]
- Kylänpää ML, Repo H, Puolakkainen PA. Inflammation and immunosuppression in severe acute pancreatitis. World J Gastroenterol 2010;16:2867-72. [Crossref] [PubMed]
- Banks PA, Freeman ML; Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis. Am J Gastroenterol 2006;101:2379-400. [Crossref] [PubMed]
- Feng YC, Wang M, Zhu F, Qin RY. Study on acute recent stage pancreatitis. World J Gastroenterol 2014;20:16138-45. [Crossref] [PubMed]
- Türkvatan A, Erden A, Türkoğlu MA, Seçil M, Yener Ö. Imaging of acute pancreatitis and its complications. Part 1: acute pancreatitis. Diagn Interv Imaging 2015;96:151-60. [Crossref] [PubMed]
- Mamisch TC, Hughes T, Mosher TJ, Mueller C, Trattnig S, Boesch C, Welsch GH. T2 star relaxation times for assessment of articular cartilage at 3 T: a feasibility study. Skeletal Radiol 2012;41:287-92. [Crossref] [PubMed]
- Tang MY, Chen TW, Zhang XM, Huang XH. GRE T2*-weighted MRI: principles and clinical applications. Biomed Res Int 2014;2014:312142.
- Youssef DM, Fawzy Mohammad F, Ahmed Fathy A, Aly Abdelbasset M. Assessment of hepatic and pancreatic iron overload in pediatric Beta-thalassemic major patients by t2* weighted gradient echo magnetic resonance imaging. ISRN Hematol 2013;2013:496985.
- Au WY, Lam WW, Chu W, Tam S, Wong WK, Liang R, Ha SY. A. T2* magnetic resonance imaging study of pancreatic iron overload in thalassemia major. Haematologica 2008;93:116-9. [Crossref] [PubMed]
- Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013;62:102-11. [Crossref] [PubMed]
- Viremouneix L, Monneuse O, Gautier G, Gruner L, Giorgi R, Allaouchiche B, Pilleul F. Prospective evaluation of nonenhanced MR imaging in acute pancreatitis. J Magn Reson Imaging 2007;26:331-8. [Crossref] [PubMed]
- Balthazar EJ, Robinson DL, Megibow AJ, Ranson JH. Acute pancreatitis: value of CT in establishing prognosis. Radiology 1990;174:331-6. [Crossref] [PubMed]
- Rahman SH, Ibrahim K, Larvin M, Kingsnorth A, McMahon MJ. Association of antioxidant enzyme gene polymorphisms and glutathione status with severe acute pancreatitis. Gastroenterology 2004;126:1312-22. [Crossref] [PubMed]
- De Campos T, Cerqueira C, Kuryura L, Parreira JG, Soldá S, Perlingeiro JA, Assef JC, Rasslan S. Morbimortality indicators in severe acute pancreatitis. JOP 2008;9:690-7. [PubMed]
- Concia M, Sprinkart AM, Penner AH, Brossart P, Gieseke J, Schild HH, Willinek WA, Mürtz P. Diffusion-weighted magnetic resonance imaging of the pancreas: diagnostic benefit from an intravoxel incoherent motion model-based 3 b-value analysis. Invest Radiol 2014;49:93-100. [Crossref] [PubMed]
- Schoennagel BP, Habermann CR, Roesch M, Hahne JD, Arndt C, Kleibeler L, Petersen KU, Graessner J, Adam G, Herrmann J. Diffusion-weighted imaging of the healthy pancreas: apparent diffusion coefficient values of the normal head, body, and tail calculated from different sets of b-values. J Magn Reson Imaging 2011;34:861-5. [Crossref] [PubMed]
- Herrmann J, Schoennagel BP, Roesch M, Busch JD, Derlin T, Doh LK, Petersen KU, Graessner J, Adam G, Habermann CR. Diffusion-weighted imaging of the healthy pancreas: ADC values are age and gender dependent. J Magn Reson Imaging 2013;37:886-91. [Crossref] [PubMed]
- Collins JM, Silva AC, Hayman LA. Arterial anatomy of the pancreas. Part 3: segmented computed tomography-angiography mapping of perineural invasion. J Comput Assist Tomogr 2010;34:961-5. [Crossref] [PubMed]
- Perry J, Haughton V, Anderson PA, Wu Y, Fine J, Mistretta C. The value of T2 relaxation times to characterize lumbar intervertebral disks: preliminary results. AJNR Am J Neuroradiol 2006;27:337-42. [PubMed]
- Takashima H, Takebayashi T, Yoshimoto M, Terashima Y, Tsuda H, Ida K, Yamashita T. Correlation between T2 relaxation time and intervertebral disk degeneration. Skeletal Radiol 2012;41:163-7. [Crossref] [PubMed]
- Lefter LP, Dajbog E, Scripcariu V, Dragomir C. Safety and efficacy of conservative management in acute severe pancreatitis. Chirurgia (Bucur) 2006;101:135-9. [PubMed]
- Romanova AL, Nemeth AJ, Berman MD, Guth JC, Liotta EM, Naidech AM, Maas MB. Magnetic resonance imaging versus computed tomography for identification and quantification of intraventricular hemorrhage. J Stroke Cerebrovasc Dis 2014;23:2036-40. [Crossref] [PubMed]
- Furlan A, Marin D, Bae KT, et al. Focal liver lesions hyperintense on T1-weighted magnetic resonance images. Semin Ultrasound CT MR 2009;30:436-49. [Crossref] [PubMed]
- Xiao B, Zhang XM. Magnetic resonance imaging for acute pancreatitis. World J Radiol 2010;2:298-308. [Crossref] [PubMed]
- Kim YK, Kim CS, Han YM. Role of fat-suppressed t1-weighted magnetic resonance imaging in predicting severity and prognosis of acute pancreatitis: an intraindividual comparison with multidetector computed tomography. J Comput Assist Tomogr 2009;33:651-6. [Crossref] [PubMed]
- Yildirim AO, Ince M, Eyi YE, Tuncer SK, Kaldirim U, Eroglu M, Oztas E, Cayci T, Kilic A, Inal V, Yamanel L, Yasar M. The effects of glycyrrhizin on experimental acute pancreatitis in rats. Eur Rev Med Pharmacol Sci 2013;17:2981-7. [PubMed]
- Zhang XM, Feng ZS, Zhao QH, Xiao CM, Mitchell DG, Shu J, Zeng NL, Xu XX, Lei JY, Tian XB. Acute interstitial edematous pancreatitis: Findings on non-enhanced MR imaging. World J Gastroenterol 2006;12:5859-65. [Crossref] [PubMed]
- Khan AA, Parekh D, Cho Y, Ruiz R, Selby RR, Jabbour N, Genyk YS, Mateo R. Improved prediction of outcome in patients with severe acute pancreatitis by the APACHE II score at 48 hours after hospital admission compared with the APACHE II score at admission. Acute Physiology and Chronic Health Evaluation. Arch Surg 2002;137:1136-40. [Crossref] [PubMed]
- Schwenzer NF, Machann J, Haap MM, Martirosian P, Schraml C, Liebig G, Stefan N, Häring HU, Claussen CD, Fritsche A, Schick F. T2* relaxometry in liver, pancreas, and spleen in a healthy cohort of one hundred twenty-nine subjects-correlation with age, gender, and serum ferritin. Invest Radiol 2008;43:854-60. [Crossref] [PubMed]


