
Simultaneous bilateral central retinal artery occlusion in a patient with dilated cardiomyopathy
Introduction
Central retinal artery occlusion (CRAO) is a retinal arterial occlusive disease that causes acute painless monocular loss of visual acuity. It usually presents in older patients with vascular risk factors. The most common cause of unilateral CRAO is embolization of thrombus from the ipsilateral internal carotid artery (1). However, there have been few reports of simultaneous bilateral CRAO which results from systemic inflammatory conditions (2-4). Here, we report the case of young patient with simultaneous bilateral CRAO caused by dilated cardiomyopathy (DCM).
Case presentation
A 29-year-old man presented with sudden-onset loss of visual acuity in both eyes and dysarthria. His initial blood pressure was 132/82 mmHg. He had no risk factors for cardiovascular disease, family history, or any connective tissue disorder symptoms. On ocular examination, his visual acuity was hand motion at 1 m bilaterally, with no restriction of extraocular movement. A normal pupillary reaction was observed with no unusual findings in the anterior segment. Fundus examination revealed bilateral pallor of the optic disc and confluent peripapillary cotton wool spots. In addition, an edematous white retina with a cherry-red spot was observed in both eyes (Figure 1A,1B). On neurologic examination, another neurologic deficit was not observed, except loss of visual acuity in both eyes and dysarthria.
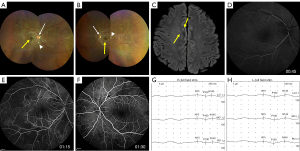
Electrocardiography revealed sinus rhythm with left ventricular hypertrophy. Brain magnetic resonance imaging (MRI) and magnetic resonance angiography revealed multiple tiny scattered acute ischemic stroke in the right frontal cortex and no steno-occlusive lesions in the intracranial or extracranial arteries, except bilateral central retinal artery (Figure 1C). Initial blood test results including infection, vasculitis (including double-stranded deoxyribonucleic acid antibody, rheumatoid factor, antineutrophil cytoplasmic antibody, antinuclear antibody, and serum tests for systemic lupus erythematosus, lupus-like disease and Sjogren’s syndrome), coagulopathy (including prothrombin G20210A, Factor V Leiden, antithrombin III, anticardiolipin antibody IgG and IgM, lupus anticoagulant, homocysteine, protein S activity, and protein C activity) and inflammation markers were within the reference ranges, with the exception of slightly elevated D-dimer (0.59 mg/L, reference <0.5 mg/L). In addition, cerebrospinal fluid tests with immunoglobulin G index and aquaporin-4 antibody results were within the reference ranges. Fundus fluorescein angiography revealed delayed arm-retinal circulation time and arteriovenous transit time in both eyes (Figure 1D-1F). Subsequent digital subtraction angiography confirmed occlusion in both central retinal arteries (Figure S1A,S1B). The visual evoked potential (VEP) test demonstrated prolonged latency and decreased amplitude of the P100 wave in both eyes (Figure 1G,1H).
Transthoracic echocardiography revealed a dilated left ventricle (LV) and left atrium (LA) with moderate LV systolic dysfunction [ejection fraction (EF) =35%], suggestive of DCM. Heart MRI revealed clear DCM, which was characterized by dilated LV, hypokinesia of the LV (EF =23%), and delayed enhancement in the mid-wall (Figure S2A,S2B). There was no evidence of common etiologies of cryptogenic stroke, such as paroxysmal atrial fibrillation on Holter monitoring, patent foramen ovale on transcranial Doppler monitoring and transesophageal echocardiography, or aortic arch atheroma and obstructive coronary artery disease on cardiac computer tomography angiography. Moreover, there was no evidence of other causes for CRAO such as using intravenous drug or cocaine, having history of migraine, systemic hypotension, or malignant hypertension. Therefore, it was considered that simultaneous bilateral CRAO and multiple tiny scattered acute ischemic stroke in the right frontal cortex were caused by cardiogenic embolism due to DCM. Although LA or LV thrombus was not observed, warfarin was initiated for secondary prevention of the embolic source of the heart. Early neurological deterioration was not observed during the following 2 weeks; therefore, the patient was discharged. After 3 months of anticoagulation treatment, although his visual acuity was counting fingers at 1 m bilaterally on ocular examination, follow-up VEP continuously showed prolonged latency and decreased amplitude of the P100 wave in both eyes.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
We described a case of simultaneous bilateral CRAO and multiple tiny scattered acute ischemic stroke in the right frontal cortex caused by DCM. CRAO refers to a compromise in blood flow from the central retinal artery to the inner layers of the retina, which causes infarction of the retina. Unilateral CRAO is a rare disease with an incidence of 1–2:100,000 and the occurrence of simultaneous bilateral CRAO in younger patients is even rarer (1,5). Most unilateral CRAO resulted from local thrombus formation or thromboembolism. However, bilateral CRAO reported rarely and resulted from lupus, Takayasu’s arteritis, and Wegener’s granulomatosis (2-4). Except for a previous report of an old man with bilateral CRAO and noncompaction cardiomyopathy without thrombus which is resulted by abnormal development of heart (6), there are no reports of younger patients presenting with bilateral CRAO due to DCM.
DCM, especially left ventricular EF <30%, is a definite cardioembolic source of ischemic stroke (7). DCM is related to cardioembolism in 66.7% of patients (8). The pathomechanism of stroke in patients with DCM might be associated with blood stasis, altered myocardial kinetics, decreased cardiac output, and arrhythmia, which is risk factors for thrombosis (9). In the current case, patient had multiple tiny scattered cortical infarctions with simultaneous bilateral CRAO and DCM with dilated and hypokinesia of the LV. Therefore, we thought that DCM with dilated and hypokinesia of the LV might have promoted a thrombogenesis, leading to multiple tiny scattered right frontal cortical infarctions with simultaneous bilateral CRAO. Also, we thought that the elevated D-dimer levels might be due to secondary reactions of the hypercoagulable state. Recent guideline suggested that anticoagulation in patients with DCM (reduced EF) and ischemic stroke in sinus rhythm with no evidence of LA or LV thrombus is not well established, and may be considered risk factor for recurrent thromboembolism (10). In the current case, although follow-up VEP continuously showed prolonged latency and decreased amplitude of the P100 wave in both eyes after 3 months of anticoagulation treatment, his visual acuity was subtle improved.
In conclusion, sudden simultaneous bilateral CRAO may be caused by thromboembolism that originates from DCM-associated thrombosis. Cardiac evaluation to detect DCM-associated thrombi may aid in the accurate diagnosis and treatment of simultaneous bilateral CRAO.
Acknowledgments
Funding: This research was supported by a grant from Kyung Hee University in 2022 (No. KHU-20220786) and a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (No. HI20C1405).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-456/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mac Grory B, Schrag M, Biousse V, Furie KL, Gerhard-Herman M, Lavin PJ, Sobrin L, Tjoumakaris SI, Weyand CM, Yaghi SAmerican Heart Association Stroke Council. Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Hypertension; and Council on Peripheral Vascular Disease. Management of Central Retinal Artery Occlusion: A Scientific Statement From the American Heart Association. Stroke 2021;52:e282-94. [Crossref] [PubMed]
- Chandran K, Shenoy SB, Kulkarni C, Mathew NR. Bilateral simultaneous Central Retinal Artery Occlusion (CRAO) in a patient with Systemic Lupus Erythematosus (SLE). Am J Ophthalmol Case Rep 2020;19:100833. [Crossref] [PubMed]
- Costello F, Gilberg S, Karsh J, Burns B, Leonard B. Bilateral simultaneous central retinal artery occlusions in wegener granulomatosis. J Neuroophthalmol 2005;25:29-32. [Crossref] [PubMed]
- Vukkadala T, Balaji A, Azad SV, Kumar V. Bilateral central retinal artery occlusion in a patient with diagnosed Takayasu's arteritis. Indian J Ophthalmol Case Rep 2021;1:71.
- Park SJ, Choi NK, Seo KH, Park KH, Woo SJ. Nationwide incidence of clinically diagnosed central retinal artery occlusion in Korea, 2008 to 2011. Ophthalmology 2014;121:1933-8. [Crossref] [PubMed]
- Jin-Poi T, Shatriah I, Ng SL, Zurkurnai Y, Yunus R. Noncompaction cardiomyopathy manifesting as retinal artery occlusion. JAMA Ophthalmol 2013;131:263-5. [Crossref] [PubMed]
- Kim W, Kim EJ. Heart Failure as a Risk Factor for Stroke. J Stroke 2018;20:33-45. [Crossref] [PubMed]
- Vemmos K, Ntaios G, Savvari P, Vemmou AM, Koroboki E, Manios E, Kounali A, Lip GY. Stroke aetiology and predictors of outcome in patients with heart failure and acute stroke: a 10-year follow-up study. Eur J Heart Fail 2012;14:211-8. [Crossref] [PubMed]
- Mazzone M, La Sala M, Portale G, Ursella S, Forte P, Carbone L, Testa A, Pignataro G, Covino M, Gentiloni Silveri N. Review of dilated cardiomyopathies. Dilated cardiomyopathies and altered prothrombotic state: a point of view of the literature. Panminerva Med 2005;47:157-67. [PubMed]
- Kleindorfer DO, Towfighi A, Chaturvedi S, Cockroft KM, Gutierrez J, Lombardi-Hill D, Kamel H, Kernan WN, Kittner SJ, Leira EC, Lennon O, Meschia JF, Nguyen TN, Pollak PM, Santangeli P, Sharrief AZ, Smith SC Jr, Turan TN, Williams LS. 2021 Guideline for the Prevention of Stroke in Patients With Stroke and Transient Ischemic Attack: A Guideline From the American Heart Association/American Stroke Association. Stroke 2021;52:e364-467. [Crossref] [PubMed]


