
Analysis of deep inspiration breath-hold technique to improve dosimetric and clinical advantages in postoperative intensity-modulated radiation therapy for thymomas
Introduction
Thymomas are relatively rare tumors with an incidence of 0.15 per 100,000 person-years. They are usually located in the anterior mediastinum. In such tumors, local recurrence is more common compared to distant metastases. Further, the rarest cases (0–2.4%) have been observed to be associated with lymph node metastasis (1,2). The Masaoka classification has been largely used in clinical staging of thymomas. Benign and malignant cases are usually distinguished based on apparent capsular invasiveness. Radiation therapy is considered one of the essential treatment modalities for invasive thymomas. Patients in the advanced stages are routinely subjected to postoperative radiation of microscopically (R1) and macroscopically (R2) positive resection margins because of their high recurrence rates. Due to the indolent nature of the tumors and their sensitivity to radiation and/or chemotherapy, most thymoma patients have a good prognosis. The 5-year overall survival (OS) rates for patients with stages I, II, III, and IV thymomas have been reported to be 93.3%, 88.7%, 74.6%, and 43.4%, respectively (3). The long natural history of thymoma highlights the importance of minimizing radiation-induced toxicities, such as radiation pneumonitis (RP), radiation-induced esophagitis (RIE), and heart-related cardiovascular disease. To minimize the acute and late complications of adjuvant radiotherapy, involved-field instead of extended-field radiation therapy has been the trend of postoperative radiotherapy for thymomas. Acute or late complications can be further reduced by improving radiation techniques such as three-dimensional (3D) conformal and image-guided radiation therapy (4). Conventional radiation treatment under free-breathing (FB) requires an extra treatment margin to account for the respiration-induced target movement. Training patients with deep inspiration breath-hold (DIBH) has been identified as a successful method to decrease the internal target volume (ITV) by controlling tumor movement, and, in turn, minimizing the volume of lung tissue included in the treatment fields and the administered dose. This method is effective and can be applied to various thoracic tumors (5-9). To the best of our knowledge, this is the first study to compare dosimetric parameters for thymoma patients receiving postoperative radiation therapy during two respiratory states (FB and DIBH). We present the following article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-21-1101/rc).
Methods
Patients
This was a retrospective cohort study, in which 26 consecutive patients with thymoma who received adjuvant radiotherapy with the DIBH technique were recruited between January and December 2017 and analyzed. The inclusion criteria were patients who underwent thoracoscopic thymectomy, with pathological diagnosis of Masaoka stage II or III thymomas. Pregnant women, patients treated previously with chest radiotherapy, those who had difficulty holding their breath for more than 10 s, and those who did not complete the whole course of radiotherapy were excluded. All patients provided written informed consent and authorized the legitimate use of images for the study. This study was approved by the Ethics Committee of the First Affiliated Hospital of College of Medicine at Zhejiang University. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
DIBH technique
Before computed tomography (CT) simulation, patients were trained in and practiced thoracic DIBH using visual and auditory feedback glasses (bt300, Seiko Epson Corporation, Suwa, Nagano, Japan) for at least 30 min. Thoracic DIBH is a type of DIBH that uses the diaphragm and chest muscles during inspiration followed by holding of the breath. The evaluation standard of training was that patients can hold their breath at the end of maximum (100%) inspiration repeatedly and stably for at least 20 s. After assuming a supine position on the CT bench and being immobilized using a thermoplastic mask, the patients were initially instructed to perform quiet tidal breathing. This was followed by verbal coaching of the patients to perform a slow, deep inspiration, a slow, deep expiration, and a second slow DIBH. The Varian Real-time Position Management (RPM) System (Varian Medical Systems, Palo Alto, CA, USA) was used to facilitate and monitor the maneuvering of DIBH. Doctors determined the breath-holding amplitude and the range of the gating window according to the patient’s level of mastery and the location of the tumor, with 3–5 cm (the infrared reflecting marker is generally placed below the xiphoid) and 2–3 mm, respectively, generally adopted.
CT simulation
A research-dedicated Siemens scanner (40-slice Sensation Open, Siemens, Erlangen, Germany) was used for all patients to undergo CT simulation. Each patient underwent two CT scans, in FB and DIBH, respectively. The typical scan acquisition time from the mid-neck to the upper abdomen was 15 s using a slice acquisition time of 1 second, table speed of 2.5 cm/s, and slice thickness of 0.5 cm.
Target delineation, organs at risks (OAR), and treatment planning
The CT series was transferred to an Eclipse treatment planning workstation (version 11.0; Varian Medical Systems, USA). Clinical target volume (CTV) was defined as a tumor bed with a margin extension of 1–2 cm. The target motion during DIBH was limited to no more than 3 mm (10,11).
In this study, a 3 mm margin was set to compensate for suppressed CTV motion during DIBH to form the ITV, and a 10 mm ITV margin was set for FB-10 mm. The DIBH and FB-10 mm are considered conventional treatment models. In addition, to ensure identical planning target volumes (PTV) between FB and DIBH to observe the thoracic expansion advantages, we copied another FB CT together with the structure sets, and set a 3 mm ITV margin for FB-3 mm, which is used to simulate a comparable plan to DIBH. A 3 mm set-up error in all the ITVs led to the formation of PTV based on our clinical experience (Figure 1).
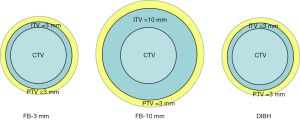
The heart, lungs, esophagus, and spinal cord were considered OARs. The heart volume was defined as the entire visible myocardium from the apex of the heart to the infundibulum of the ventricle, excluding the root of the aorta, pulmonary artery and veins, venae cavae, and epicardial fat and pericardium (12). The lung volume was automatically delineated using the auto-contouring tool of the treatment planning system (TPS). The delineation of the spinal cord was contoured from the C7 to T12 vertebrae.
A template-based 7-field TPS was developed for each of the contours, with AAA algorithm, optimization performed for dose-volume optimization (DVO), and a 100% prescription dose of 50 Gy to cover 95% of the PTV. The limitation on the maximum dose (Dmax) within the PTV was set to less than 110% of the prescription dose. Dose-volume histograms (DVHs) were generated for all the three plans (FB-3 mm, FB-10 mm, and DIBH) and the following dosimetric parameters of OARs were noted and recorded: lung mean dose (Dmean), percentage volume bilateral lung receiving doses 5 Gy (V5) and 20 Gy (V20), heart Dmean, percentage volumes of the heart receiving doses 10 Gy (V10) and 40 Gy (V40), Dmean, dose to 2% of the volume (D2%), and Dmax of esophagus and spine. The dose constraints to OARs were as follows: lungs V20 <37%, lungs Dmean <15 Gy, heart V40 <37%, esophagus V50 <35%, esophagus Dmean <35 Gy, and spinal cord Dmax <45 Gy, and all treatment plans were clinically acceptable.
For consistency, the delineation of CTV and OARs was performed by an oncologist, and treatment plans were designed by a medical physicist. The targets were reviewed by another oncologist to ensure reasonable target delineation, and the treatment plans were reviewed by a senior physicist.
Dosimetric study
A standard schedule of 50 Gy in 2 Gy fractions was considered for the study. The dosimetric parameters evaluated during FB and DIBH were as follows: the mean dose (Dmean) and maximum dose (Dmax) of OARs, percentage volume of the lung receiving x Gy (Vx, x=5, 20), and percentage volume of the heart receiving x Gy (Vx, x=10, 40). The parameters during FB were used as control values.
Normal tissue complication probability calculation
The Lyman-Kutcher-Burman (LKB) probit model was used for calculating normal tissue complication probability (NTCP) (13). According to this model, if a fraction v of an organ is uniformly irradiated at dose D, NTCP is expressed using the below formula:
where t is defined as:
and TD50 (v) is defined as:
For lung toxicity, we considered pneumonitis as the endpoint and used TD50 =30.8 Gy, m =0.37, and n =0.99 with an α/β ratio of 3 Gy (14). The considered endpoints for heart toxicity were pericarditis and long-term mortality. The NTCP for pericarditis was calculated using the LKB model with TD50 =50.6 Gy, m =0.13, n =0.64, and an α/β ratio of 2.5 Gy (15,16). For long-term mortality, the parameters considered were as follows: TD50 =52.3 Gy, m =0.28, n =1, and α/β ratio of 3 Gy. The best approximation to the Erikson breast dose-effect curve (17) was due to this last value using the LKB model with TD50 and n fixed as in Gagliardi et al. (16,18). Further, we used MATLAB version 9.0 (MathWorks, Natick, MA, USA) to calculate the above-mentioned model.
Statistical analysis
Comparisons of OARs dose parameters and volumes between DIBH and FB-3 mm and FB-10 mm, and NTCPs for lung and heart between FB-10 mm and DIBH were performed using paired sample Student’s t-tests. Pearson correlation coefficients were used to determine the associations between the lung volume increment and all the OARs parameters. A P value below 0.05 was considered statistically significant.
Results
Patient characteristics
A total of 20 patients were included in this study, including 15 males and 5 females with a median age of 50 years (range, 31–65 years). Six patients were identified to have Masaoka stage II, and 14 had stage III. Regarding pathologic tumor types, nine patients had B2, five had B3, three had squamous cell carcinoma, two had AB, and one had A.
Volume comparison
The total lung volume was 2,884±166 and 4,216±198 mL in FB and DIBH, respectively, and was identified to have significantly increased by 1,332 mL, and the percentage increment was 31% on DIBH acquisitions compared to FB (P<0.001) (Table 1). The mean heart volume was 636±35 and 552±25 mL in FB and DIBH, respectively. Although the heart volume was found to have decreased by 12% in DIBH compared to that in FB acquisitions, this difference was not statistically significant (P=0.059). The mean volumes of CTV were the same in three CT sets. The mean volumes of PTV were 578±33 and 307±45 mL FB and DIBH, respectively. The PTV volume significantly reduced by 48.8% on DIBH compared to that on FB-10 mm (P<0.001), which was mainly caused by the difference of ITV.
Table 1
| Volume (mL) | FB-3 mm, mean ± SD | FB-10 mm, mean ± SD | DIBH, mean ± SD | P value | |
|---|---|---|---|---|---|
| DIBH vs. FB-3 mm | DIBH vs. FB-10 mm | ||||
| Lung | 2,884±166 | 2,884±166 | 4,216±198 | <0.001 | <0.001 |
| Heart | 636±35 | 636±35 | 552±25 | 0.06 | 0.06 |
| CTV | 58±25 | 58±25 | 58±22 | 0.96 | 0.96 |
| PTV | 308±49 | 578±33 | 307±45 | 0.97 | <0.001 |
CTV, clinical target volume; PTV, planning target volume; OARs, organs at risk; FB, free breath; DIBH, deep inspiration breath hold; SD, standard deviation
Dosimetric parameters of OARs
Dosimetric parameters along with dose distributions of PTV and relevant normal tissues were calculated using a cumulative DVH between FB and DIBH (Table 2). All lung parameters (V5, V20, Dmean) were found significantly improved in DIBH (both compared to FB-3 mm and FB-10 mm). A significant improvement of Dmean, V10, and V40 of the heart was observed in DIBH compared to FB-10 mm. However, dosimetric parameters were comparable between DIBH and FB-3 mm. This indicated that DIBH’s advantage in terms of protecting the heart was mainly due to the shrinkage of ITV. The Dmean, Dmax, and D2% of the esophagus were reduced by 7 Gy, 10 Gy, and 5 Gy, respectively. Significant improvements in Dmean (P<0.001), Dmax (P=0.002), and D2% (P<0.001) of the esophagus were observed in DIBH compared to those in FB-10 mm. The Dmean between DIBH and FB-3 mm were comparable (P=0.35). The results in the spinal cord were similar to those in the esophagus, as shown in Table 2.
Table 2
| OARs | Parameter | FB-3 mm, mean ± SD | FB-10 mm, mean ± SD | DIBH, mean ± SD | P value | |
|---|---|---|---|---|---|---|
| DIBH vs. FB-3 mm | DIBH vs. FB-10 mm | |||||
| Lung | V5 | 54%±2.85% | 63%±2.92% | 47%±2.90% | <0.001 | <0.001 |
| V20 | 15%±1.37% | 22%±1.50% | 12%±1.32% | 0.004 | <0.001 | |
| Dmean (Gy) | 10.28±0.58 | 13.70±0.89 | 8.76±0.57 | <0.001 | <0.001 | |
| Heart | V10 | 48%±4.84% | 52%±4.95% | 40%±5.48% | 0.27 | <0.001 |
| V40 | 15%±2.34% | 25%±3.91% | 16%±3.24% | 0.85 | <0.001 | |
| Dmean (Gy) | 15.9±1.81 | 21.57±2.41 | 14.83±2.01 | 0.25 | <0.001 | |
| Esophagus | Dmean (Gy) | 17.54±0.95 | 27.76±1.47 | 20.58±1.46 | 0.35 | <0.001 |
| Dmax (Gy) | 41.16±1.85 | 47.28±1.38 | 37.29±2.29 | 0.02 | 0.002 | |
| D2% (Gy) | 38.91±1.84 | 40.09±1.42 | 35.79±1.84 | 0.002 | <0.001 | |
| Spine | Dmean (Gy) | 17.54±0.95 | 21.02±1.08 | 17.04±1.29 | 0.11 | <0.001 |
| Dmax (Gy) | 31.65±0.94 | 34.14±1.01 | 28.56±1.29 | 0.013 | <0.001 | |
| D2% (Gy) | 29.38±0.99 | 32.68±0.94 | 26.94±1.23 | 0.018 | <0.001 | |
OARs, organs at risk; FB, free breath; DIBH, deep inspiration breath hold; SD, standard deviation; V5, percentage volume receiving doses 5 Gy; V20, percentage volume receiving doses 20 Gy; Dmean, mean dose; Dmax, maximum dose; D2%, dose to 2% of the volume.
Relationship between enlarged lung volumes and OARs
The FB-3 mm and DIBH treatment plans were compared to ensure identical volumes of PTV to observe if the difference in lung volume owing to thoracic expansion had an impact on OARs. Pearson correlations determined the associations between lung volume increment and the OAR parameters. The results revealed that lung volume increment was related to a reduction in lung mean dose, with a correlation coefficient of r=0.27, P=0.03. Further, as the lung volume increased, the reduction of lung irradiation also increased. However, no significant relationship was identified between lung volume increment and lung V20, V5 decrement, and dosimetric reduction in other OARs parameters.
NTCP analysis
The NTCPs for lung were 1.1% and 0.6% in FB-10 mm and DIBH, respectively. Regarding heart conditions, the incidence of pericarditis was 0.00026% and 0.00014%, respectively, and long-term mortality of the heart were 0.25% and 0.17%, respectively in FB-10 mm and DIBH (Table 3). The NTCPs for lung toxicity were significantly lower in DIBH compared to those in FB-10 mm (P<0.001). However, the differences between the two breathing patterns were not statistically significant for long-term cardiac mortality and pericarditis.
Table 3
| Parameter | FB-10 mm | DIBH | P value |
|---|---|---|---|
| Lung NTCP | 1.07×10−2±8.25×10−4 | 6.71×10−3±4.26×10−4 | <0.001 |
| Heart NTCP-pericarditis | 2.56×10−6±1.67×10−7 | 1.39×10−6±8.82×10−7 | 0.54 |
| Heart NTCP-long term mortality | 2.52×10−3±6.31×10−4 | 1.68×10−3±5.65×10−4 | 0.33 |
NTCP, normal tissue complication probabilities; FB, free breath; DIBH, deep inspiration breath hold.
Discussion
In this study, 20 thymoma patients received CT scans using FB and DIBH methods, and dosimetric advantages were compared. The total lung volume increased in DIBH by approximately 31%. According to research reports, the total lung volume increase ranges between 45% and 52% in DIBH compared to FB (12,19). The mean volume of CTV is equivalent in both respiratory states because they are derived from the same patient tumor target, and thus the delineation differences can be excluded. With a reduced ITV margin, the average PTV volume decreases by 48.8% in DIBH compared to FB. Further, DIBH changes the treatment target’s shape but has minimal influence on the CTV volume, when compared to FB. However, the heart volume (552±25 vs. 636±35 mL) reduces by 12% on DIBH acquisitions (Table 1). In accordance with Gong et al.’s report (20), the total heart volume significantly reduces during DIBH, which is considered the result of compressed pericardium during lung inflation.
The lung is a radiosensitive organ. RP is a well-known complication in thoracic radiotherapy. Several studies have investigated the predictive dosimetric factors for RP risk during thoracic radiotherapy (21-24). For treatment-related pneumonitis, the classic predictors considered are V5, V20, and the mean lung dose (22-24).
In this study, owing to thoracic expansion and reduction of ITV, the mean total lung dose was reduced by 36.1% in DIBH compared with FB-10 mm (13.7 vs. 8.8 Gy). In addition, this study also demonstrates that when both FB-3 mm and DIBH have the same ITV, the mean total lung dose was reduced by 14.8% in DIBH compared with FB-3 mm only owing to thoracic expansion. In addition, the relative lung dosimetric parameters of V5 and V20 led to a significant decrease in DIBH. In Paumier et al.’s study, the DIBH technique was combined with intensity-modulated radiation therapy (IMRT) for patients with mediastinal Hodgkin’s disease, and a 20% reduction in the mean lung dose and 30% reduction in the lung V20 was observed (25). Specifically, DIBH has two advantages in reducing the dose to the lungs. On the one hand, lung inflation during DIBH increases the absolute lung volume, thus reducing the relative lung volume irradiated, and on the other hand, owing to suppressed breathing motion, DIBH decreases the treatment target volume through the reduced ITV margin. When considered together, a significant reduction of lung dose is achieved. As shown in Table 2, the lung volume irradiated is typically reduced during DIBH compared to FB-10 mm. Further, due to a lower diaphragm, the lower lung tissue, which is essential for breathing movement, is not considered for the treatment target. This also protects lung functionality. In the NTCP lung study, RP decreases significantly in DIBH, with a 0.5% reduction in incidence.
Radiation-induced cardiovascular disease is common among patients receiving chest radiotherapy. In this study, the mean dose of the heart was significantly reduced by 31.4% in DIBH (14.8 vs. 21.57 Gy, P=0.004), as well as in V10 and V40. In the NTCP study, pericarditis and long-term mortality of the heart were comparable in FB and DIBH, although dosimetric parameters of the heart are significantly reduced in DIBH, owing to the low occurrence probability during radiotherapy of thymoma. Paumier et al. reported that tumors located in the upper mediastinum are more likely to achieve a lower heart dose compared to the entire mediastinal masses in lymphoma patients (25). Hence, several factors, including tumor location, target design, and DIBH use, affect the dose and volume of the heart irradiated.
This study also demonstrated obvious dose reduction to the esophagus, whereby the Dmean, Dmax, and D2% were shown to have reduced by approximately 7 Gy, 10 Gy, and 5 Gy, respectively. Dosimetric indices are commonly used to predict RIE (26,27). According to Dehing-Oberije et al. (27), the Dmean and Dmax of the esophagus are the most important predictive factors for radiation-induced acute dysphagia. In Marchand et al.’s study on lung cancer, the DIBH technique achieved a 7.3% reduction in the Dmean of the esophagus (27.9 vs. 30.1 Gy), and the Dmax of the esophagus (62.5 vs. 62.4 Gy) was comparable in both respiration states (5). However, in this study on thymomas, the DIBH technique reduced Dmean, Dmax, and D2% of the esophagus. In each pairwise comparison between DIBH with FB-3 mm and FB-10 mm, there was no significant difference between the DIBH and FB-3 mm in Dmean (P=0.35), but an obvious difference was found between the DIBH and FB-10 mm (P<0.001), suggesting that the Dmean of the esophageal dose was determined by the irradiation target volume or ITV. The reason for the different results of Dmax or D2% compared with lung cancer is that the tumor location is different. Thymoma is an anterior mediastinal tumor, and thus it is difficult to avoid the esophagus when implementing the treatment plan.
Several studies on the spinal cord have revealed an extremely low incidence of radiation spinal cord injury (28,29). Schultheiss et al. analyzed more than 300 cases of radiation myelopathy from 77 papers, and the data suggested that a higher biological dose is associated with a short latent period of radiation myelopathy (30). Our study demonstrated that DIBH can also reduce the Dmean, Dmax, and D2% of the spinal cord in thymoma radiotherapy. These findings are similar to those on the esophagus, possibly because the anatomical position of the spinal cord is close to that of the esophagus. Briefly, comparing the results of Dmean between FB-3 mm, FB-10 mm, and DIBH, reducing the ITV through the DIBH technique can decrease the rate of injury to the esophagus and spinal cord.
This study had several limitations. First, our study cohort size was modest, although comparable to most of the published DIBH case series. Second, this was a single-center study comparing two breathing patterns rather than a comprehensive multicenter study. Third, two sets of CT scan images (one in FB and another in DIBH) were taken for each patient to judge whether the DIBH CT is justified by comparing the diaphragm position and lung volume of the two images. However, DIBH is not indicated for patients with poor comprehension or hearing or those unable to cooperate, and thus patient selection should be carefully evaluated before CT simulation.
Conclusions
This study suggests that postoperative IMRT with DIBH for invasive thymomas can remarkably reduce the total irradiation dose to the lungs and reduce the incidence of pneumonitis. Doses to the spinal cord and esophagus can also be decreased, albeit by a relatively smaller degree. The application of DIBH in thymoma has a protective effect on the lung, heart, esophagus, and spine mainly by reducing treatment ITV. This study also found that even if ITV is the same as FB, DIBH can still reduce lung injury by expanding lung volume. We suggest the use of DIBH in postoperative radiation for invasive thymomas for improved protection to the OARs and reduction in long-term complications.
Acknowledgments
Funding: This work was supported by the Natural Science Foundation of Zhejiang Province of China (No. LSY19H160004).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-21-1101/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-21-1101/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Human Research Ethics Committee of the First Affiliated Hospital of College of Medicine of Zhejiang University Hospital and informed consent was provided by all the patients. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Engels EA, Pfeiffer RM. Malignant thymoma in the United States: demographic patterns in incidence and associations with subsequent malignancies. Int J Cancer 2003;105:546-51. [Crossref] [PubMed]
- Marx A, Weis CA, Ströbel P. Thymomas. Pathologe 2016;37:412-24. [Crossref] [PubMed]
- Mariano C, Ionescu DN, Cheung WY, Ali RH, Laskin J, Evans K, Carolan H, Murray N. Thymoma: a population-based study of the management and outcomes for the province of British Columbia. J Thorac Oncol 2013;8:109-17. [Crossref] [PubMed]
- Gomez D, Komaki R. Technical advances of radiation therapy for thymic malignancies. J Thorac Oncol 2010;5:S336-43. [Crossref] [PubMed]
- Marchand V, Zefkili S, Desrousseaux J, Simon L, Dauphinot C, Giraud P. Dosimetric comparison of free-breathing and deep inspiration breath-hold radiotherapy for lung cancer. Strahlenther Onkol 2012;188:582-9. [Crossref] [PubMed]
- Boda-Heggemann J, Knopf AC, Simeonova-Chergou A, Wertz H, Stieler F, Jahnke A, Jahnke L, Fleckenstein J, Vogel L, Arns A, Blessing M, Wenz F, Lohr F. Deep Inspiration Breath Hold-Based Radiation Therapy: A Clinical Review. Int J Radiat Oncol Biol Phys 2016;94:478-92. [Crossref] [PubMed]
- Bergom C, Currey A, Desai N, Tai A, Strauss JB. Deep Inspiration Breath Hold: Techniques and Advantages for Cardiac Sparing During Breast Cancer Irradiation. Front Oncol 2018;8:87. [Crossref] [PubMed]
- Chen M, Zang S, Yu H, Ning L, Huang H, Bu L, Ge J, Xu M, Tang Q, Zhao F, Yao G, Yan S. Immobilization-assisted abdominal deep inspiration breath-hold in post-mastectomy radiotherapy of left-sided breast cancer with internal mammary chain coverage. Quant Imaging Med Surg 2021;11:3314-26. [Crossref] [PubMed]
- Goyal U, Saboda K, Roe D, Gonzalez VJ. Prone Positioning With Deep Inspiration Breath Hold for Left Breast Radiotherapy. Clin Breast Cancer 2021;21:e295-301. [Crossref] [PubMed]
- Onishi H, Kuriyama K, Komiyama T, Tanaka S, Sano N, Aikawa Y, Tateda Y, Araki T, Ikenaga S, Uematsu M. A new irradiation system for lung cancer combining linear accelerator, computed tomography, patient self-breath-holding, and patient-directed beam-control without respiratory monitoring devices. Int J Radiat Oncol Biol Phys 2003;56:14-20. [Crossref] [PubMed]
- Nakamura K, Shioyama Y, Nomoto S, Ohga S, Toba T, Yoshitake T, Anai S, Terashima H, Honda H. Reproducibility of the abdominal and chest wall position by voluntary breath-hold technique using a laser-based monitoring and visual feedback system. Int J Radiat Oncol Biol Phys 2007;68:267-72. [Crossref] [PubMed]
- Hayden AJ, Rains M, Tiver K. Deep inspiration breath hold technique reduces heart dose from radiotherapy for left-sided breast cancer. J Med Imaging Radiat Oncol 2012;56:464-72. [Crossref] [PubMed]
- Lyman JT. Complication probability as assessed from dose-volume histograms. Radiat Res Suppl 1985;8:S13-9. [Crossref] [PubMed]
- Seppenwoolde Y, Lebesque JV, de Jaeger K, Belderbos JS, Boersma LJ, Schilstra C, Henning GT, Hayman JA, Martel MK, Ten Haken RK. Comparing different NTCP models that predict the incidence of radiation pneumonitis. Normal tissue complication probability. Int J Radiat Oncol Biol Phys 2003;55:724-35. [Crossref] [PubMed]
- Martel MK, Sahijdak WM, Ten Haken RK, Kessler ML, Turrisi AT. Fraction size and dose parameters related to the incidence of pericardial effusions. Int J Radiat Oncol Biol Phys 1998;40:155-61. [Crossref] [PubMed]
- Gagliardi G, Constine LS, Moiseenko V, Correa C, Pierce LJ, Allen AM, Marks LB. Radiation dose-volume effects in the heart. Int J Radiat Oncol Biol Phys 2010;76:S77-85. [Crossref] [PubMed]
- Eriksson F, Gagliardi G, Liedberg A, Lax I, Lee C, Levitt S, Lind B, Rutqvist LE. Long-term cardiac mortality following radiation therapy for Hodgkin's disease: analysis with the relative seriality model. Radiother Oncol 2000;55:153-62. [Crossref] [PubMed]
- Gagliardi G, Lax I, Ottolenghi A, Rutqvist LE. Long-term cardiac mortality after radiotherapy of breast cancer--application of the relative seriality model. Br J Radiol 1996;69:839-46. [Crossref] [PubMed]
- Lorchel F, Dumas JL, Noël A, Wolf D, Bosset JF, Aletti P. Dosimetric consequences of breath-hold respiration in conformal radiotherapy of esophageal cancer. Phys Med 2006;22:119-26. [Crossref] [PubMed]
- Gong G, Wang R, Guo Y, Zhai D, Liu T, Lu J, Chen J, Liu C, Yin Y. Reduced lung dose during radiotherapy for thoracic esophageal carcinoma: VMAT combined with active breathing control for moderate DIBH. Radiat Oncol 2013;8:291. [Crossref] [PubMed]
- Huang P, Yan H, Hu Z, Liu Z, Tian Y, Dai J. Predicting radiation pneumonitis with fuzzy clustering neural network using 4DCT ventilation image based dosimetric parameters. Quant Imaging Med Surg 2021;11:4731-41. [Crossref] [PubMed]
- Zhang XJ, Sun JG, Sun J, Ming H, Wang XX, Wu L, Chen ZT. Prediction of radiation pneumonitis in lung cancer patients: a systematic review. J Cancer Res Clin Oncol 2012;138:2103-16. [Crossref] [PubMed]
- Ullah T, Patel H, Pena GM, Shah R, Fein AM. A contemporary review of radiation pneumonitis. Curr Opin Pulm Med 2020;26:321-5. [PubMed]
- Roeder F, Friedrich J, Timke C, Kappes J, Huber P, Krempien R, Debus J, Bischof M. Correlation of patient-related factors and dose-volume histogram parameters with the onset of radiation pneumonitis in patients with small cell lung cancer. Strahlenther Onkol 2010;186:149-56. [Crossref] [PubMed]
- Paumier A, Ghalibafian M, Gilmore J, Beaudre A, Blanchard P, el Nemr M, Azoury F, al Hamokles H, Lefkopoulos D, Girinsky T. Dosimetric benefits of intensity-modulated radiotherapy combined with the deep-inspiration breath-hold technique in patients with mediastinal Hodgkin's lymphoma. Int J Radiat Oncol Biol Phys 2012;82:1522-7. [Crossref] [PubMed]
- Ozgen A, Hayran M, Kahraman F. Mean esophageal radiation dose is predictive of the grade of acute esophagitis in lung cancer patients treated with concurrent radiotherapy and chemotherapy. J Radiat Res 2012;53:916-22. [Crossref] [PubMed]
- Dehing-Oberije C, De Ruysscher D, Petit S, Van Meerbeeck J, Vandecasteele K, De Neve W, Dingemans AM, El Naqa I, Deasy J, Bradley J, Huang E, Lambin P. Development, external validation and clinical usefulness of a practical prediction model for radiation-induced dysphagia in lung cancer patients. Radiother Oncol 2010;97:455-61. [Crossref] [PubMed]
- Bütof R, Löck S, Soliman M, Haase R, Perrin R, Richter C, Appold S, Krause M, Baumann M. Dose-volume predictors of early esophageal toxicity in non-small cell lung cancer patients treated with accelerated-hyperfractionated radiotherapy. Radiother Oncol 2020;143:44-50. [Crossref] [PubMed]
- Schultheiss TE. Repair of radiation damage and radiation injury to the spinal cord. Adv Exp Med Biol 2012;760:89-100. [Crossref] [PubMed]
- Schultheiss TE, Higgins EM, El-Mahdi AM. The latent period in clinical radiation myelopathy. Int J Radiat Oncol Biol Phys 1984;10:1109-15. [Crossref] [PubMed]


