Reversal of left ventricular “rigid body rotation” during dipyridamole-induced stress in a patient with stable angina: a case from the three-dimensional speckle tracking echocardiographic MAGYAR-Stress Study
Introduction
The left ventricular (LV) twist is defined as the wringing motion of the heart around its long-axis in systole caused by oppositely directed counterclockwise apical and clockwise basal rotations resulted from the movement of two orthogonally oriented muscular bands (1). In some clinical circumstances, rotation at both basal and apical levels of the LV occurred in the same clockwise or counterclockwise direction during systole resulting the near absence of LV twist as called left ventricular “rigid body rotation” (LV-RBR) (2-13). Three-dimensional (3D) speckle-tracking echocardiography (STE) is a new technique with potential for noninvasive assessment of LV myocardial movements (14,15). Hereby we present that LV-RBR normalization of LV rotational mechanics could be demonstrated at maximum hyperaemia during dipyridamole-induced stress by 3DSTE in a patient with stable angina.
Case presentation
A 60-year-old man is presented, who was referred to our Cardiac Catheterization Laboratory due to stable angina. Only hypertension and hyperlipidemia were in his anamnesis. Coronary angiography showed left dominant coronary system with 30–40% lesions in the proximal left anterior descending (LAD) coronary artery, while left circumflex and right coronary (RC) arteries seemed to be intact. Fractional flow reserve measurements were performed showing 0.85 in LAD and 0.87 in RC suggesting haemodinamically non-significant lesions. The patient has been included in the MAGYAR-Stress Study (Motion Analysis of the heart and Great vessels bY three-dimensionAl speckle-tRacking echocardiography during Stress protocols), in which among others drug-induced stress-related pathophysiologic consequences were aimed to be examined (‘magyar’ means ‘Hungarian’ in Hungarian language). Informed consent was obtained from the patient and the study protocol was approved by the institution’s human research committee.
Following complete two-dimensional (2D) Doppler echocardiography 3DSTE was performed with the same Toshiba ArtidaTM echocardiography equipment (Toshiba, Tokyo, Japan) using a 1–4 MHz matrix phased-array PST-25SX transducer (15). Six wedge-shaped subvolumes were acquired within a single breath-hold from the apical window to create full-volume 3D datasets at rest and at peak hyperaemia. Dipyridamole was used as a vasodilator stress agent using standard international protocols. During chamber quantifications 3D Wall Motion Tracking software version 2.7 was used. The apical two-(AP2CH) and four-chamber (AP4CH) views and three short-axis views at different LV levels from the LV base to apex were automatically selected from the 3D echocardiographic pyramidal dataset at end-diastole by the software. Two points of the LV endocardium at the edges of the mitral valve and one at the LV apex were marked manually on the AP2CH and AP4CH views. Then, the 3D endocardial surface was automatically reconstructed and tracked in 3D space throughout the cardiac cycle. Manual adjustments were also performed, if needed. Curves were generated by the software for quantification of global peak apical, midventricular and basal LV rotations at rest and at peak hyperaemia (Figure 1).
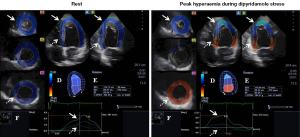
No wall motion abnormalities could be detected both in resting conditions and at peak hyperaemia by visual assessment suggesting a negative stress test result. Interestingly, apical and basal LV rotations were in the same counterclockwise direction suggesting LV-RBR at rest. At peak hyperaemia basal LV rotation become clockwise-directed, while apical rotation remained counterclockwise oriented (Figure 1). Following stress at recovery phase, all these changes in rotational mechanics returned back to LV-RBR.
Discussion
The evaluation of LV function by visual assessment is restricted by its known subjective nature. STE allows quantitative analysis of myocardial global and segmental deformations by assessing strain and rotational characteristics (15). Although 2DSTE has emerged due to its angle independency, this methodology does not allow to see the heart as a 3D organ. 3DSTE overcomes this limitation from a single 3D data acquisition from which all strain and rotational parameters could be calculated at the same time (15).
In healthy subjects, LV systole is associated with a counterclockwise rotation of the LV apex and a clockwise rotation of the LV base resulting in a towel-wringing motion as called LV twist (net difference between LV apex and LV base) (1). LV twist is the result of the movement of two orthogonally oriented mascular bands of a helical myocardial structure and responsible for up to 40% of LV stroke volume in physiologic studies (16). In the presented case, both LV apical and basal rotations were in the same counterclockwise direction at rest confirming near absence of LV twist (LV-RBR). Recently, LV-RBR could be demonstrated in different cardiomyopathies [noncompaction (2-7); dilated (8) and hypertensive (9) with reduced systolic funtion], congenital heart diseases [hypoplastic right heart syndrome (10), univentricular heart (11), Ebstein anomaly (12)] and amyloidosis (13). Interestingly, in a woman with postpartum cardiomyopathy with noncompaction phenotype and LV-RBR, 6-month heart failure treatment was associated with reversal of LV-RBR proceeding normally directed LV rotations (7). No structural or functional cardiac alterations, as well as LV wall depositions could be confirmed in our case. Although our knowledge is limited regarding the real prevalence of LV-RBR, the effect of classic risk factors (for instance hypertension) or a subclinical disease (focal oedema, inlammation, etc.) could explain its presence. Moreover, there is no literature, which could support the fact that a non-significant coronary artery disease and related haemodynamic changes are associated with LV-RBR, but obviously could not be excluded.
To the best of authors’ knowledge this is the first time to demonstrate normalization of LV rotational mechanics during dipyridamole-induced stress. In normal situation subepicardial myocardial fibers run in a left-handed direction leading to a clockwise rotation of the LV base and a counterclockwise rotation of the LV apex. Myocardial fibers on the subendocardial side run in a right-handed direction, and contraction of these fibers will cause the LV base to rotate in a counterclockwise direction and the LV apex to rotate in a clockwise direction. Due to greater radius of rotation of the subepicardium it consequently provides greater torque than the subendocardium resulting in a significantly expressed rotation of the subepicardium (16). In the presented case counterclockwise rotation of the LV base could be detected at rest which turned to normally directed clockwise basal LV rotation theoretically due to dipyridamole-induced vasodilation via altered blood supply at maximum hyperaemia. This case could highlight our attention on the importance on the assessment of changes in myocardial deformation during stress imaging.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this case report.
References
- Nemes A, Kalapos A, Domsik P, Forster T. Left ventricular rotation and twist of the heart. Clarification of some concepts. Orv Hetil 2012;153:1547-51. [Crossref] [PubMed]
- van Dalen BM, Caliskan K, Soliman OI, Nemes A, Vletter WB, Ten Cate FJ, Geleijnse ML. Left ventricular solid body rotation in non-compaction cardiomyopathy: a potential new objective and quantitative functional diagnostic criterion? Eur J Heart Fail 2008;10:1088-93. [Crossref] [PubMed]
- van Dalen BM, Caliskan K, Soliman OI, Kauer F, van der Zwaan HB, Vletter WB, van Vark LC, Ten Cate FJ, Geleijnse ML. Diagnostic value of rigid body rotation in noncompaction cardiomyopathy. J Am Soc Echocardiogr 2011;24:548-55. [Crossref] [PubMed]
- Peters F, Khandheria BK, Libhaber E, Maharaj N, Dos Santos C, Matioda H, Essop MR. Left ventricular twist in left ventricular noncompaction. Eur Heart J Cardiovasc Imaging 2014;15:48-55. [Crossref] [PubMed]
- Nemes A, Kalapos A, Domsik P, Forster T. Identification of left ventricular "rigid body rotation" by three-dimensional speckle-tracking echocardiography in a patient with noncompaction of the left ventricle: a case from the MAGYAR-Path Study. Echocardiography 2012;29:E237-40. [Crossref] [PubMed]
- Kalapos A, Domsik P, Forster T, Nemes A. Comparative evaluation of left ventricular function by two-dimensional echocardiography and three-dimensional speckle-tracking echocardiography in noncompaction cardiomyopathy. Results from the MAGYAR-Path Study. Orv Hetil 2013;154:1352-9. [Crossref] [PubMed]
- Peters F, Khandheria BK, dos Santos C, Govender S, Botha F, Essop MR. Peripartum cardiomyopathy associated with left ventricular noncompaction phenotype and reversible rigid body rotation. Circ Heart Fail 2013;6:e62-3. [Crossref] [PubMed]
- Popescu BA, Beladan CC, Calin A, Muraru D, Deleanu D, Rosca M, Ginghina C. Left ventricular remodelling and torsional dynamics in dilated cardiomyopathy: reversed apical rotation as a marker of disease severity. Eur J Heart Fail 2009;11:945-51. [Crossref] [PubMed]
- Maharaj N, Khandheria BK, Peters F, Libhaber E, Essop MR. Time to twist: marker of systolic dysfunction in Africans with hypertension. Eur Heart J Cardiovasc Imaging 2013;14:358-65. [Crossref] [PubMed]
- Nemes A, Havasi K, Forster T. "Rigid body rotation" of the left ventricle in hypoplastic right-heart syndrome: a case from the three-dimensional speckle-tracking echocardiographic MAGYAR-Path Study. Cardiol Young 2015;25:768-72. [Crossref] [PubMed]
- Nemes A, Havasi K, Domsik P, Kalapos A, Forster T. Can univentricular heart be associated with "rigid body rotation"? A case from the three-dimensional speckle-tracking echocardiographic MAGYAR-Path study. Hellenic J Cardiol 2015;56:186-8. [PubMed]
- Nemes A, Havasi K, Domsik P, Kalapos A, Forster T. Left ventricular rigid body rotation in Ebstein’s anomaly from the MAGYAR-Path Study. Arq Bras Cardiol 2016. In press. [Crossref]
- Nemes A, Földeák D, Domsik P, Kalapos A, Sepp R, Borbényi Z, Forster T. Different patterns of left ventricular rotational mechanics in cardiac amyloidosis-results from the three-dimensional speckle-tracking echocardiographic MAGYAR-Path Study. Quant Imaging Med Surg 2015;5:853-7. [PubMed]
- Zhou Z, Ashraf M, Hu D, Dai X, Xu Y, Kenny B, Cameron B, Nguyen T, Xiong L, Sahn DJ. Three-dimensional speckle-tracking imaging for left ventricular rotation measurement: an in vitro validation study. J Ultrasound Med 2010;29:903-9. [PubMed]
- Nemes A, Kalapos A, Domsik P, Forster T. Three-dimensional speckle-tracking echocardiography -- a further step in non-invasive three-dimensional cardiac imaging. Orv Hetil 2012;153:1570-7. [Crossref] [PubMed]
- Nakatani S. Left ventricular rotation and twist: why should we learn? J Cardiovasc Ultrasound 2011;19:1-6. [Crossref] [PubMed]


