
Early assessment of subclinical myocardial injury in systemic lupus erythematosus by two-dimensional longitudinal layer speckle tracking imaging
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease with cardiac involvement and is characterized by the formation of autoantibodies and deposition of immune complexes. It is associated with higher cardiovascular morbidity and mortality (1,2). The diagnosis of myocardial insufficiency remains a challenge, because symptoms in patients with SLE are often mild, and myocardial involvement may be present even asymptomatically, particularly at the early stage. Previous studies have found that in patients with SLE, the detection rate of myocardial damage during autopsy is up to 50%, while the clinical diagnosis rate is only approximately 7–10% (3). The current commonly used tools, such as transthoracic echocardiography and laboratory data, have low sensitivity for detecting subclinical myocardial dysfunction. Speckle tracking echocardiography (STE) has been shown to be a more reproducible and sensitive technology than conventional echocardiography. The latter is an angle-independent quantification of myocardial deformation, which does not require the use of the Doppler technique (4). A previous study showed that STE can be used to evaluate changes in left ventricular function in patients with SLE (5). The left ventricular wall is composed of 3 layers of myocardium, and the endocardial layer is most prone to ischemic injury. The purpose of this study was to quantitatively analyze the changes of layer-specific myocardial strain in patients with SLE, and provide a technique for the diagnosis of myocardial damage.
We present the following article in accordance with the Standards for Reporting Diagnostic accuracy studies (STARD) reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-21-805/rc).
Methods
Study population
This was a single-center prospective study. The case group was recruited as a convenience sample, and the control group was recruited randomly. A total of 69 patients with SLE admitted to Shenzhen People’s Hospital between May 2020 and June 2021 with left ventricular ejection fraction (LVEF) >50% were selected as Group A. All of the above patients met the diagnostic criteria for SLE recommended by the American Society of Rheumatology in 1997 (6). The SLE disease activity index was evaluated using the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI 2000) scoring system. Based on the scores, participants were divided into 2 subgroups: an inactive stage group (A1, SLEDAI-2000: 0–4 points) and an active-stage group (A2, SLEDAI-2000: ≥5 points). The exclusion criteria were as follows: patients with severe valvular heart disease, essential hypertension, diabetes, cardiomyopathy, coronary heart disease, hyperthyroidism, congenital heart disease, and poor image quality. A total of 30 healthy volunteers who had no abnormalities were selected as the control group. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Ethics Board of Shenzhen People’s Hospital, and written informed consent was provided by all individual participants.
Image acquisition
Echocardiographic studies were performed with a commercially available system (Vivid E95 M5sc1.4-4.6 mHz transducer GE Vingmed, Horton, Norway). Patients were scanned in the left decubitus position while breathing calmly while connected to the synchronous electrocardiogram, and the frame rates were set at 51–70 frames/second. The standard two-dimensional (2D) images consisting of 4 cardiac cycles were saved in cine-loop digital format for offline analysis. Left ventricular end-diastolic/systolic anteroposterior diameter (LVDd/LVDds), left ventricular end-diastolic volume (LVEDV), left ventricular end-diastolic septal thickness (IVSd), left ventricular end-diastolic posterior wall thickness (LVPWd), left atrium anteroposterior diameter (LAD), and left atrium maximum volume (LAVmax) were measured. The LVEF was measured using the modified Simpson biplane method. Peaks E and A of the mitral flow velocity were measured using a pulse Doppler. Tissue Doppler imaging was used to measure septal and lateral mitral annular early myocardial relaxation velocities from the apical 4-chamber view. The measurements and calculated formulas of the parameters in our study followed the 2015 American Society of Echocardiography and the European Association of Cardiovascular Imaging recommendations for chamber quantification (7).
Offline analysis
The images were imported into EchoPAC (version 203, GE, Vingmed Ultrasound, Horton, Norway) software, QAnalysis-2D Strain mode was used for tracking of myocardial motion, the endocardial boundary was manually traced, and the software automatically divided the left ventricular myocardium into 3 layers. The unsatisfactory delineation of segments could be adjusted again to ensure satisfactory tracking. According to a bull's eye diagram, layer strain data of 17 segments were obtained. The system automatically calculated values of global endocardial longitudinal strain (GLSendo), global longitudinal strain (GLSglobal), global epicardial longitudinal strain (GLSepi), and peak strain dispersion (PSD) (Figure 1), and then calculated transmural gradient of longitudinal strain (TGLS) = GLSendo − GLSepi. Then, the right ventricle (RV) endocardium in focus on the right ventricular apical 4-chamber view was manually traced. Only RV free wall (RVFW) segmental strain was analyzed. The parameters of GLSglobal, GLSendo, and GLSepi were measured in the RVFW for basal, middle, and apical segments, respectively. The above operations and data measurements were made according to relevant guidelines (8). The analysis was performed by an experienced sonographer, who had no knowledge of the clinical data.
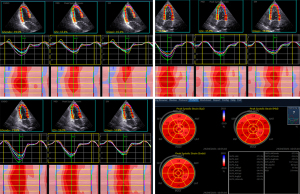
Intra- and inter-observer variability
A total of 15 participants were randomly selected and remeasured by 2 observers blinded to patient clinical data. Intraobserver variability was measured for the same sonographer on offline data at different points in time (the interval was 1 week).
Statistical analysis
The software SPSS 25.0 (IBM Corp., Chicago, IL, USA) was used for statistical analysis. Data are presented as mean ± SD, numbers, and median (interquartile range), respectively. The chi-square test (categorical variables) or Student’s t-test (continuous variables) were used to determine differences between 2 groups. The Mann-Whitney U test was used for nonnormally distributed continuous variables. Comparisons of means between the 3 groups were performed by analysis of variance (ANOVA) with a least significant difference post hoc correction for multiple comparisons. The correlation between variables was detected using Pearson’s test for data consistent with a normal distribution, while Spearman’s test was used for abnormally distributed data. The areas under the receiver operating characteristic (ROC) curves (AUC) were used for early detection of myocardial injury. A P value <0.05 was considered statistically significant. Intraclass correlation coefficient (ICC) and Bland-Altman analysis were used to estimate inter- and intra-observer variability. All participants had complete parameters.
Results
Comparison of basic clinical data and echocardiography data
We included 69 patients with SLE, based on previous annual hospital admissions. There were 10 males and 59 females in Group A (age: 18–61 years, average: 38.78±11.27 years). There were 37 cases in the A1 group (4 male, 33 females, age: 39.68±10.86 years) and 32 cases in the A2 group (6 males, 26 females, age: 37.75±11.82 years). There were 6 males and 24 females in Group B (age: 26–55 years, average: 41.73±8.81 years). Compared with the control group, patients with SLE had lower LVEF and septal E', and LVDs, LAD, and IVSd were increased (all P<0.05). There were no statistically significant differences in other conventional ultrasound indicators and clinical data. Patients with inactive SLE (A1) and active SLE (A2) were compared with the control group. Heart rate (HR), LAD, and IVSd in active patients with SLE were increased and septal E' was decreased, with no statistically significant differences in the other indicators. Compared with the A1 group, systolic blood pressure (SBP), SLEDAI, erythrocyte sedimentation rate (ESR), and high-sensitivity C-reactive protein (Hs-CRP) in the A2 group were increased, while complements C3 and C4 were decreased (P<0.05), and there was no statistically significant difference in the remaining indicators (Tables 1,2).
Table 1
| Variables | A (n=69) | A1 (n=37) | A2 (n=32) | B (n=30) | P1 | P2 | P3 | P4 |
|---|---|---|---|---|---|---|---|---|
| Age (years) | 38.78±11.27 | 39.68±10.86 | 37.75±11.82 | 41.73±8.81 | 0.206 | 0.432 | 0.143 | 0.454 |
| Male (n) | 10 | 4 | 6 | 6 | 0.556 | 0.324 | 1.000 | 0.496 |
| Height (cm) | 160.49±6.48 | 158.92±8.18 | 162.31±7.26 | 160.13±8.08 | 0.359 | 0.474 | 0.216 | 0.044 |
| Weight (kg) | 57.23±10.13 | 55.78±8.45 | 58.91±11.70 | 56.10±10.78 | 0.618 | 0.901 | 0.286 | 0.212 |
| BSA (m2) | 1.59±0.15 | 1.56±0.12 | 1.62±0.17 | 1.57±0.17 | 0.681 | 0.671 | 0.215 | 0.084 |
| HR (bpm) | 78.12±11.96 | 76.03±10.90 | 80.53±10.69 | 73.70±10.83 | 0.067 | 0.383 | 0.015 | 0.088 |
| SBP (mmHg) | 118.14±16.64 | 114.38±14.92 | 122.50±17.69 | 113.80±11.01 | 0.381 | 0.815 | 0.057 | 0.025 |
| Duration (years) | 8.68±7.16 | 9.59±7.62 | 7.63±6.14 | – | – | – | – | 0.262 |
| SLEDAI | 5.46±3.91 | 2.57±1.32 | 8.81±3.14 | – | – | – | – | 0.000 |
| ESR (mm/h) | 28.19±21.64 | 20.14±13.45 | 37.50±25.49 | – | – | – | – | 0.001 |
| Hs-CRP (mg/L) | 10.94±11.24 | 7.63±8.69 | 14.78±12.64 | – | – | – | – | 0.007 |
| C3 (g/L) | 0.74±0.19 | 0.83±0.13 | 0.63±0.20 | – | – | – | – | 0.000 |
| C4 (g/L) | 0.13±0.07 | 0.15±0.07 | 0.11±0.07 | – | – | – | – | 0.010 |
P1: significantly different (P<0.05) compared with Groups A and B. P2: significantly different (P<0.05) compared with the Groups A1 and B. P3: significantly different (P<0.05) compared with Groups A2 and B. P4: significantly different (P<0.05) compared with Groups A1 and A2. BSA, body surface area; HR, heart rate; SBP, systolic blood pressure; SLEDAI, Systemic Lupus Erythematosus Disease Activity Index; ESR, erythrocyte sedimentation rate; Hs-CRP, high-sensitivity C-reactive protein; C3, complement 3; C4, complement 4.
Table 2
| Variables | A (n=69) | A1 (n=37) | A2 (n=32) | B (n=30) | P1 | P2 | P3 | P4 |
|---|---|---|---|---|---|---|---|---|
| LVDd (mm) | 45.06±3.18 | 45.27±4.03 | 44.81±3.60 | 43.57±3.79 | 0.077 | 0.073 | 0.409 | 0.621 |
| LVDs (mm) | 28.29±3.25 | 28.57±3.27 | 27.97±3.24 | 26.63±3.42 | 0.024 | 0.019 | 0.115 | 0.455 |
| LVEF (%) | 67.19±4.41 | 67.08±4.54 | 67.31±4.32 | 69.40±5.42 | 0.035 | 0.050 | 0.080 | 0.841 |
| LVEDV (mL) | 89.59±17.63 | 87.84±12.16 | 91.63±22.40 | 87.53±12.11 | 0.561 | 0.939 | 0.322 | 0.335 |
| IVSd (mm) | 8.84±1.08 | 8.57±0.96 | 9.16±1.14 | 8.60±1.00 | 0.301 | 0.899 | 0.037 | 0.020 |
| LVPWd (mm) | 7.99±1.18 | 7.78±0.89 | 8.22±1.43 | 8.00±1.17 | 0.955 | 0.454 | 0.464 | 0.127 |
| E (m/s) | 0.84±0.18 | 0.83±0.17 | 0.84±0.20 | 0.86±0.20 | 0.513 | 0.463 | 0.699 | 0.732 |
| A (m/s) | 0.68±0.18 | 0.65±0.15 | 0.71±0.21 | 0.65±0.18 | 0.554 | 0.909 | 0.220 | 0.160 |
| E/A | 1.29±0.36 | 1.35±0.42 | 1.23±0.26 | 1.38±0.37 | 0.280 | 0.704 | 0.114 | 0.119 |
| Septal E' (cm/s) | 9.26±2.47 | 9.56±2.80 | 8.91±1.99 | 10.53±2.34 | 0.019 | 0.109 | 0.010 | 0.262 |
| Latal E' (cm/s) | 13.04±3.08 | 13.43±3.08 | 12.59±3.06 | 13.87±3.15 | 0.277 | 0.569 | 0.109 | 0.264 |
| LAD (mm) | 31.97±3.24 | 31.46±3.22 | 32.56±3.20 | 30.50±1.94 | 0.023 | 0.179 | 0.060 | 0.117 |
| LADI (mm/m2) | 20.28±2.53 | 20.28±2.31 | 20.27±2.80 | 19.57±2.22 | 0.190 | 0.240 | 0.268 | 0.975 |
| LAVmax (mL) | 30.26±10.90 | 29.73±9.26 | 30.88±12.66 | 29.17±9.10 | 0.636 | 0.829 | 0.526 | 0.655 |
| LAVI (mL/m2) | 19.06±6.51 | 19.12±5.93 | 18.98±7.24 | 18.59±5.95 | 0.736 | 0.733 | 0.810 | 0.925 |
P1: significantly different (P<0.05) compared with Groups A and B. P2: significantly different (P<0.05) compared with the Groups A1 and B. P3: significantly different (P<0.05) compared with Groups A2 and B. P4: significantly different (P<0.05) compared with Groups A1 and A2. LV, left ventricular; LVDd, LV end-diastolic anteroposterior diameter; LVDs, LV end-systolic anteroposterior diameter; LVEF, left ventricular ejection fraction; LVEDV, LV end-diastolic volume; IVSd, LV end-diastolic septal thickness; LVPWd, LV end-diastolic posterior wall thickness; LAD, left atrium anteroposterior diameter; LADI, left atrial anteroposterior diameter index; LAVmax, left atrium maximum volume; LAVI, left atrium maximum volume index.
Comparison of myocardial strain parameters
- Compared with Group B, GLSglobal (A: −18.80%±2.41%, B: −21.19%±2.16%), GLSendo (A: −21.15%±2.47%, B: −24.09%±2.49%), GLSepi (A: −16.58%±2.39%, B: −18.50%±1.77%), and TGLS (A: −4.56%±1.24%, B: −5.59%±1.39%) were decreased in Group A, while PSD (A: 36.61±10.85 ms, B: 30.00±8.54 ms) was increased (P<0.05). Compared with Group B, GLSendo (A1: −22.14%±2.21%), GLSglobal (A1: −19.54%±2.21%), GLSepi (A1: −17.17%±2.23%), and TGLS (A1: −4.97±1.16) in Group A1 were also decreased, while PSD (A1: 35.16±11.42 ms) was increased. Compared to Group B, GLSglobal (−17.95%±2.39%), GLSendo (−20.00%±2.27%), GLSepi (−15.91%±2.42%), PSD (38.28±10.06 ms), and TGLS (−4.09%±1.17%) in Group A2 were decreased, and PSD was increased significantly. Compared with the A1 group, the strain parameters in Group A2 were all reduced except for PSD (P<0.05) (Table 3).
Table 3
Layer speckle strain parameters in study groupsVariables A (n=69) A1 (n=37) A2 (n=32) B (n=30) P1 P2 P3 P4 GLSglobal (%) −18.80±2.41 −19.54±2.21 −17.95±2.39 −21.19±2.16 0.000 0.004 0.000 0.013 GLSendo (%) −21.15±2.47 −22.14±2.21 −20.00±2.27 −24.09±2.49 0.000 0.001 0.000 0.000 GLSepi (%) −16.58±2.39 −17.17±2.23 −15.91±2.42 −18.50±1.77 0.000 0.014 0.000 0.018 PSD (ms) 36.61±10.85 35.16±11.42 38.28±10.06 30.00±8.54 0.004 0.042 0.020 0.207 TGLS (%) −4.56±1.24 −4.97±1.16 −4.09±1.17 −5.59±1.39 0.000 0.044 0.000 0.040 P1: significantly different (P<0.05) compared with Groups A and B. P2: significantly different (P<0.05) compared with Groups A1 and B. P3: significantly different (P<0.05) compared with Groups A2 and B. P4: significantly different (P<0.05) compared with Groups A1 and A2. GLSglobal, global longitudinal strain; GLSendo, global endocardial longitudinal strain; GLSepi, global epicardial longitudinal strain; PSD, peak strain dispersion; TGLS, transmural gradient of longitudinal strain. - Compared with Group B, some segmental strain reductions were statistically significant, while some data were not statistically significant in patients with SLE. Comparisons of means between the 3 groups using ANOVA with least significant difference post hoc correction for multiple comparisons showed that the strain changes of the differences between some segments were still statistically significant between 3 groups (Tables S1-S3).
- Compared with Group B, although the layer strain values of the free wall of the RV were reduced in patients with SLE, the differences were not statistically significant, and the reduction was still not significant in active patients (Table S4).
An ROC curve was used to analyze the value of strain parameters in early assessment of subclinical myocardial injury
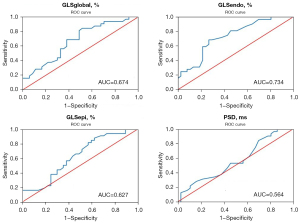
GLSendo was more capable of early and sensitive identification of subclinical myocardial injury in patients with SLE, with the AUC was 0.809 [95% confidence interval (CI): 0.714 to 0.905], other parameters for detecting myocardial injury were GLSglobal (AUC =0.765), GLSepi (AUC =0.736), PSD (AUC =0.692), and LVEF (AUC =0.385) (Table 4 and Figure 2). The GLSendo was also superior to GLSglobal, GLSepi, PSD, and LVEF in the early assessment of subclinical myocardial injury in patients with active SLE, and the AUC was 0.734 (95% CI: 0.617 to 0.851), while the other AUCs were about 0.674, 0.627, 0.564, and 0.517, respectively. The optimal cut-off point of GLSendo for the assessment of myocardial injury in patients with active SLE was −21.35%, with a sensitivity of 71.9% and a specificity of 62.2% (Table 5, Figure 3).
Table 4
| Variables | GLS global | GLSendo | GLSepi | PSD (ms) | LVEF (%) |
|---|---|---|---|---|---|
| AUC | 0.765 | 0.809 | 0.736 | 0.692 | 0.385 |
| AUC 95% CI | 0.664–0.866 | 0.714–0.905 | 0.634–0.839 | 0.580–0.804 | 0.253–0.516 |
| Cutoff value | −20.25% | −22.85% | −18.05% | 30.50% | – |
| Sensitivity | 71% | 82.6% | 69.6% | 71% | – |
| Specificity | 63.3% | 70% | 60% | 60% | – |
ROC, receiver operating characteristic; SLE, systemic lupus erythematosus; AUC, area under the curve; CI, confidence interval; GLSglobal, global longitudinal strain; GLSendo, global endocardial longitudinal strain; GLSepi, global epicardial longitudinal strain; PSD, peak strain dispersion: LVEF, left ventricular ejection fraction
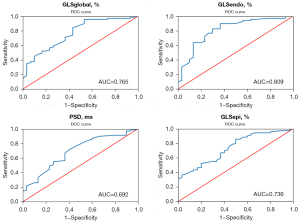
Table 5
| Variables | GLSglobal | GLSendo | GLSepi | PSD (ms) | LVEF (%) |
|---|---|---|---|---|---|
| AUC | 0.674 | 0.734 | 0.627 | 0.564 | 0.517 |
| AUC 95% CI | 0.547–0.800 | 0.617–0.851 | 0.495–0.759 | 0.427–0.700 | 0.380–0.655 |
| Cutoff value | −19.10% | −21.35% | −16.85% | – | – |
| Sensitivity | 68.8% | 71.9% | 62.5% | – | – |
| Specificity | 62.2% | 62.2% | 56.8% | – | – |
ROC, receiver operating characteristic; SLE, systemic lupus erythematosus; AUC, are under the curve; CI, confidence interval; GLSglobal, global longitudinal strain; GLSendo, global endocardial longitudinal strain; GLSepi, global epicardial longitudinal strain; PSD, peak strain dispersion; LVEF, left ventricular ejection fraction.
Correlation analysis
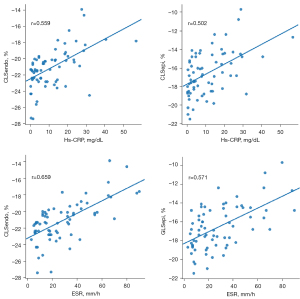
Moderate correlations were observed between PSD, SLEDAI, ESR, Hs-CRP, and complement C3 (correlation coefficients 0.535, 0.428, 0.659, 0.559, and −0.440, respectively), but no significant correlations were found between LVEF and complement C4 (correlation coefficients −0.350 and −0.259, respectively). The correlation coefficients of GLSglobal with PSD, ESR, Hs-CRP, complement C3, and LVEF were about 0.506, 0.622, 0.542, −0.359, and −0.350, respectively, and GLSglobal had no significant correlation with SLEDAI and complement C4. The correlation coefficients of GLSepi with PSD, ESR, Hs-CRP, and LVEF were about 0.524, 0.571, 0.502, and −0.307 respectively, while there was no significant correlation with SLEDAI, complement C3, and complement C4 (Table 6, Figure 4).
Table 6
| Variables | PSD | LVEF | SLEDAI | ESR | Hs-CRP | C3 | C4 |
|---|---|---|---|---|---|---|---|
| GLSglobal | 0.506 | −0.350 | – | 0.622 | 0.542 | −0.359 | – |
| GLSendo | 0.535 | −0.350 | 0.428 | 0.659 | 0.559 | −0.440 | – |
| GLSepi | 0.524 | −0.307 | – | 0.571 | 0.502 | – | – |
GLSglobal, global longitudinal strain; GLSendo, global endocardial longitudinal strain; GLSepi, global epicardial longitudinal strain; PSD, peak strain dispersion; LVEF, left ventricular ejection fraction; SLEDAI, Systemic Lupus Erythematosus Disease Activity Index; ESR, erythrocyte sedimentation rate; Hs-CRP, high-sensitivity C-reactive protein; C3, complement 3; C4, complement 4.
Intra- and inter-observer variability
The ICCs for repeated measurements by the same observer and between 2 different observers were excellent for GLSendo, GLSglobal, and GLSepi (intraobserver: 0.944, 0.921, and 0.892, respectively, and interobserver: 0.927, 0.920, and 0.867, respectively) (Table 7). Bland-Altman analysis showed good interobserver and intraobserver repeatability and consistency in the analysis of various indexes of strain (Figures 5,6).
Table 7
| Variables | GLSglobal | GLSendo | GLSepi |
|---|---|---|---|
| Intraobserver | 0.921 | 0.944 | 0.892 |
| Interobserver | 0.920 | 0.927 | 0.867 |
ICCs, intraclass correlation coefficients; GLSglobal, global longitudinal strain; GLSendo, global endocardial longitudinal strain; GLSepi, global epicardial longitudinal strain.


Discussion
In this study, the intimal, epicardial myocardial, and overall LV myocardial function were all impaired in both active and inactive patients with SLE, and the myocardial injury was more obvious in patients with active SLE, especially in the intimal myocardium. The ROC curve analysis found that GLSendo was superior to GLSglobal, and that GLSepi can sensitively detect subclinical myocardial injury in patients with SLE at an early stage, which highlighted its superiority for use in active-stage patients. These results suggest that assessment of layer speckled strain can be of valuable help as an additional clinical tool in the diagnosis of subclinical myocardial injury in patients with SLE.
Effect of LV layer myocardial strain in patients with SLE
The LV wall of the heart comprises 3 myocardial layers: the inner oblique, middle circular, and outer oblique myocardial layers. The 3 layers function differently in normal myocardial deformations, and the endocardium undergoes greater dimensional changes (both thickening and shortening) during systole than does the epicardium (9-11).
A previous study showed (12) that LV whole strain can evaluate LV systolic function of patients with SLE and found that GLSglobal was reduced in patients with SLE. We obtained the same result. The GLSglobal as a measure of longitudinal fiber performance, which is vulnerable to the influence of ischemia and fibrosis. It can detect subtle changes in LV systolic function. The LV myocardium is mainly composed of longitudinal fibers (13). Some studies (14,15) have shown that the longitudinal peak strain obtained using 2D speckle imaging technology has excellent clinical value in predicting different heart disease models; however, when analyzing myocardial function, we should not only focus on the function of the myocardium as a whole, but also consider the differences between the myocardium layers of the myocardium. If the technique allows a specific layer of myocardial function analysis, it has the potential to increase the understanding of the morphology and pathophysiology of myocardial ischemia and to help improve the characteristics of patients with SLE. In this study, longitudinal layer strain analysis was conducted on patients with SLE, and it was found that the layers of myocardial function of patients with SLE were impaired to varying degrees. The decrease of myocardial strain in both the endocardium and epicardium in patients with SLE may be due to several factors: first, the myocardial layer deformation may not be independent. Contraction of the nonischemic myocardium can lead to deformation of adjacent ischemic muscles by passive translation or tethered motion. Conversely, the ischemic myocardium may have a negative effect on the contraction of the adjacent nonischemic myocardium; second, with the progression of the disease, the subendocardial myocardium is involved initially, the middle myocardium is involved later, and the epicardial myocardium is affected finally. We also found that the myocardial damage was more obvious in the active phase of the disease. It may be related to the mechanism of SLE involving the myocardium: first, the inflammatory reaction to immune complex deposition in patients; second, coronary atherosclerotic action occurs; in addition, long-term medications such as glucocorticoids can accelerate or lead to atherosclerosis, and antiphospholipid antibodies lead to arterial thrombosis (16,17). When the disease is active, the activation of macrophages is more obvious, and the activation of macrophages enhances the proinflammatory process of SLE coronary disease (18), which may further worsen cardiac function. The PSD is a new parameter derived from 2D-STI to reflect the synchronicity of myocardial contraction. The smaller the value, the better the synchronicity. The PSD has a high application value as an indicator in the evaluation of myocardial coordination (13). In this study, it was found that the PSD in the SLE group was higher than that in the control group, suggesting that the subclinical synchronization of LV wall motion decreased in patients with SLE. The reason may be that deposition of antigen and/or antibody complexes activate an inflammatory response that may involve the conduction system in the heart, leading to changes in its structure or function.
The application of 2D dot stratification technology to evaluate the myocardial injury and prognosis of patients with SLE has great potential value, which needs to be verified by further studies with large samples.
Endocardial myocardial strain is an early and sensitive indicator of subclinical myocardial injury in SLE
Immunofluorescence experiments in patients with SLE have shown that there are fine granular immune complexes and complement deposits in the perimyocardial tissue, supporting the hypothesis that lupus myocarditis can be mediated by immune complexes (19). The endocardial myocardium of healthy people has the most obvious deformation, and the endocardium is the first to be affected by ischemia. We found that the ROC curve showed that GLSendo was more sensitive than GLSepi in the early detection of subclinical myocardial injury in patients with SLE, and this was still reflected in the active subgroup, with AUCs of 0.809 and 0.734, respectively. Coronary artery lesions and endocarditis caused by antigen and/or antibody complex in patients with SLE mainly cause collagen fibrous degeneration of the inner myocardial interstitium and microvascular lesions in the subendocardial layer. Longitudinal myocardial strain function of the endocardial layer can be impaired in the early stage of the disease, and over time, the strain function of the medial and outer layers of the myocardium become impaired, with the expansion of the extent of damage to the whole layer of the myocardium and to the supply to the myocardium of the coronary artery. The TGLS is defined as the difference of longitudinal strain between the overall endocardial myocardium and epicardial myocardium of the LV, and its use has been shown to reflect specific endocardial injury (20). In this study, TGLS in SLE was lower than that in the control group, and the decrease of SLE in the active phase was more obvious. The TGLS is a good indicator of endocardial myocardium-specific injury in patients with SLE.
These results indicate that the endocardial myocardium in SLE is the most prone to specific injury, and the endocardial myocardium strain in the layer speckle tracking technique can detect subclinical myocardial injury in an early and sensitive way, which can provide an important reference value for early clinical intervention and treatment.
Effect of inflammatory activity on myocardial strain in SLE
The activity of SLE disease is assessed by the SLEDAI score, where 0–4 points indicates basically no activity; 5–9 points light activity; 10–14 points moderate activity; and ≥15 severe activity. Previous studies have shown that the SLEDAI score is significantly correlated with cardiac damage in patients with SLE, and the higher disease activity in patients with SLE, the greater the damage to the heart (21). In this study, SLE was divided into an active and inactive group according to the SLEDAI score, and it was found that the subclinical myocardial injury of patients in the active stage was more obvious than that of patients in the inactive stage, which is consistent with the above research results. A possible reason is that the infiltration of inflammatory cells and the degeneration of fibrin and the edema of connective tissue between muscle bundles are more significant in active-stage SLE patients (22). It is also possible that related antibodies such as antimyocardial antibodies are deposited in myocardial cells during the active phase, thus affecting myocardial function. The above features can lead to vascular endothelial damage and coronary artery lesions, prompting corresponding regional ischemic injury (23,24).
When SLE is active, a large amount of C3 and C4 in serum is consumed and, at the same time, deposited in the skin basement membrane zone. The C3 and C4 levels decreased significantly during SLE activity. The level of C3 and C4 can be used as an important indicator to observe the disease activity of SLE (25). In this study, the levels of C3 and C4 in the active subgroup were lower than those in the inactive subgroup. In addition, ESR and Hs-CRP in the active subgroup in this study were higher than those in the inactive subgroup. Previous studies have shown that ESR in patients with active SLE is mostly increased, while ESR in clinical remission is basically normal, suggesting that ESR detection can dynamically observe changes in disease activity in patients with SLE (26,27). The Hs-CRP is an acute response protein, which has been shown to be associated with disease activity in autoimmune diseases such as rheumatoid arthritis and vasculitis. The Hs-CRP level was correlated with SLEDAI score, which in turn is associated with myocarditis (28). In addition, using correlation analysis, we found that GLSendo and GLSepi had a certain correlation with the inflammatory indexes of disease activity, while endometrial cardiomyopathy had a slightly stronger correlation with the inflammatory indexes (such as ESR and Hs-SRP) of disease activity. The reason may be related to the mechanism of the above-mentioned diseases affecting the myocardial function (16,17,22-24). In our study, the ICCs for repeated measurements by the same observer and between 2 different observers were excellent for layer speckle strains. In conclusion, subclinical myocardial injury is present in patients with SLE, especially when the disease is in the active phase, and the layer strain technique can quantitatively assess the subclinical myocardial injury in these patients. The endocardial myocardial strain index is not only more sensitive in the evaluation of subclinical myocardial injury in patients with SLE, but also has a better correlation with clinical test index in reflecting the degree of disease activity, which is an important reference value for early intervention treatment of patients.
Limitations
There were several limitations to this study. The sample size was relatively small. Most patients in the active phase subgroup were moderately active, while few patients were severe active, and this study did not provide further detail of the latter subgroup.
Although this study included some indicators of disease activity, other indicators, such as serum factor, were not included. This study included hierarchical strain data and clinical data, no strong correlation was observed for patients who underwent alternations between periods of inactivity and activity for a long time, or patients with receiving different drug treatments. The patients included in this study were not patients diagnosed with SLE for the first time and had already been treated with drugs, and as such the effects of drugs could not be separately reported. While there may be associations with these factors, such associations need to be verified with larger samples.
Image quality can affect the results of stratified strain data analysis. Patients whose image quality was too poor to be analyzed were excluded from this study.
At present, there is no imaging technology as the gold standard for the detection of SLE myocardial injury. Magnetic resonance imaging (MRI) has become an important cardiac imaging technology with its unique advantages. However, the number of patients willing to undergo MRI was small in this study, and as such we cannot compare our results with MRI-based results. Therefore, this result needs to be verified further.
Acknowledgments
Funding: This study was supported by the National Natural Science Foundation of China (No. 81771841) and the Project of Innovation of the Science and Technology Commission of Shenzhen City (No. JCYJ20190807145609482).
Footnote
Reporting Checklist: The authors have completed the Standards for Reporting Diagnostic accuracy studies (STARD) reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-21-805/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-21-805/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Institutional Ethics Board of the Shenzhen People’s Hospital, and written informed consent was provided by all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ward MM. Premature morbidity from cardiovascular and cerebrovascular diseases in women with systemic lupus erythematosus. Arthritis Rheum 1999;42:338-46. [Crossref] [PubMed]
- Abu-Shakra M, Gladman DD, Urowitz MB. Mortality studies in SLE: how far can we improve survival of patients with SLE. Autoimmun Rev 2004;3:418-20. [Crossref] [PubMed]
- Knockaert DC. Cardiac involvement in systemic inflammatory diseases. Eur Heart J 2007;28:1797-804. [Crossref] [PubMed]
- Tops LF, Delgado V, Marsan NA, Bax JJ. Myocardial strain to detect subtle left ventricular systolic dysfunction. Eur J Heart Fail 2017;19:307-13. [Crossref] [PubMed]
- Guşetu G, Pop D, Pamfil C, Bǎlaj R, Mureşan L, Cismaru G, Matuz R, Roşu R, Zdrenghea D, Rednic S. Subclinical myocardial impairment in SLE: insights from novel ultrasound techniques and clinical determinants. Med Ultrason 2016;18:47-56. [Crossref] [PubMed]
- Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:1725. [Crossref] [PubMed]
- Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233-70. [Crossref] [PubMed]
- Voigt JU, Pedrizzetti G, Lysyansky P, Marwick TH, Houle H, Baumann R, Pedri S, Ito Y, Abe Y, Metz S, Song JH, Hamilton J, Sengupta PP, Kolias TJ, d'Hooge J, Aurigemma GP, Thomas JD, Badano LP. Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. J Am Soc Echocardiogr 2015;28:183-93. [Crossref] [PubMed]
- Adamu U, Schmitz F, Becker M, Kelm M, Hoffmann R. Advanced speckle tracking echocardiography allowing a three-myocardial layer-specific analysis of deformation parameters. Eur J Echocardiogr 2009;10:303-8. [Crossref] [PubMed]
- Sabbah HN, Marzilli M, Stein PD. The relative role of subendocardium and subepicardium in left ventricular mechanics. Am J Physiol 1981;240:H920-6. [PubMed]
- Leitman M, Lysiansky M, Lysyansky P, Friedman Z, Tyomkin V, Fuchs T, Adam D, Krakover R, Vered Z. Circumferential and longitudinal strain in 3 myocardial layers in normal subjects and in patients with regional left ventricular dysfunction. J Am Soc Echocardiogr 2010;23:64-70. [Crossref] [PubMed]
- Gegenava T, Gegenava M, Steup-Beekman GM, Huizinga TWJ, Bax JJ, Delgado V, Marsan NA. Left Ventricular Systolic Function in Patients with Systemic Lupus Erythematosus and Its Association with Cardiovascular Events. J Am Soc Echocardiogr 2020;33:1116-22. [Crossref] [PubMed]
- Langeland S, D'hooge J, Wouters PF, Leather HA, Claus P, Bijnens B, Sutherland GR. Experimental validation of a new ultrasound method for the simultaneous assessment of radial and longitudinal myocardial deformation independent of insonation angle. Circulation 2005;112:2157-62. [Crossref] [PubMed]
- Witkowski TG, Thomas JD, Debonnaire PJ, Delgado V, Hoke U, Ewe SH, Versteegh MI, Holman ER, Schalij MJ, Bax JJ, Klautz RJ, Marsan NA. Global longitudinal strain predicts left ventricular dysfunction after mitral valve repair. Eur Heart J Cardiovasc Imaging 2013;14:69-76. [Crossref] [PubMed]
- Mignot A, Donal E, Zaroui A, Reant P, Salem A, Hamon C, Monzy S, Roudaut R, Habib G, Lafitte S. Global longitudinal strain as a major predictor of cardiac events in patients with depressed left ventricular function: a multicenter study. J Am Soc Echocardiogr 2010;23:1019-24. [Crossref] [PubMed]
- Gustafsson J, Gunnarsson I, Börjesson O, Pettersson S, Möller S, Fei GZ, Elvin K, Simard JF, Hansson LO, Lundberg IE, Larsson A, Svenungsson E. Predictors of the first cardiovascular event in patients with systemic lupus erythematosus - a prospective cohort study. Arthritis Res Ther 2009;11:R186. [Crossref] [PubMed]
- Abu-Shakra M, Codish S, Zeller L, Wolak T, Sukenik S. Atherosclerotic cardiovascular disease in systemic lupus erythematosus: the Beer Sheva experience. Isr Med Assoc J 2008;10:43-4. [PubMed]
- Labonte AC, Kegerreis B, Geraci NS, Bachali P, Madamanchi S, Robl R, Catalina MD, Lipsky PE, Grammer AC. Identification of alterations in macrophage activation associated with disease activity in systemic lupus erythematosus. PLoS One 2018;13:e0208132. [Crossref] [PubMed]
- Tincani A, Rebaioli CB, Taglietti M, Shoenfeld Y. Heart involvement in systemic lupus erythematosus, anti-phospholipid syndrome and neonatal lupus. Rheumatology (Oxford) 2006;45:iv8-13. [Crossref] [PubMed]
- Chen Z, Li C, Li Y, Rao L, Zhang X, Long D, Li C. Layer-specific strain echocardiography may reflect regional myocardial impairment in patients with hypertrophic cardiomyopathy. Cardiovasc Ultrasound 2021;19:15. [Crossref] [PubMed]
- Yip GW, Shang Q, Tam LS, Zhang Q, Li EK, Fung JW, Yu CM. Disease chronicity and activity predict subclinical left ventricular systolic dysfunction in patients with systemic lupus erythematosus. Heart 2009;95:980-7. [Crossref] [PubMed]
- Miner JJ, Kim AH. Cardiac manifestations of systemic lupus erythematosus. Rheum Dis Clin North Am 2014;40:51-60. [Crossref] [PubMed]
- Chen PY, Chang CH, Hsu CC, Liao YY, Chen KT. Systemic lupus erythematosus presenting with cardiac symptoms. Am J Emerg Med 2014;32:1117-9. [Crossref] [PubMed]
- Chin CW, Chin CY, Ng MX, Le TT, Huang FQ, Fong KY, Thumboo J, Tan RS. Endothelial function is associated with myocardial diastolic function in women with systemic lupus erythematosus. Rheumatol Int 2014;34:1281-5. [Crossref] [PubMed]
- Kenyon KD, Cole C, Crawford F, Kappler JW, Thurman JM, Bratton DL, Boackle SA, Henson PM. IgG autoantibodies against deposited C3 inhibit macrophage-mediated apoptotic cell engulfment in systemic autoimmunity. J Immunol 2011;187:2101-11. [Crossref] [PubMed]
- Ward MM, Guthrie LC, Alba MI. Clinically important changes in individual and composite measures of rheumatoid arthritis activity: thresholds applicable in clinical trials. Ann Rheum Dis 2015;74:1691-6. [Crossref] [PubMed]
- Lee SS, Singh S, Magder LS, Petri M. Predictors of high sensitivity C-reactive protein levels in patients with systemic lupus erythematosus. Lupus 2008;17:114-23. [Crossref] [PubMed]
- Lee SS, Singh S, Link K, Petri M. High-sensitivity C-reactive protein as an associate of clinical subsets and organ damage in systemic lupus erythematosus. Semin Arthritis Rheum 2008;38:41-54. [Crossref] [PubMed]


