
Sagittal morphology of the cervical spine in adolescent idiopathic scoliosis: a retrospective case-control study
Introduction
As a 3-dimensional deformity of the spine, adolescent idiopathic scoliosis (AIS) is a common type of scoliosis with an incidence of 2–3% in the general population (1-4). The clinical symptoms of AIS are characterized by disfigurement of the torso with waist or shoulder asymmetry, rib rotation, and trunk imbalance (5,6). The main diagnostic criterium of AIS is the maximal Cobb angle >10º. When scoliosis occurs in the coronal plane of the spine, the sagittal plane shape will change accordingly. Due to the complex anatomical structure and movement mode of the human body, the sagittal profile changes in each segment of the spine are different. Many radiological indexes have been used to evaluate the sagittal spinal and pelvic alignment in the treatment of spinal diseases (7). In patients who have thoracic scoliosis, coronal deformity is frequently associated with sagittal alignment. Current research of AIS sagittal plane is mostly focused on the interaction between different spinal segments (8), with few studies on the spinal sagittal morphology and coronal scoliosis. As the most flexible segment of the spine, the cervical spine which is rich in peripheral nerves and blood vessels has the main function of maintaining horizontal line of sight. Disorders of the structure and function of the cervical spine will affect the sagittal balance of the body, leading to cervical spondylosis and neck pain to decrease the life quality of the patients (9,10). Moreover, the incidence of cervical curvature straightening or kyphosis or thoracic curvature straightening in AIS patients is higher than that in normal adolescents and children (11), and decreases in cervical lordosis and thoracic kyphosis are commonly related to AIS (12). Cervical spine deformity has also been reported to be closely associated with the thoracic deformity, especially on the sagittal plane (1,13), and sagittal alignment of the cervical spine is changed after deformity correction in patients with AIS (14). Therefore, it is of great clinical significance to study the changes of cervical sagittal curvature in patients with AIS. This retrospective study was consequently performed to analyze the imaging characteristics of cervical sagittal curvature and spinal coronal deformity in AIS patients so as to reveal the clinical significance. We present the following article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-21-902/rc).
Methods
Subjects
This retrospective case-control study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of the Third Hospital of Hebei Medical University, and the legal guardians of all patients had signed the informed consent to participate. Between October 2018 and September 2020, patients with AIS who underwent anteroposterior and lateral X-ray radiography of the whole spine in our hospital were enrolled. The inclusion criteria were patients with AIS, aged between 12 and 17 years, who underwent anteroposterior and lateral X-ray radiography, and Cobb angle >10°. The exclusion criteria were patients with congenital scoliosis (including hemivertebrae, butterfly vertebrae, and failure of segmentation or spinal dyssegmentation), other developmental thoracic deformity (funnel chest etc.), spinal cord deformity, upper limb and girdle bone malformation, scoliosis with definitive reasons (secondary to neurofibromatosis, Marfan syndrome, and syringomyelia), diseases or functional and structural abnormalities in the pelvic, hip and lower limbs, spinal tumor lesions, history of spinal trauma and infection, and metabolic bone disease. The enrollment of patients is shown in Figure 1. The patients were divided into two groups according to the size of the Cobb angle: group A with a Cobb angle ≤45° and group B with a Cobb angle >45°.
Data measurement
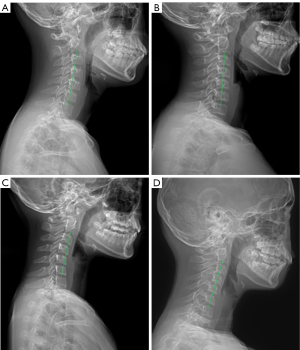
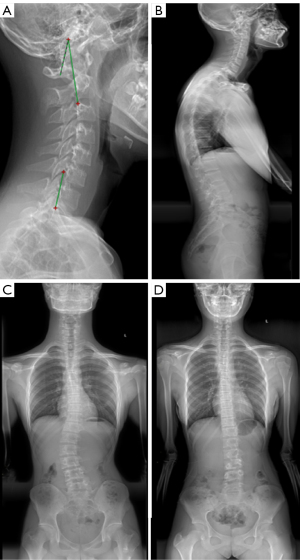
All patients had undergone full-length standing anteroposterior and lateral X-ray radiography of the whole spine. All data were saved, extracted, and measured through the picture archiving and communication systems (PACS). The maximal Cobb angle was measured on the anteroposterior radiograph, namely, the angle between the parallel lines of the upper endplate and the lower endplate. The morphology of cervical spine in the sagittal plane was evaluated in four types classified according to the centroid method (four types: lordosis as shown in Figure 2A, straightening in Figure 2B, kyphosis in Figure 2C, and sigmoid in Figure 2D). On the lateral X-ray radiograph, the following parameters were measured: cervical lordosis (CL, the angle formed between the tangent line of the lower end plate of C2 and that of the inferior end plate of C7 using the Jackson physiological stress line method as shown in Figure 3A, in a patient with a large Cobb angle in Figure 3B,3C, in a patient with a small Cobb angle in Figure 3D, and in patients with different Cobb angles and cervical lordosis for comparison in Figure 4), C1-C2 angle (the angle between the line connecting the anterior and posterior arch of atlas and the line passing through the inferior endplate of C2 vertebral body), T1 slope (the sagittal angle between a horizontal line and the superior end plate of T1), thoracic kyphosis (TK, the angle between the parallel line of T1 vertebral upper endplate and T12 vertebral lower endplate), lumbar lordosis (LL, the angle between the parallel line of superior endplate of L1 vertebral body and the parallel line of superior endplate of sacrum), sacral slope (SS, the angle between the parallel line and the horizontal line of the superior sacral endplate), pelvic tilt (PT), pelvic incidence (PI), cervical sagittal vertical axis [cSVA, with cSVA >40 mm being defined as cervical sagittal imbalance (15)], and sagittal vertical axis [SVA, with SVA >50 mm as sagittal imbalance (16)]. These spinopelvic alignment parameters had been defined in the literature (1,17).
Two researchers independently measured the above parameters, and an average of the parameters was calculated for analysis. When the error was large between two measurements, the values were measured again for calculation of the average value.
Statistical analysis
The statistical analysis was performed with the SPSS 21.0 software (IBM, Chicago, IL, USA). Measurement data were presented as mean ± standard deviation if in normal distribution and tested with the student t-test. If measurement data were in skew distribution, they were presented as median and interquartile range and tested with the Mann-Whitney-U test. Enumeration data were presented as numbers and percentages and tested with the Chi square test. The correlation analysis between measurement data was performed with the Spearman correlation analysis (bilateral sides) with |r| <0.3 as weak correlation, 0.3≤ |r| <0.5 as mild correlation, 0.5≤ |r| <0.8 as moderate correlation, |r| ≥0.8 as marked correlation, and |r| >0.95 as highly marked correlation. Comparison between groups was conducted with the Mann-Whitney-U test. The significant P was set at <0.05.
Results
A total of 454 patients with AIS were enrolled (Figure 1), including 146 (32.2%) male and 308 (67.8%) female patients, with an age range of 12–17 (median 14) years. Patients between 13–14 years accounted for 41.2%. According to the sagittal morphology of the cervical spine, a lordosis spine was in 132 (29.1%) patients, straightening in 228 (50.2%), kyphosis in 85 (18.7%), and S type in 9 (2.0%) (Figures 2,3).
There were 414 patients in group A, including 128 (30.9%) male and 286 (69.1%) female patients, and 40 patients in group B, including 18 (45.0%) male and 22 (55.0%) female patients (Table 1). No significant (P>0.05) differences existed in the age and sex component between the two groups. In group B, the main curve was in the thoracic spine (17/40), and other curves included lumbar curve in 6 cases, thoracolumbar curve in 5, double curve in 11, and triple curve in 1.
Table 1
| Parameters | Group A, median [IQR] | Group B, median [IQR] | 95% CI | P | |
|---|---|---|---|---|---|
| Group A | Group B | ||||
| No. | 414 | 40 | – | – | – |
| Male/female | 128/286 | 18/22 | – | – | 0.077a |
| Age (years) | 14 [3] | 14 [3] | 14.20–14.51 | 13.83–14.87 | 0.996 |
| Cobb angle | 24 [12] | 51.2 [9] | 24.56–26.13 | 50.14–54.08 | 0.000 |
| CL angle | 5.1 [19] | 6.0 [29] | 3.38–5.96 | 2.01–13.23 | 0.540 |
| C1-C2 angle | 31.0 [14] | 30.9 [14] | 30.21–32.28 | 28.41–34.49 | 0.966 |
| cSVA | 17.1 [13] | 18.7 [14] | 16.72–18.46 | 15.27–21.15 | 0.780 |
| T1S | 20.0 [10] | 22.0 [14] | 19.70–21.16 | 18.07–24.10 | 0.559 |
| TK | 34.5 [16] | 35.5 [25] | 33.25–35.40 | 29.50–39.61 | 0.910 |
| LL | 48.0 [15] | 53.0 [17] | 47.20–49.28 | 47.23–56.00 | 0.107 |
| SS | 36.0 [10] | 39.5 [10] | 35.21–36.69 | 36.90–42.22 | 0.009 |
| PT | 8.0 [9] | 7.1 [11] | 8.08–9.54 | 5.34–10.17 | 0.419 |
| PI | 44.0 [13] | 48.3 [11] | 43.74–45.78 | 44.43–50.69 | 0.037 |
| SVA | 8.0 [32] | 11.3 [43] | 2.41–6.91 | 5.80–23.68 | 0.032 |
AIS, adolescent idiopathic scoliosis; QR, interquartile range; CL, cervical lordosis; cSVA, cervical sagittal vertical axis; T1S, T1 slope; TK, thoracic kyphosis; LL, lumbar lordosis; SS, sacral slope; PT, pelvic tilt; PI, pelvic incidence; SVA, sagittal vertical axis; CI, confidence interval. a, means Chi-Square Test result; other Ps are of Mann-Whitney U Test result.
Correlation analysis was performed between the Cobb angle and the spinal sagittal parameters (Table 2). In group A, the Cobb angle (median 24, 95% CI: 24.56–26.13) was in a medium negative correlation with the CL angle (median 5.1, 95% CI: 3.38–5.96, r=−0.637, P<0.001), in a weak positive correlation (|r| <0.3, P<0.05) with the C1-C2 angle (median 31.0, 95% CI: 30.21–32.28), T1 slope (median 20.0, 95% CI: 19.70–21.16) and TK (median 34.5, 95% CI: 33.25–35.40), but in no apparent correlation with the other parameters. In group B, the Cobb angle (median 51.2, 95% CI: 50.14–54.08) was in a mild positive correlation (P<0.05) with PT (median 7.1, 95% CI: 5.34–10.17, r=0.398) and PI (median 48.3, 95% CI: 44.43–50.69, r=0.360) but in no apparent correlation with the other parameters.
Table 2
| Parameters | Cobb angle (Group A) | Cobb angle (Group B) | |||
|---|---|---|---|---|---|
| r | P | r | P | ||
| CL | −0.637 | 0.000** | 0.150 | 0.355 | |
| C1-C2 angle | 0.119 | 0.015* | 0.054 | 0.740 | |
| cSVA | 0.078 | 0.112 | 0.155 | 0.339 | |
| T1S | −0.255 | 0.000** | 0.056 | 0.731 | |
| TK | −0.238 | 0.000** | −0.073 | 0.655 | |
| LL | 0.007 | 0.884 | −0.107 | 0.510 | |
| SS | 0.067 | 0.173 | 0.065 | 0.691 | |
| PT | 0.043 | 0.385 | 0.398 | 0.011* | |
| PI | 0.064 | 0.197 | 0.360 | 0.023* | |
| SVA | 0.017 | 0.734 | 0.056 | 0.732 | |
*, P<0.05; **, P<0.01. CL, cervical lordosis; cSVA, cervical sagittal vertical axis; T1S, T1 slope; TK, thoracic kyphosis; LL, lumbar lordosis; SS, sacral slope; PT, pelvic tilt; PI, pelvic incidence; SVA, sagittal vertical axis.
The CL angle was significantly (P<0.05) different between male and female patients in both groups (Table 3). Correlation analysis was performed between the CL angle and other parameters (Table 4). In Group A, the CL angle (median 5.1, 95% CI: 3.38–5.96) was in a significantly (P<0.01) positive correlation with the T1 slope (median 20.0, 95% CI: 19.70–21.16, r=0.586), TK (median 34.5, 95% CI: 33.25–35.40, r=0.490), and SVA (median 8.0, 95% CI: 2.41–6.91, r=0.135), in a significantly (P<0.01) negative correlation with cSVA (median 17.1, 95% CI: 16.72–18.46, r=−0.128) and C1-C2 angle (median 31.0, 95% CI: 30.21–32.28, r=−0.155), but in no significant correlation with other parameters. In group B, the CL angle (median 6.0, 95% CI: 2.01–13.23) was in a significantly (P<0.05) positive correlation with T1 slope (median 22.0, 95% CI: 18.07–24.10, r=0.661), TK (median 35.5, 95% CI: 29.50–39.61, r=0.608), LL (median 53.0, 95% CI: 47.23–56.0, r=0.425), SS (median 39.5, 95% CI: 36.90–42.22, r=0.434), and SVA (median 11.3, 95% CI: 5.80–23.68, r=0.335), but in no significant (P>0.05) correlation with other parameters.
Table 3
| Group | Male, median [IQR] | Female, median [IQR] | P |
|---|---|---|---|
| A | 8.8 [20] | 2.75 [17] | 0.000** |
| B | 8.4 [23] | −4.2 [30] | 0.016* |
*, P<0.05; **, P<0.01. IQR, interquartile range; CL, cervical lordosis.
Table 4
| Parameters | CL (Group A) | CL (Group B) | |||
|---|---|---|---|---|---|
| r | P | r | P | ||
| Age | −0.019 | 0.692 | −0.080 | 0.625 | |
| C1-C2 angle | −0.155 | 0.002** | −0.018 | 0.911 | |
| cSVA | −0.128 | 0.009** | 0.008 | 0.961 | |
| T1S | 0.586 | 0.000** | 0.661 | 0.000** | |
| TK | 0.490 | 0.000** | 0.608 | 0.000** | |
| LL | 0.047 | 0.344 | 0.425 | 0.006** | |
| SS | −0.039 | 0.428 | 0.434 | 0.005** | |
| PT | −0.056 | 0.252 | 0.001 | 0.994 | |
| PI | −0.064 | 0.195 | 0.239 | 0.137 | |
| SVA | 0.135 | 0.006** | 0.335 | 0.035* | |
*, P<0.05; **, P<0.01. CL, cervical lordosis; cSVA, cervical sagittal vertical axis; T1S, T1 slope; TK, thoracic kyphosis; LL, lumbar lordosis; SS, sacral slope; PT, pelvic tilt; PI, pelvic incidence; SVA, sagittal vertical axis.
Discussion
Major findings
In this study investigating the relationship between the spinal sagittal alignment and coronal deformity in patients with AIS, it was found that in patients with the Cobb angle ≤45º, the cervical lordosis significantly decreases with increase of Cobb angle, and that in patients with the Cobb angle >45º, no significant (P>0.05) correlation is found between the cervical lordosis and the Cobb angle.
Characteristics of cervical sagittal morphology
In newborns, there is only large C-shaped kyphosis in the sagittal plane of the spine. After the infant begins to learn to crawl, stand and walk, four physiological curves of the spinal sagittal plane are formed, namely cervical anterior curve, thoracic posterior curve, lumbar anterior curve, and sacral posterior curve. When the body static posture and movement mode change, the three-dimensional shape of the spine also changes for adaptation, and bad living habits and environmental factors may lead to abnormal spinal adaptation and subsequent AIS. AIS is an abnormal change of curvature in three-dimensional directions of the spine and affects the coronal, horizontal, and sagittal plane in varying degrees.
In as early as 1995, it was found that the cervical curvature of AIS patients decreased (18), which was confirmed by later research (15). The CL of AIS patients was less than that of normal adolescents while the incidence of kyphosis was significantly higher than that of normal adolescents (15). In our study with the sagittal morphology of cervical spine being divided into four types: lordosis, straightening, kyphosis and S type based on the detailed centroid method, the incidence of AIS patients with cervical straightening, kyphosis, and S-type (70.9%) was significantly higher than that of AIS patients with lordosis (29.1%). In a study investigating the incidence and risk factors of cervical kyphosis (CK) in AIS patients, it was found that CK occurred in 60.7% (68/112) of AIS patients but only in 35% (14/40) of normal population. Hiyama et al. also found a higher incidence of CK in AIS patients than in normal control subjects (59.5% vs. 41.1%) (1).
In clinical observation, we found that when the curvature of cervical spine changed, the three-dimensional structure of the whole spine changed. The cervical spine is the most easily observed spinal segment. Therefore, we studied the morphology of the cervical spine to estimate the severity of scoliosis. Scoliosis in patients with a large Cobb angle is more manifested in the body posture, and its severity is easy to observe. For patients with a small Cobb angle, the posture changes little, which is difficult to observe. Therefore, we hope to make a preliminary assessment of the severity of scoliosis through a simpler method by observing the cervical curvature, which is convenient for clinicians to screen patients and for patients and their families to observe and evaluate the changes of their condition. This can help to avoid unnecessary radiological examination. In addition, it also lays a foundation for further evaluating changes of cervical curvature after surgery and brace treatment. After surgery and brace treatment, if we do not pay attention to the recovery of cervical curvature, the correction effect of patients will be affected. Therefore, strengthening the study on cervical curvature still has some clinical value.
Effect of Cobb angle on the cervical sagittal parameters
Spinal coronal scoliosis can affect some sagittal parameters (TK, LL, and PI). In patients with the Cobb angle of coronal thoracic curvature ≥20º, the incidence rate of CK is high (13). In the study investigating the incidence of CK in AIS patients, the incidence of CK increased with increase of the Cobb angle, however, no correlation was found between these two parameters. We thought that there was a certain relationship between cervical sagittal morphology and coronal deformity of spine. In clinical practice, we found that in patients with the Cobb angle <45, the anterior curvature of the cervical spine tended to become smaller (straightened or even kyphosis) as the Cobb angle or scoliosis increased. However, in patients with the Cobb angle >45º, the anterior curvature of the cervical spine appeared to enlarge and tended to become “normalized” (Figure 4). Of course, it’s not really normal. Rather, the head moves anteriorly to compensate for increased scoliosis. If the cervical anterior curvature decreased with increase of the Cobb angle, the CK should be more apparent in patients with the Cobb angle greater than 45°, however, it had been observed clinically that these patients exhibited cervical lordosis, straightening, or only mild kyphosis. Moreover, the Cobb angle of 45° is one of the important surgical indications for clinical treatment (19,20). Thus, in our study, AIS patients were divided into two groups based on the criteria of Cobb angle of 45º for correlation analysis, and a certain relationship was confirmed between cervical curvature and coronal deformity. When the Cobb angle was ≤45º, a moderate negative correlation existed between CL and Cobb angle (r=−0.637), suggesting that the cervical curvature decreased with increase of the Cobb angle.
In patients with the Cobb angle ≤45º, the larger the Cobb angle, the more prone the cervical spine was to kyphosis. However, in patients with more severe scoliosis or the Cobb angle >45º, no significant correlation existed between the Cobb angle and CK. No significant (P>0.05) difference in CL between the two groups also reflects the tendency of the cervical curvature to being normalized in patients with the Cobb angle >45º. If group B also is in line with this law, the CL will be significantly different. The cervical spine of AIS patients will compensate to a certain extent for the imbalance of the whole spine and the body in the sagittal plane, but the compensation of the cervical spine has a certain limit. When this limit is exceeded or other plane compensation occurs, the curvature of the cervical spine will deviate from this law. This phenomenon occurs more frequently in group B with the Cobb angle >45º.
After analyzing 39 cases in group A whose cervical curvature did not decrease with the increase of Cobb angle (Table 5), it was found that in patients with enlarged thoracic kyphosis and extended range (Figure 2B,2C), CL was enlarged (14/18) probably to compensate for lumbar lordosis. In patients with thoracic vertebral straightening, CL was mostly small (9/9). Because the cervical spine was directly adjacent to the thoracic vertebra, CL would decrease with decrease of thoracic vertebra kyphosis (1,21). When the vertebral body becomes flat or wedge-shaped, the change of spinal curvature is related to the shape of the deformed vertebral body. Vertebral rotation (Figure 2D) is also to compensate for the imbalance of the spine and to replace the effect of cervical vertebral straightening on the balance of the spine to a certain extent. The compensation effect of CL is similar to that of CK, which makes the head moving forward to balance the retroverted spine. This suggests that both CL (cervical spine leading forward) and CK make the head move forward in a similar way to balance the retroverted spine. When CL (cervical spine leading forward) occurs, the degree of CK will decrease. The shape and function of the spine are complex, and the above conditions may exist at the same time, with different effects on the sagittal plane of the cervical spine.
Table 5
| Abnormal conditions | n | Large CL | Small CL |
|---|---|---|---|
| Large range of thoracic kyphosis | 18 | 14 | 4 |
| Increased thoracic curvature | 9 | 9 | 0 |
| Straightened thoracic curvature | 7 | 1b | 6 |
| Wedge-shaped or flattened vertebral body | 5 | 3 | 2 |
| Cervical scoliosis | 5 | 2 | 3 |
| Scoliosis vertebral rotation | 4 | 3 | 1a |
| Cervical spine extension | 2 | 2 | 0 |
a, the patient also has a large scope of thoracic kyphosis; b, the patient also has thoracic kyphosis decrease. CL, cervical lordosis.
The Cobb angle was also in a weak negative correlation with the T1S and TK (−0.255 and −0.238, respectively, P<0.001), but in no correlation with LL, SS, PT and PI. This may suggest some certain correlation between Cobb angle and sagittal parameters of thoracic vertebrae, but there is no linear correlation. The Cobb angle in AIS patients may have an effect on the sagittal morphology of thoracic spine, lumbar spine and pelvis, which may be related to the location of scoliosis.
Other factors affecting cervical sagittal parameters
In our study, the CL was significantly (P<0.05) decreased in women than in men in both groups (Table 4), and other studies have also confirmed that CL was related to gender (17,22). This may be due to the fact that compared with adolescent males, females have a softer spine, lower neck muscle volume, and less muscle tension around the spine compared with males (23), and the morphology of cervical spine is more easily affected by spinal deformity. Zheng et al. (23) found in their study investigating sex-specific prediction of neck muscle volume that females had 59% lower total neck muscle volume compared to that of males (510±43 vs. 814±64 cm3, P<0.0001). In the study of cervical spine alignment and range of motion (24), Liu et al. found that segmental range of motion (65.02°±15.48° vs. 61.41°±14.94°), flexion range of motion (−25.59°±9.68° vs. −24.97°±9.96°) and extension range of motion (39.42°±11.70° vs. 36.44°±10.59°) in the cervical spine were significantly greater in females than those in males and were related to cervical alignment. Gong et al. (17) found in their study investigating changes of upright body posture in the sagittal plane of men and women that marked changes in the neck, thorax and hip in different body posture were significantly present in women than in men. The C1-C2 angle is a parameter of the upper cervical spine, and its main function is to maintain the horizontal line of sight. Our study demonstrated no linear relationship between CL and C1-C2 angle regardless of the size of Cobb angle. However, multiple uncertain factors, including the patient’s posture and anatomical position, may affect the measurement accuracy of C1-C2 angle, and the exact relationship between the C1-C2 angle and Cobb angle remain to be determined. The cervical vertebra is adjacent to the thoracic vertebra, which makes the CL greatly affected by the thoracic sagittal parameters (T1S and TK) (1,21,25). Our study had confirmed this finding, demonstrating that CL positively increased with increase of T1S and TK (r=0.586 and 0.490, respectively) in patients with the Cobb angle ≤45° and increase of T1S (r=0.661) in patients with the Cobb angle >45°.
The cSVA and SVA are parameters representing the sagittal balance of the cervical spine and the whole spine, respectively. Generally, cSVA >4 cm and SVA >5 cm represent sagittal imbalance of the cervical spine and the whole spine, respectively. The cSVA and SVA were in a negative correlation with the health-related quality of life (HRQoL) score, and in cSVA >4 cm and SVA >5 cm, the HRQoL score markedly decreased (26). In our study, the CL in group A with the Cobb angle ≤45° did not have a linear correlation with cSVA (r=−0.128) or SVA (r=0.135), indicating no direct impact on CL by cSVA and SVA. In group B with the Cobb angle >45°, the CL was in a mild positive correlation with SVA (r=0.335), suggesting that the spinal sagittal balance in patients with severe scoliosis will have a certain effect on the cervical curvature. In a study by Yu et al. (27), it was found that the SVA in patients with no CK was significantly higher than that in patients with CK, whereas cSVA had no statistical difference. The study by Tang et al. (21) suggested that decrease of SVA was an independent risk factor for CK in AIS patients. The decrease of SVA means that the body has a tendency of leaning backward, which makes the cervical spine and head move forward for compensation, thus resulting in decreased CL and even kyphosis. When severe scoliosis occurs and involves a long segment of the spine, a greater degree of change is needed in the horizontal rotation and sagittal curvature for compensation, resulting in a certain kyphosis tendency of the cervical spine. The increase of cSVA indicates extension of cervical spine, which, just like decrease of CL, compensates for spinal retroversion.
In our series, CL did not have a linear relationship with LL and SS in patients with the Cobb angle ≤45°, but had a medium positive correlation with LL (r=0.425) and SS (r=0.434) in patients with the Cobb angle >45º, suggesting that CL will increase with increase of LL and SS. In patients with severe scoliosis, lumbosacral imbalance may be more serious, which has a certain impact on the sagittal morphology of cervical spine. In group B with the Cobb angle >45º in our study, the main curve was in the thoracic spine (17/40), and other curves accounted for over 1/2 of the cohort (23/40). The correlation of CL with LL or SS was medium, which may be due to the serious scoliosis of the patients in this group because more than half of the patients do not have the main curve in the thoracic spine.
Our study suggested that spinal coronal deformity is related to the cervical sagittal morphology in AIS patients with the Cobb angle ≤45°. In AIS patients with the Cobb angle >45º, the spinal coronal deformity is not related to the cervical spine but is mildly correlated with the pelvic parameters. It is suggested that there may be different modes of sagittal balance adjustment in patients with different severity of scoliosis.
Strengths and limitations
Our study investigated the relationship of the Cobb angle with the cervical sagittal parameters by dividing AIS patients into two groups with a Cobb angle ≤45° or >45°. It was thus found that in patients with Cobb angle ≤45º, the more severe the coronal scoliosis, the smaller the CL was, resulting in straightening or kyphosis of the cervical spine. The limitations of our study included the retrospective and one center design, no observation on the dynamic changes of AIS condition, no control, and Chinese patients enrolled only, which may all affect the outcomes of the study. Future studies will have to solve these issues for better conclusions.
In conclusion, the Cobb angle is in a moderate negative correlation with cervical lordosis in AIS patients with Cobb angle ≤45°, and is correlated with the pelvic parameters rather than cervical lordosis in AIS patients with Cobb angle >45°. The cervical lordosis is in a Moderate positive correlation with the T1 slope and thoracic kyphosis regardless of the Cobb angle. In AIS patients with Cobb angle >45°, the cervical lordosis is also positively correlated with lumbar lordosis and sacral slope. The sagittal morphology of the cervical spine in AIS patients is affected by the spinal coronal deformity, and the interaction mode of the two has a certain law to follow, which has a certain guiding significance for the treatment of AIS.
Acknowledgments
Funding: Tracking project of Hebei Provincial Health Commission (GZ2020050); Medical science research project of Hebei Province (20200082).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-21-902/rc).
Conflicts of Intertest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-21-902/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of the Third Hospital of Hebei Medical University, and the legal guardians of all patients had signed the informed consent to participate.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hiyama A, Sakai D, Watanabe M, Katoh H, Sato M, Mochida J. Sagittal alignment of the cervical spine in adolescent idiopathic scoliosis: a comparative study of 42 adolescents with idiopathic scoliosis and 24 normal adolescents. Eur Spine J 2016;25:3226-33. [Crossref] [PubMed]
- Hu Z, Vergari C, Gajny L, Liu Z, Lam TP, Zhu Z, Qiu Y, Man GCW, Yeung KH, Chu WCW, Cheng JCY, Skalli W. Comparison of 3D and 2D characterization of spinal geometry from biplanar X-rays: a large cohort study. Quant Imaging Med Surg 2021;11:3306-13. [Crossref] [PubMed]
- Negrini S, Aulisa AG, Aulisa L, Circo AB, de Mauroy JC, Durmala J, Grivas TB, Knott P, Kotwicki T, Maruyama T, Minozzi S, O'Brien JP, Papadopoulos D, Rigo M, Rivard CH, Romano M, Wynne JH, Villagrasa M, Weiss HR, Zaina F. 2011 SOSORT guidelines: Orthopaedic and Rehabilitation treatment of idiopathic scoliosis during growth. Scoliosis 2012;7:3. [Crossref] [PubMed]
- Zhang Y, Shu S, Gu Q, Liu Z, Zhu Z, Qiu Y, Bao H. Cervical vertebral maturation (CVM) stage as a supplementary indicator for the assessment of peak height velocity (PHV) in adolescent idiopathic scoliosis (AIS). Quant Imaging Med Surg 2020;10:96-105. [Crossref] [PubMed]
- Hresko MT. Clinical practice. Idiopathic scoliosis in adolescents. N Engl J Med 2013;368:834-41. [Crossref] [PubMed]
- Lou EH, Hill DL, Raso JV, Moreau M, Hedden D. How quantity and quality of brace wear affect the brace treatment outcomes for AIS. Eur Spine J 2016;25:495-9. [Crossref] [PubMed]
- Schwab F, Lafage V, Patel A, Farcy JP. Sagittal plane considerations and the pelvis in the adult patient. Spine (Phila Pa 1976) 2009;34:1828-33. [Crossref] [PubMed]
- Matsubayashi Y, Chikuda H, Oshima Y, Taniguchi Y, Fujimoto Y, Shimizu T, Tanaka S. C7 sagittal vertical axis is the determinant of the C5-C7 angle in cervical sagittal alignment. Spine J 2017;17:622-6. [Crossref] [PubMed]
- Norheim EP, Carreon LY, Sucato DJ, Lenke LG, Glassman SD. Cervical Spine Compensation in Adolescent Idiopathic Scoliosis. Spine Deform 2015;3:327-31. [Crossref] [PubMed]
- Zhu C, Yang X, Zhou B, Wang L, Zhou C, Ling T, Liu L, Song Y. Cervical kyphosis in patients with Lenke type 1 adolescent idiopathic scoliosis: the prediction of thoracic inlet angle. BMC Musculoskelet Disord 2017;18:220. [Crossref] [PubMed]
- Ofiram E, Garvey TA, Schwender JD, Wroblewski JM, Winter RB. Cervical degenerative changes in idiopathic scoliosis patients who underwent long fusion to the sacrum as adults: incidence, severity, and evolution. J Orthop Traumatol 2009;10:27-30. [Crossref] [PubMed]
- Roussouly P, Labelle H, Rouissi J, Bodin A. Pre- and post-operative sagittal balance in idiopathic scoliosis: a comparison over the ages of two cohorts of 132 adolescents and 52 adults. Eur Spine J 2013;22:S203-15. [Crossref] [PubMed]
- Hu X, Lieberman IH. Prevalence and Factors Affecting Cervical Deformity in Adolescent Idiopathic Scoliosis Patients: A Single-Center Retrospective Radiological Study. Int J Spine Surg 2018;12:22-5. [Crossref] [PubMed]
- Luo SG, Zhong ZM, Zhu SY, Chen JT. The change of cervical sagittal alignment after surgery for adolescent idiopathic scoliosis. Clin Neurol Neurosurg 2018;171:21-5. [Crossref] [PubMed]
- Knott PT, Mardjetko SM, Techy F. The use of the T1 sagittal angle in predicting overall sagittal balance of the spine. Spine J 2010;10:994-8. [Crossref] [PubMed]
- Mac-Thiong JM, Transfeldt EE, Mehbod AA, Perra JH, Denis F, Garvey TA, Lonstein JE, Wu C, Dorman CW, Winter RB. Can c7 plumbline and gravity line predict health related quality of life in adult scoliosis? Spine (Phila Pa 1976) 2009;34:E519-27. [Crossref] [PubMed]
- Gong H, Sun L, Yang R, Pang J, Chen B, Qi R, Gu X, Zhang Y, Zhang TM. Changes of upright body posture in the sagittal plane of men and women occurring with aging - a cross sectional study. BMC Geriatr 2019;19:71. [Crossref] [PubMed]
- Kuntz C 4th, Levin LS, Ondra SL, Shaffrey CI, Morgan CJ. Neutral upright sagittal spinal alignment from the occiput to the pelvis in asymptomatic adults: a review and resynthesis of the literature. J Neurosurg Spine 2007;6:104-12. [Crossref] [PubMed]
- El-Hawary R, Chukwunyerenwa C. Update on evaluation and treatment of scoliosis. Pediatr Clin North Am 2014;61:1223-41. [Crossref] [PubMed]
- Negrini S, Negrini F, Fusco C, Zaina F. Idiopathic scoliosis patients with curves more than 45 Cobb degrees refusing surgery can be effectively treated through bracing with curve improvements. Spine J 2011;11:369-80. [Crossref] [PubMed]
- Tang Y, Xu X, Zhu F, Chen C, Wang F, Lu M, Huang X. Incidence and Risk Factors of Cervical Kyphosis in Patients with Adolescent Idiopathic Scoliosis. World Neurosurg 2019;127:e788-92. [Crossref] [PubMed]
- Zeng Z, Hai Y, Bi Y, Wang B, Liu M, Liu Y. Characteristics of sagittal spinopelvic alignment in asymptomatic Han Chinese adults. Exp Ther Med 2018;16:4107-13. [Crossref] [PubMed]
- Zheng L, Siegmund G, Ozyigit G, Vasavada A. Sex-specific prediction of neck muscle volumes. J Biomech 2013;46:899-904. [Crossref] [PubMed]
- Liu B, Wu B, Van Hoof T, Okito JP, Liu Z, Zeng Z. Are the standard parameters of cervical spine alignment and range of motion related to age, sex, and cervical disc degeneration? J Neurosurg Spine 2015;23:274-9. [Crossref] [PubMed]
- Canavese F, Turcot K, De Rosa V, de Coulon G, Kaelin A. Cervical spine sagittal alignment variations following posterior spinal fusion and instrumentation for adolescent idiopathic scoliosis. Eur Spine J 2011;20:1141-8. [Crossref] [PubMed]
- Tang JA, Scheer JK, Smith JS, Deviren V, Bess S, Hart RA, Lafage V, Shaffrey CI, Schwab F, Ames CP. ISSG. The impact of standing regional cervical sagittal alignment on outcomes in posterior cervical fusion surgery. Neurosurgery 2012;71:662-9; discussion 669. [Crossref] [PubMed]
- Yu M, Silvestre C, Mouton T, Rachkidi R, Zeng L, Roussouly P. Analysis of the cervical spine sagittal alignment in young idiopathic scoliosis: a morphological classification of 120 cases. Eur Spine J 2013;22:2372-81. [Crossref] [PubMed]



