
Shear wave elastography-based liver fibrosis assessment in patients with chronic hepatitis E displays elevated liver stiffness regardless of previous antiviral therapy
Introduction
Hepatitis E virus (HEV) has four genotypes that are known to cause hepatitis E in humans. In industrialized countries, zoonotic transmitted genotype 3 causes a usually asymptomatic and self-limiting disease (1). In patients with preexisting chronic liver disease or cirrhosis, hepatitis E is often described as a cause of decompensation of preexisting chronic liver disease or cirrhosis (2). In addition to acute hepatitis, chronic hepatitis E is a disease that predominantly affects immunocompromised patients, out of which the largest group are solid organ transplant recipients. Due to its often asymptomatic course with only lightly elevated transaminases, the exact time point of infection remains unknown in many HEV patients and diagnosis of a chronic infection may be late. It has been described that chronic hepatitis E can take an aggressive course and lead to cirrhosis in a short period of time (3). Knowledge about the clinical course of HEV-related liver disease is sparse and relies on scattered reports of patients with diverse underlying diseases such as human immunodeficiency virus (HIV) or transplanted patients who were examined by diverse diagnostic approaches such as liver biopsy or transient elastography (TE).
As listed in Table 1, most studies that provide evidence for the presence of fibrosis in chronic HEV patients were histological findings in single or few cases. Biopsies were often performed due to other indications than fibrosis assessment such as suspected (liver) transplant rejection (5,8). Kamar et al. were among the first to describe two cases of rapidly progressing fibrosis leading to cirrhosis in two patients with chronic hepatitis E in 2008 (3). Various cases with a rapid progression from no or mild fibrosis to advanced fibrosis or cirrhosis have been reported (3,6). A recent study by Beer et al. aimed at further describing the histopathological changes in chronic hepatitis E. Seven out of 19 chronic HEV infected patients, who underwent liver biopsy, displayed F2-fibrosis, one had F3-fibrosis and one patient had incomplete cirrhosis (13). These findings add evidence that chronic hepatitis E poses a substantial risk for liver fibrosis and cirrhosis. Nevertheless, liver biopsy is associated with substantial costs and procedural risks (19). Therefore, liver fibrosis assessment is preferably performed in a non-invasive way. Non-invasive methods of liver stiffness measurement (LSM) are clinical scores that contain laboratory parameters or ultrasound (US)-based techniques such as TE or 2D-shear wave elastography (2D-SWE) (20). As summarized in Table 1, few studies have reported liver fibrosis in chronic hepatitis E using TE for fibrosis measurement: Neukam et al. found a regression of stiffness values after therapy of chronic hepatitis E in two patients, which indicates that non-invasive LSM can be utilized for follow-up examinations (9). Choi et al. described an elevated average in TE-measured liver stiffness in a cohort of 17 renal transplant recipients with chronic hepatitis E (12).
Table 1
| Study | Number of chronic HEV patients with fibrosis/cirrhosis | Cause of immunosuppression/underlying disease | Means of fibrosis/cirrhosis detection | Presence of cirrhosis/grading of fibrosis | Outcome/course of fibrosis if reported |
|---|---|---|---|---|---|
| Haagsma et al. 2008 (4) | 1 | KTx | Histological | Cirrhosis | N.S. |
| Kamar et al. 2008 (3) | 2 | 1 KPTx, 1 KTx | Histological | Cirrhosis | Death (sepsis); progression from no fibrosis to cirrhosis |
| Haagsma et al. 2008 (5) | 2 | LTx | Histological | Cirrhosis and fibrosis | Liver transplant failure |
| Pischke et al. 2010 (6) | 1 | LTx | Histological | F2 | Progression of fibrosis from F0 to F2 within 2 years |
| Kamar et al. 2011 (7) | 38 | solid organ transplant recipients | N.S. | Fibrosis and cirrhosis | N.S. |
| Schlosser et al. 2012 (8) | 1 | LTx | Histological | Cirrhosis | Death (sepsis) |
| Neukam et al. 2013 (9) | 2 | HIV | TE + histological | Cirrhosis | Regression of TE values after therapy with RBV 1,200 mg/d 23 weeks, relapse; relapse after 24 weeks of RBV (100 mg/d) treatment |
| Ingiliz et al. 2016 (10) | 1 | HIV | TE + histological | Cirrhosis | SVR after 5 months RBV 800 mg/d |
| Mazzola et al. 2017 (11) | 1 | LTx | Histological | F3/4 | Regression from F4 to F1 after therapy (PEG-IFN + RBV, long-term RBV), no SVR |
| Choi et al. 2018 (12) | 17 | KTx | TE | Mean TE of 11.2 kPa | N.S. |
| Beer et al. 2019 (13) | 19 | 17 solid organ transplant recipients, 1 CD4 deficiency, 1 ulcerative colitis | Histological | 7 F2, 1 F3, 1 incomplete cirrhosis | SVR in 17 of 19 patients after 8 weeks of RBV |
| Pischke et al. 2012 (14) | 4 | HTx | Histological | 1 with clinical signs of cirrhosis, 1 F2 | SVR after 5 months RBV in 3 patients; RBV treatment failure in 1 patient |
| Liu et al. 2016 (15) | 15 | N.S. | N.S. | N.S. | N.S. |
| Jagjit Singh et al. 2013 (16) | 1 | HIV | Histological | Cirrhosis | PEG- IFN 24 weeks, SVR |
| Miyoshi et al. 2016 (17) | 1 | Burkitt’s lymphoma | Histological | F2 | Refractory to 8 months RBV 8 mg/kg/d |
| Schulz et al. 2020 (18) | 1 | KTx, lymphoma | US | Cirrhosis | RBV discontinued after short period due to low platelets |
HEV, hepatitis E virus; KTx, kidney transplantation; N.S., not stated; KPTx, kidney-pancreas transplantation; LTx, liver transplantation; F, fibrosis stage; HIV, human immunodeficiency virus; TE, transient elastography; RBV, ribavirin; SVR, sustained virological response; PEG-IFN, pegylated interferon; CD, cluster of differentiation; HTx, heart transplantation; US, ultrasound.
2D-SWE is a modern, non-invasive US-based technique that allows to assess liver stiffness in an accurate way. It is an established method for detection and quantification of fibrosis in frequent liver diseases such as non-alcoholic fatty liver disease (NAFLD) and has also shown good accuracy in the assessment of less common liver diseases such as hepatic alpha1-antitrypsin deficiency or sinusoidal obstruction syndrome (20-22). Since it provides real-time visualization of the examined tissue, confounding factors such as ascites, cholestasis, signs of right heart failure or infiltrative malignancies can be excluded which is an advantage over TE. In contrast to liver biopsy, 2D-SWE is easily repeatable for follow-up examinations.
Antiviral treatment is crucial in chronic hepatitis E especially in the vulnerable group of solid-organ transplant recipients in order to prevent the development of liver cirrhosis or hepatocellular carcinoma (HCC) (23). It has been shown that fibrosis can decrease during the course of treatment (11). A vaccine was approved in China, but its efficacy in genotype 3 patients is not fully understood though (24).
The aim of this study was to quantify liver fibrosis by measuring liver stiffness in patients with chronic active hepatitis E and in patients with sustained virological response after ribavirin (RBV) treatment using SWE and laboratory-based fibrosis scores and to provide further evidence on the natural course of disease. We present the following article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-21-1178/rc).
Methods
Patients with a history of or active chronic hepatitis E who presented in the outpatient department of Charité-Unversitätsmedizin Berlin for hepatology or kidney transplantation, which is located in an area of high HEV prevalence (25), were enrolled in this cross-sectional study in the time period from May 2019 until February 2021. Chronic hepatitis E was in accordance with EASL (European Association for the Study of the Liver) clinical practice guidelines defined as HEV replication for at least 3 months (23). Other common reasons for elevated liver enzymes were excluded in all patients. A control group of kidney-transplant recipients without a history of chronic hepatitis E was included.
After fasting at least 4 hours, enrolled patients received an abdominal US including grayscale US, Doppler measurement of the portal venous blood flow and 2D-SWE examination using Canon (former Toshiba) Aplio 500 US system (Canon Medical systems Corporation, Otawara, Tochigi, Japan). All US examinations were conducted by an experienced operator with experience in liver US and sonoelastography (>6,000 US, >2,000 SWEs). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committees of the Universities of Berlin (approval number: EA4/109/21) and informed consent was taken from all individual participants.
2D-SWE
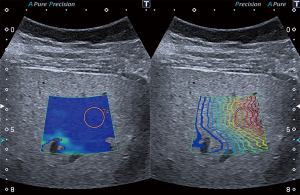
Liver stiffness can be indirectly determined by measuring the velocity of induced shear waves since the speed of shear wave propagation is proportional to tissue elasticity. 2D-SWE allows real-time imaging of shear wave propagation in a focused area. Elasticity is expressed in meters per second (m/s) or converted into kilopascals (kPa). Each 2D-SWE measurement was conducted with the patient in a supine position during a transient breath hold avoiding large vessels and ascites according to the guidelines of the European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) (26). A circular shaped region of interest (ROI) with a diameter of 1 cm was then assigned at least 1 cm below the liver capsule and 3–5 cm from the transducer in a right intercostal position as depicted in Figure 1. 2D-SWE value was defined as median value of at least 5 reliable SWE measurements. A measurement was considered to be of good quality when parallel lines in real time propagation mode were observed and a homogenous color filling of the elastogram was present.
Laboratory testing
A routine liver panel was analyzed in all patients including: alanine aminotransaminase (ALT), aspartate aminotransaminase (AST), total bilirubin, gamma-glutamyl transpeptidase (GGT), blood count, international normalized ratio (INR) and creatinine. In addition, laboratory-based fibrosis scores aspartate aminotransferase-to-platelet ratio index (APRI) and Fibrosis-4 (FIB-4) score were calculated. FIB-4 encompasses age, aminotransferases and platelets whereas APRI includes AST and platelets (20).
Statistical analysis
Data collection and statistical analysis was performed with IBM SPSS Statistics for Windows, (Version 27.0. Armonk, IBM Corp, USA). The significance level was set at α=5% for all comparisons. As group sizes were below 30 biological values non-normal distribution was assumed and verified using D’Agostino-Pearson normality test. Non-parametric Mann-Whitney test was used for two-group comparison.
Patient characteristics
Patient characteristics are displayed in Table 2. A majority of 11 patients (78.6%) was male, median age was 59.5 years (range, 22–75 years).
Table 2
| Characteristics | All patients (n=14) | Patients with active chronic hepatitis E (n=8) | Patients after therapy (n=6) | P value |
|---|---|---|---|---|
| Gender | Female 3 (21.4%); male 11 (78.6%) | Female 1 (12.5%); male 7 (87.5%) | Female 3 (50%); male 3 (50%) | |
| Age (years) | 59.5 (range, 22–75) | 59 (range, 22–75) | 59.5 (range, 29–73) | |
| Laboratory findings | ||||
| Total bilirubin (<1.2 mg/dL) | 0.48 (0.17–1.82); n=13 | 0.55 (0.3–1.82); n=7 | 0.32 (0.17–0.6) | |
| ALT (<31 U/L) | 20 (6–678) | 25 (6–678) | 19 (8–24) | N.S. |
| AST (<35 U/L) | 22.5 (13–615) | 25.5 (13–615) | 22.5 (22–36) | N.S. |
| GGT (5–35 U/L) | 59.5 (17–161) | 72 (21–161) | 22 (14–72) | N.S. |
| Platelets (150–370/nL) | 195 (112–523); n=13 | 183.5 (112–523) | 230 (160–262); n=5 | 0.02 |
| Creatinine (0.5–0.9 mg/dL) | 1.37 (0.77–4.18) | 1.61 (0.77–4.18) | 1.29 (0.85–1.45) | N.S. |
| INR 0.9–1.25 | 1.03 (0.9–1.27); n=12 | 1.075 (0.9–1.27); n=6 | 1.15 (1–1.22); n=3 | N.S. |
| US findings | ||||
| PV maximum velocity | 22 (17–30); n=12 | 23 (19–30); n=7 | 22 (17–24); n=5 | N.S. |
| Spleen length | 105 (80–163); n=13 | 96 (81–163); n=7 | 110 (80–124) | N.S. |
| 2D-SWE (m/s) | 1.59 (1.32–1.76) | 1.59 (1.4–1.74) | 1.54 (1.32–1.76) | N.S. |
| 2D-SWE (kPa) | 7.4 (5.1–9.3) | 7.4 (5.8–9) | 7.1 (5.1–9.3) | N.S. |
| Fibrosis scores | ||||
| FIB-4 | 1.6 (1.15–4.17) | 1.9 (0.38–4.17) | 1.695 (0.53–2.47) | N.S. |
| APRI | 0.3 (0.1–12.4) | 0.35 (0.1–12.4) | 0.3 (0.2–0.4) | N.S. |
| Cause of immunosuppression | 12 kidney transplant recipients; 1 multivisceral transplant recipient; 1 non-Hodgkin lymphoma | 6 kidney transplant recipients; 1 multivisceral transplant recipient; 1 non-Hodgkin lymphoma | 6 kidney transplant recipients |
ALT, alanine aminotransaminase; AST, aspartate aminotransaminase; GGT, gamma-glutamyl transpeptidase; INR, international normalized ratio; US, ultrasound; PV, portal vein; 2D-SWE, two-dimensional shear wave elastography; N.S., not significant; FIB-4, fibrosis-4; APRI, aspartate aminotransferase-to-platelet ratio index.
Seven out of 13 solid organ transplant-recipients had received prior rejection treatment. At the time of 2D-SWE examination, 6 patients were receiving RBV-treatment for chronic hepatitis E. Two patients had discontinued RBV: one patient due to side effects, one patient due to ineffectiveness. Five patients had cleared HEV after RBV therapy and one patient had reached viral clearance after reduction of his immunosuppressive medication. The immunosuppressive regimen of the solid organ transplant-recipients included a triple therapy with tacrolimus, mycophenolic acid (MPA) and corticosteroid in 8 patients, three patients had a dual therapy with tacrolimus and MPA, one patient received a triple therapy with cyclosporine A, MPA and corticosteroid and one patient had a dual therapy with tacrolimus and sirolimus.
2D-SWE and US
2D-SWE measurement revealed a median stiffness of 1.59 m/s or 7.4 kPa respectively. According to the cut-off values proposed by Ferraioli et al., who recently compared 367 patients using TE and 2D-SWE, median stiffness corresponded to an F2 fibrosis (27). Ten patients (71.4%) displayed liver fibrosis: 8 patients had significant (F2) fibrosis (57.1%) and 2 patients had severe (F3-F4) fibrosis (14.3%). Median spleen length was 105 mm, median blood flow velocity in the portal vein 22 cm/s.
Results
Comparison of treated versus untreated patients
2D-SWE values remain elevated also after successful viral clearance
Five patients had cleared HEV after completing antiviral therapy with RBV before undergoing 2D-SWE examination. One patient had reached viral clearance by reduction of his immunosuppressive medication. These patients displayed a median stiffness of 1.54 m/s, 7.1 kPa respectively. Treatment with RBV was terminated 1, 1, 4, 39 and 53 months respectively prior to SWE examinations. In one patient, immunosuppressive therapy was reduced 10 months prior to 2D-SWE examination (leading to virus clearance). In these 6 patients, the median time period between the end of therapy and 2D-SWE measurement was 7 months. All patients who had cleared the virus displayed normal ALT values at the time of 2D-SWE measurement.
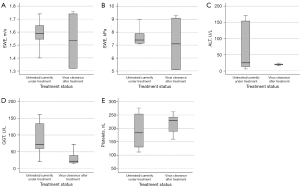
8 patients were either under antiviral treatment or untreated at the time of 2D-SWE examination. In this group, median liver stiffness was 1.59 m/s or 7.4 kPa respectively as depicted in Figure 2A,2B. This difference between the two groups was not statistically significant (P=0.85, 0.95 respectively).
Viral clearance is associated with a non-significant reduction of liver transaminases
ALT (Figure 2C) and AST were lower in the group of successfully treated patients (median ALT in patients with active chronic hepatitis E was 25 versus 19 U/L in successfully treated patients; AST 25.5 versus 22.5 U/L) without reaching statistical significance (P=0.35, 0.95 respectively). Furthermore, total bilirubin was 0.55 mg/dL (normal <1.2 mg/dL) in patients with active chronic hepatitis E versus 0.32 mg/dL in successfully treated patients also not reaching statistical significance (P=0.1).
Successfully treated patients displayed a statistically significant (P=0.02) lower GGT (Figure 2D) of median 22 U/L than patients with active chronic hepatitis E (median 72 U/L).
Thrombocytes (Figure 2E) were lower in active chronic hepatitis E patients with 183.5/nL versus 230/nL in treated patients (not significant, P=0.62). INR was 1.075 in treated patients versus 1.15 in patients with viral clearance (P=0.91). Creatinine was 1.61 mg/dL in untreated patients, 1.29 mg/dL in successfully treated patients (P=0.28).
Median APRI and FIB-4 scores were without signs of advanced fibrosis in both groups (APRI 0.35 and FIB-4 1.9 in patients with active chronic hepatitis E APRI 0.3 and FIB-4 1.695 in successfully treated patients). Differences between the two groups were not statistically significant (P=0.8 for both scores).
Viral clearance is associated with a larger spleen length (not statistically significant)
Spleen length was higher in successfully treated patients with a median of 110 versus 96 mm in patients without successful therapy. This was not statistically significant (P=0.53). Portal vein velocity was 22 cm/s in successfully treated patients versus 23 cm/s in patients with active chronic hepatitis E (P=0.27).
Comparison of 2D-SWE in hepatitis E patients with a control group without hepatitis E
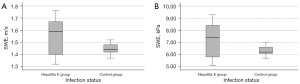
The liver stiffness in the group of 14 patients with a history of hepatitis E was compared with a group of solid-organ transplant recipients without signs of hepatitis E. The control group consisted of 13 kidney transplant recipients and one patient who had received a kidney and a liver transplant. In the control group, 8 patients were female (57%). Median age was 61 years (matched with the hepatitis E group). In 2D-SWE measurement, the control group displayed a liver stiffness of 1.44 m/s, 6.2 kPa respectively. Compared to the hepatitis E group (depicted in Figure 3), this was significantly lower (P=0.04).
Discussion
The assessment of fibrosis is crucial for the clinical management of chronic liver diseases since it provides information on severity and prognosis of liver disease. Non-invasive LSM can detect and grade fibrosis accurately and is established in clinical routine for the assessment of liver disease severity, identification of patients with worse prognosis, monitoring disease progression or response to treatment (26,28). Cut-off values for fibrosis stages vary among the different LSM methods and systems and also depend on the etiology of liver disease. Due to its rareness, no specific cut-off values have been reported for chronic hepatitis E.
To our knowledge, this is the first study applying 2D-SWE for fibrosis assessment in chronic hepatitis E. Based on liver stiffness cut-off values defined for other chronic liver diseases, 71.4% of our patients displayed liver fibrosis underlining that the burden of clinically relevant liver disease is high in patients with chronic hepatitis E. At the time of 2D-SWE examination, 6 patients had already cleared the virus after RBV therapy (in 5 cases) or reduction of immunosuppression (in one case). A median liver stiffness of 1.59 m/s was not significantly higher in untreated patients and patients under treatment than in successfully treated patients whose median stiffness was 1.54 m/s. However, both groups displayed signs of liver fibrosis in 2D-SWE. Liver stiffness was significantly higher than that in a control group of kidney-transplant recipients without a history of hepatitis E which implicates a clinical relevance of chronic hepatitis E in these patients. Possible reasons for elevated liver stiffness also in untreated patients may be a long period of undetected chronic hepatitis E or a rapid progression of fibrosis as it has been described anecdotally (3).
Since chronic hepatitis E is a rather infrequent clinical presentation of HEV infection, data on its clinical course are sparse. As described in Table 1, the majority of available information on fibrosis assessment was gathered via liver biopsy, only a few reported cases were examined non-invasively using TE (9,12). Table 1 shows that if fibrosis assessment was performed in patients with chronic hepatitis E, a majority of patients displayed liver fibrosis or often already cirrhosis.
In a Mexican cohort of 12 HEV-RNA positive individuals, advanced fibrosis in TE was found in 26.1% (29). Using laboratory-based fibrosis scores, Shah et al. determined a higher FIB-4 score in a cohort of 86 Nepalese HIV-patients with positive HEV-IgG compared to HEV-IgG negative controls, suggesting an increase liver fibrosis profile in this group (30). These two studies suggest that previous contact to or active replication of HEV is associated with liver fibrosis. However, both surveys lack information whether patients had a chronic course of hepatitis E which would increase the probability of HEV being the main cause for the detected liver fibrosis.
In a long-term observation of 4–14 years of 55 patients with hepatitis E, Liu et al. describe 15 patients who were still RNA-positive after 6 months. Since underlying diseases, virus-associated treatment or liver stiffness assessment of these 15 patients was not specified (15), the clinical implications that are caused by the detected chronic HEV replication in this cohort remain unclear.
Published data on fibrosis assessment, in hepatitis E patients suggest that those patients, who are examined, tend to display fibrosis which can already be advanced. As depicted in Table 1, most published reports achieve fibrosis detection by liver biopsy. Since this procedure contains the risk of complications, fibrosis assessment in other chronic liver diseases such as NASH or other forms of viral hepatitis has shifted toward non-invasive LSM which was applied in our cohort.
Established surrogates for inflammation in the liver, ALT and AST, were lower in the group of treated patients without reaching statistical significance. Albeit in a small cohort, GGT levels were significantly lower in the group of treated patients. GGT may have a role as a follow-up parameter in the assessment of liver disease in patients with chronic hepatitis E. ALT levels >5 times the upper limit of normal (ULN) may lead to confounding results in 2D-SWE measurement (26). In our cohort, the only patient who had an ALT >5 times the ULN displayed a lower liver stiffness (1.55 m/s; 7.1 kPa) than the median stiffness of patients with active chronic hepatitis E and all patients with chronic hepatitis E.
Limitations of this study are the absence of histological examination or TE. There were no serial measurements. The small cohort size is mainly caused by the infrequent chronic course of hepatitis E and our findings can therefore only be interpreted as indicative.
Conclusions
It is known that chronic hepatitis E can lead to liver fibrosis and cirrhosis. However, there is still only limited knowledge about the natural course of disease and about factors that influence progression of fibrosis. Our findings clearly underline that the burden of liver fibrosis is high in patients with chronic hepatitis E. Even after successful treatment, persisting elevated liver stiffness can be detected. We believe that with current knowledge these patients should continue to undergo surveillance US every six months as it is recommended in successfully treated patients with hepatitis C associated advanced fibrosis (31). 2D-SWE is a helpful tool in fibrosis detection and grading as it is accurate, non-invasive and easily repeatable. Further studies with larger numbers of patients and over longer time periods would be helpful to better understand the course of chronic hepatitis E and its clinical impact.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-21-1178/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-21-1178/coif). FT reports that the laboratory of FT has received funding from Allergan, BMS, Inventiva and Gilead. FT has received honoraria or consulting fees from Allergan, Alnylam, Bayer, Gilead, BMS, Boehringer, Intercept, Ionis, Inventiva, Merz, Pfizer, NGM, CSL Behring, Novo Nordisk, Novartis, Falk. The consulting activities are unrelated to the current study. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committees of the Universities of Berlin (approval number: EA4/109/21) and informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kamar N, Izopet J, Pavio N, Aggarwal R, Labrique A, Wedemeyer H, Dalton HR. Hepatitis E virus infection. Nat Rev Dis Primers 2017;3:17086. [Crossref] [PubMed]
- Tseng TC, Liu CJ, Chang CT, Su TH, Yang WT, Tsai CH, Chen CL, Yang HC, Liu CH, Chen PJ, Chen DS, Kao JH. HEV superinfection accelerates disease progression in patients with chronic HBV infection and increases mortality in those with cirrhosis. J Hepatol 2020;72:1105-11. [Crossref] [PubMed]
- Kamar N, Mansuy JM, Cointault O, Selves J, Abravanel F, Danjoux M, Otal P, Esposito L, Durand D, Izopet J, Rostaing L. Hepatitis E virus-related cirrhosis in kidney- and kidney-pancreas-transplant recipients. Am J Transplant 2008;8:1744-8. [Crossref] [PubMed]
- Gérolami R, Moal V, Colson P. Chronic hepatitis E with cirrhosis in a kidney-transplant recipient. N Engl J Med 2008;358:859-60. [Crossref] [PubMed]
- Haagsma EB, van den Berg AP, Porte RJ, Benne CA, Vennema H, Reimerink JH, Koopmans MP. Chronic hepatitis E virus infection in liver transplant recipients. Liver Transpl 2008;14:547-53. [Crossref] [PubMed]
- Pischke S, Suneetha PV, Baechlein C, Barg-Hock H, Heim A, Kamar N, Schlue J, Strassburg CP, Lehner F, Raupach R, Bremer B, Magerstedt P, Cornberg M, Seehusen F, Baumgaertner W, Klempnauer J, Izopet J, Manns MP, Grummer B, Wedemeyer H. Hepatitis E virus infection as a cause of graft hepatitis in liver transplant recipients. Liver Transpl 2010;16:74-82. [Crossref] [PubMed]
- Kamar N, Garrouste C, Haagsma EB, Garrigue V, Pischke S, Chauvet C, et al. Factors associated with chronic hepatitis in patients with hepatitis E virus infection who have received solid organ transplants. Gastroenterology 2011;140:1481-9. [Crossref] [PubMed]
- Schlosser B, Stein A, Neuhaus R, Pahl S, Ramez B, Krüger DH, Berg T, Hofmann J. Liver transplant from a donor with occult HEV infection induced chronic hepatitis and cirrhosis in the recipient. J Hepatol 2012;56:500-2. [Crossref] [PubMed]
- Neukam K, Barreiro P, Macías J, Avellón A, Cifuentes C, Martín-Carbonero L, Echevarría JM, Vargas J, Soriano V, Pineda JA. Chronic hepatitis E in HIV patients: rapid progression to cirrhosis and response to oral ribavirin. Clin Infect Dis 2013;57:465-8. [Crossref] [PubMed]
- Ingiliz P, Mayr C, Obermeier M, Herbst H, Polywka S, Pischke S. Persisting hepatitis E virus infection leading to liver cirrhosis despite recovery of the immune system in an HIV-infected patient. Clin Res Hepatol Gastroenterol 2016;40:e23-5. [Crossref] [PubMed]
- Mazzola A, Tran Minh M, Charlotte F, Hdiji A, Bernard D, Wendum D, Calmus Y, Conti F. Chronic Hepatitis E Viral Infection After Liver Transplantation: A Regression of Fibrosis After Antiviral Therapy. Transplantation 2017;101:2083-7. [Crossref] [PubMed]
- Choi M, Hofmann J, Köhler A, Wang B, Bock CT, Schott E, Reinke P, Nickel P. Prevalence and Clinical Correlates of Chronic Hepatitis E Infection in German Renal Transplant Recipients With Elevated Liver Enzymes. Transplant Direct 2018;4:e341. [Crossref] [PubMed]
- Beer A, Holzmann H, Pischke S, Behrendt P, Wrba F, Schlue J, Drebber U, Neudert B, Halilbasic E, Kreipe H, Lohse A, Sterneck M, Wedemeyer H, Manns M, Dienes HP. Chronic Hepatitis E is associated with cholangitis. Liver Int 2019;39:1876-83. [Crossref] [PubMed]
- Pischke S, Stiefel P, Franz B, Bremer B, Suneetha PV, Heim A, Ganzenmueller T, Schlue J, Horn-Wichmann R, Raupach R, Darnedde M, Scheibner Y, Taubert R, Haverich A, Manns MP, Wedemeyer H, Bara CL. Chronic hepatitis e in heart transplant recipients. Am J Transplant 2012;12:3128-33. [Crossref] [PubMed]
- Liu L, Liu Y, Du Y, Liu C, Li W, Li H, Jia T, Chang L. Analysis of long-term follow-up and examination of pathological liver tissue in chronic hepatitis E. Dig Liver Dis 2016;48:684-6. [Crossref] [PubMed]
- Jagjit Singh GK, Ijaz S, Rockwood N, Farnworth SP, Devitt E, Atkins M, Tedder R, Nelson M. Chronic Hepatitis E as a cause for cryptogenic cirrhosis in HIV. J Infect 2013;66:103-6. [Crossref] [PubMed]
- Miyoshi M, Kakinuma S, Tanabe Y, Ishii K, Li TC, Wakita T, Tsuura Y, Watanabe H, Asahina Y, Watanabe M, Ikeda T. Chronic Hepatitis E Infection in a Persistently Immunosuppressed Patient Unable to Be Eliminated after Ribavirin Therapy. Intern Med 2016;55:2811-7. [Crossref] [PubMed]
- Schulz M, Biedermann P, Bock CT, Hofmann J, Choi M, Tacke F, Hanitsch LG, Mueller T. Rituximab-Containing Treatment Regimens May Imply a Long-Term Risk for Difficult-To-Treat Chronic Hepatitis E. Int J Environ Res Public Health 2020;17:341. [Crossref] [PubMed]
- Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith ADAmerican Association for the Study of Liver Diseases. Liver biopsy. Hepatology 2009;49:1017-44. [Crossref] [PubMed]
- Schulz M, Tacke F. Identifying High-Risk NASH Patients: What We Know so Far. Hepat Med 2020;12:125-38. [Crossref] [PubMed]
- Schulz M, Kleinjans M, Strnad P, Demir M, Holtmann TM, Tacke F, Wree A. Shear Wave Elastography and Shear Wave Dispersion Imaging in the Assessment of Liver Disease in Alpha1-Antitrypsin Deficiency. Diagnostics (Basel) 2021;11:629. [Crossref] [PubMed]
- Schulz M, Vuong LG, Müller HP, Maibier M, Tacke F, Blau IW, Wree A. Shear Wave Elastography in the Detection of Sinusoidal Obstruction Syndrome in Adult Patients Undergoing Allogenic Hematopoietic Stem Cell Transplantation. Diagnostics (Basel) 2021;11:928. [Crossref] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines on hepatitis E virus infection. J Hepatol 2018;68:1256-71. [Crossref] [PubMed]
- Zhu FC, Zhang J, Zhang XF, Zhou C, Wang ZZ, Huang SJ, et al. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: a large-scale, randomised, double-blind placebo-controlled, phase 3 trial. Lancet 2010;376:895-902. [Crossref] [PubMed]
- Schulz M, Beha D, Plehm K, Zöllner C, Hofmann J, Schott E. High prevalence of anti-hepatitis E virus antibodies in outpatients with chronic liver disease in a university medical center in Germany. Eur J Gastroenterol Hepatol 2016;28:1431-6. [Crossref] [PubMed]
- Dietrich CF, Bamber J, Berzigotti A, Bota S, Cantisani V, Castera L, Cosgrove D, Ferraioli G, Friedrich-Rust M, Gilja OH, Goertz RS, Karlas T, de Knegt R, de Ledinghen V, Piscaglia F, Procopet B, Saftoiu A, Sidhu PS, Sporea I, Thiele M. EFSUMB Guidelines and Recommendations on the Clinical Use of Liver Ultrasound Elastography, Update 2017 (Long Version). Ultraschall Med 2017;38:e48. [Crossref] [PubMed]
- Ferraioli G, Maiocchi L, Dellafiore C, Tinelli C, Above E, Filice C. Performance and cutoffs for liver fibrosis staging of a two-dimensional shear wave elastography technique. Eur J Gastroenterol Hepatol 2021;33:89-95. [Crossref] [PubMed]
- European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016;64:1388-402. [Crossref] [PubMed]
- Viera-Segura O, Realpe-Quintero M, Panduro A, Roman S, Jose-Abrego A, Gonzalez-Aldaco K, Trujillo-Ochoa JL, Fierro NA. First detection of hepatitis E virus genotype 3 as a common infectious agent in patients with chronic liver damage in Mexico. Ann Hepatol 2019;18:571-7. [Crossref] [PubMed]
- Shah SM, Baniya JB, Gupta BP, Shrestha A, Rodin H, Boonstra A, Debes JD. Short article: Association between liver fibrosis and hepatitis E seroprevalence among HIV-positive individuals in Nepal. Eur J Gastroenterol Hepatol 2019;31:503-5. [Crossref] [PubMed]
- European Association for the Study of the Liver. Clinical Practice Guidelines Panel: Chair; EASL Governing Board representative. EASL recommendations on treatment of hepatitis C: Final update of the series. J Hepatol 2020;73:1170-218. [Crossref]


