
Prognostic usefulness of clinical features and pretreatment 18F-FDG PET/CT metabolic parameters in patients with angiosarcoma
Introduction
Angiosarcoma is a highly malignant vascular tumor of endothelial cell origin constituting 1–2% of soft tissue sarcomas with a poor prognosis of a 24–34% 5-yr survival (1-9). These tumors can occur at any site in the body with great heterogeneity (10). Its pathogenesis is related to exposure to chemicals, radiation, chronic lymphedema, trauma and vasodilation (9,11). Integrated 18F-2-fluoro-2-deoxy-D-glucose (18F-FDG) positron emission tomography/X-ray computed tomography (PET/CT), computed tomography (CT), magnetic resonance imaging (MRI) and ultrasonography are all diagnostic methods, but the final diagnosis requires pathology and immunohistochemistry. Because of the low incidence of angiosarcoma, scant evidence-based medicine data exist regarding morphological and functional imaging of these diseases.
Integrated 18F-FDG PET/CT is the most widely used functional and metabolic imaging technique and provides more comprehensive and accurate information for clinicians presently (12,13). 18F-FDG PET/CT has contributed to the detection of heterogeneous mesenchymal tumors of soft tissue or bone (14) and has great advantages in clinical grading, differentiation of benign and malignant tumors, clinical stage and restage, evaluation of local recurrence and monitoring of curative effects in sarcomas (15-18). Studies of 18F-FDG PET/CT on angiosarcoma remain scarce because of the rarity of this tumor.
This retrospective study of 19 cases of pathologically diagnosed angiosarcoma before treatment explored the relationship among the clinical characteristics, laboratory examinations, 18F-FDG PET/CT parameters and the prognosis of angiosarcoma.
We present the following article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-21-563/rc).
Methods
Patients
In 19 patients (10 women and 9 men) with histopathologically verified angiosarcoma, 18F-FDG PET/CT was performed for the initial diagnosis before treatment between November 2014 and February 2020, and the outcome data were evaluated retrospectively.
Clinical characteristics and survival data were obtained from medical records and telephone call follow-up. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This retrospective study was approved by the Ethics Committee of the First Affiliated Hospital, Zhejiang University School of Medicine (No. IIT2020639A) and the requirement to obtain written informed consent was waived.
Patients who had any treatment for their tumor before 18F-FDG PET/CT, had undergone 18F-FDG PET/CT at another institution, or had incomplete clinical data were excluded from the study.
Patients ranged in age from 27 to 79 years (median age, 59 years) with different angiosarcoma types covering all tumor grades and subtypes. We recorded the age at presentation, sex, underlying diseases, sites of primary tumors, KPS, ECOG, time from onset to diagnosis, laboratory examinations, sites and sizes of primary tumors, treatment modalities, and outcome (survival) for each patient. The time from onset to diagnosis was defined as interval from the date when the patient reported chief complaint in admission records and the date of angiosarcoma diagnosis pathologically. Laboratory examinations included the white blood cell count, platelet count, hemoglobin (Hb) levels, C-reactive protein (CRP), lactate dehydrogenase (LDH), ferritin (Fer) levels in serum, glutamic-pyruvic transaminase (GPT) and creatinine (Cr) (Table 1).
Table 1
| Characteristics | n (range or %) |
|---|---|
| Age, median (interquartile range), years | 59 [27–79] |
| Sex, male/female | 9/10 (47.4/52.6) |
| Underling diseases | |
| Hypertension | 6 (31.6) |
| Local trauma history | 1 (5.3) |
| Breast cancer and local radiotherapy | 1 (5.3) |
| Hepatitis B cirrhosis | 2 (10.5) |
| Family history | 1 (5.3) |
| None | 10 (52.6) |
| Sites of primary tumors | |
| Cutaneous | 5 (26.4) |
| Right atrium | 4 (21.0) |
| Liver | 4 (21.0) |
| Bone | 2 (10.5) |
| Artery (pulmonary artery/Iliac artery) | 2 (10.5) |
| Spleen | 1 (5.3) |
| Breast | 1 (5.3) |
| Size of primary tumors, median (interquartile range), cm | 5.7 (1.4–12.5) |
| Initial KPS, median [interquartile range] | 80 [30–90] |
| Initial ECOG | |
| 1/2/3/4 | 10 (52.6)/5 (26.4)/2 (10.5)/2 (10.5) |
| Time from onset to diagnosis, median [interquartile range], week | 5 [1–60] |
| laboratory examination, median [interquartile range] | |
| White-cell count (109/L) | 6.6 [2.4–15.2] |
| Platelet count (109/L) | 250 [14–424] |
| Hemoglobin (g/dL) | 110 [53–148] |
| C-reactive protein (mg/L) | 17.9 [2.7–76.1] |
| Lactate dehydrogenase (U/L) | 199 [148–709] |
| Ferritin (ng/mL) | 351 [47.2–3,143.4] |
| Glutamic-pyruvic transaminase (U/L) | 21 [14–515] |
| Creatinine (μmol/L) | 68 [42–258] |
| Treatment modalities | |
| Surgery | 6 (31.6) |
| Chemotherapy | 6 (31.6) |
| Surgery+ chemotherapy | 2 (10.5) |
| TACE (liver)* | 2 (10.5) |
| None | 3 (15.8) |
| Survival time, median (interquartile range), month | 2.5 [1–24] |
*, treatment by transcatheter arterial chemoembolization (TACE) in patients with primary hepatic angiosarcoma. KPS, Karnofsky Performance Status; ECOG, Eastern Cooperative Oncology Group.
Histologic features and AJCC stage
All the patients had undergone biopsy of the tumor to establish the tumor grade and subtype (based on surgical specimens in 8 patients and biopsy specimens in 11 patients). The histologic features of the tumor were graded according to the Federation Nationale des Centres de Lutte Contre le Cancer (FNCLCC) system (14) by an experienced sarcoma pathologist. The FNCLCC grade was determined by three parameters: differentiation, mitotic activity and extent of necrosis. Each parameter was scored as follows: differentiation [1–3], mitotic activity [1–3], and necrosis [0–2]. The scores were added to determine the grade. All the patients were assigned a clinical stage on 18F-FDG PET/CT imaging using the seventh edition of the AJCC staging system (Table 2).
Table 2
| Characteristics | N (%) |
|---|---|
| Differentiation | |
| Grade 1 | 2 (10.5) |
| Grade 2 | 13 (68.4) |
| Grade 3 | 4 (20.1) |
| Mitotic activity | |
| Grade 1 | 3 (15.8) |
| Grade 2 | 14 (10.5) |
| Grade 3 | 2 (73.7) |
| Necrosis | |
| Grade 0 | 1 (5.3) |
| Grade 1 | 11 (57.9) |
| Grade 2 | 7 (36.8) |
| FNCLCC | |
| Grade 1 | 2 (10.5) |
| Grade 2 | 7 (36.8) |
| Grade 3 | 10 (52.6) |
| T classification | |
| T1 | 7 (36.8) |
| T2 | 3 (15.8) |
| T3 | 5 (26.3) |
| T4 | 4 (20.1) |
| N classification | |
| N0 | 9 (47.4) |
| N1 | 10 (52.6) |
| M classification | |
| M0 | 8 (42.1) |
| M1 | 11 (57.9) |
| Bone and marrow | 10 (52.6) |
| Lung | 8 (42.1) |
| Liver | 2 (10.5) |
| Spleen | 1 (5.3) |
| Single organ metastasis | 3 (15.8) |
| Multiple organ metastasis | 8 (42.1) |
| AJCC stage | |
| IB | 1 (5.3) |
| II | 3 (15.8) |
| IIIB | 2 (10.5) |
| IV | 13 (68.4) |
FNCLCC, Federation Nationale des Centres de Lutte Contre le Cancer; AJCC, American Joint Committee on Cancer.
PET/CT imaging
All the patients were scanned on a dedicated PET/CT scanner (Biograph 16; Siemens, Germany). The patients had been fasting for at least 4–6 h and blood glucose levels were required to be less than 10 mmol/L before 18F-FDG injection (3.75–5.55 MBq/kg). Scanning was started from the basal skull to mid-thigh after an uptake time of 40–60 min. Low-dose CT without intravenous or oral contrast scans were performed using a sixteen-slice helical CT with a continuous spiral technique (120 KeV; automatic current regulation adjusted to thickness and density of each patient’s body; section thickness of 5 mm). PET scans were obtained for 3 min per frame and were reconstructed using iterative algorithm (Siemens). Additional scan of the extremities was acquired if the patient had tumors or suspected metastases on the lower extremities below the mid-thigh.
Measurements of the metabolic PET parameters
Experienced nuclear medicine physicians used volume viewer software on a dedicated workstation (MedEx), which provides automatic delineation of the region of interest (ROI) using an isocontour threshold method based on the SUV, to assess the initial diagnostic staging of 18F-FDG PET/CT images. The metabolic tumor volume (MTV) was defined as the total tumor volume segmented via the threshold SUV. Two MTV segmentation methods were applied as an isocontour at an early SUV of 2.5 (MTV2.5) or using fixed thresholds of 40% (MTV40%) of the maximum intratumoral 18F-FDG activity. Total lesion glycolysis (TLG), another functional tumor parameter which determined the metabolic activity of tumors, was defined as a product of the SUVavg multiplied by the MTV. Using two threshold SUVs, ten parameters were then measured and recorded: pSUVmax (SUVmax of the primary lesion); pSUVavg (SUVavg of the primary lesion); pMTV40% (MTV40% of the primary lesion); pMTV2.5 (MTV2.5 of the primary lesion); pTLG40% (TLG40% of the primary lesion); pTLG2.5 (TLG2.5 of the primary lesion); wMTV40% (MTV40% of whole body); wMTV2.5 (MTV2.5 of whole body); wTLG40% (TLG40% of whole body); wTLG2.5 (TLG2.5 of whole body) (Table 3).
Table 3
| Characteristics | Median (interquartile range) |
|---|---|
| Primary tumor (n=19) | |
| SUVmax | 6.8 (3.4–39.8) |
| SUVavg | 5.3 (1.5–24.1) |
| MTV40% | 144.2 (9.7–876.9) |
| TLG40% | 944.9 (39.8–5,049.5) |
| MTV2.5 | 240.3 (13.3–3,072.3) |
| TLG2.5 | 2,012.6 (16.5–17,756.9) |
| Metastatic tumor (n=13) | |
| MTV40% | 285.5 (0*–2,786.5) |
| TLG40% | 1,084.9 (0–8,283.3) |
| MTV2.5 | 920.0 (0–5,711.4) |
| TLG2.5 | 3,616.9 (0–19,418.74) |
| Whole body (n=19) | |
| MTV40% | 315.6 (9.7–2,960.6) |
| TLG40% | 2,714.2 (90.4–9,496.4) |
| MTV2.5 | 870.3 (21.4–5,906.3) |
| TLG2.5 | 5,070.6 (66.0–20,974.2) |
*, FDG uptake of lung metastatic lesions was too low to measure MTV and TLG in one patient. 18F-FDG, 18F-2-fluoro-2-deoxy-D-glucose; PET/CT, positron emission tomography/X-ray computed tomography; SUVmax, maximum standardized uptake value; SUVavg, average standardized uptake value; MTV2.5, metabolic tumor volume using SUVmax cut-off value of 2.5; MTV40%, metabolic tumor volume using 40% of SUVmax as threshold; TLG40%, total lesion glycolysis using SUVmax cut-off value of 2.5; TLG2.5, total lesion glycolysis using 40% of SUVmax as threshold.
Statistical analysis
All the statistical analyses were conducted using IBM SPSS Statistics software (version 20; SPSS Inc., Chicago, USA). Overall survival was measured from the date of initial diagnosis to death (18 patients) or the last follow-up (1 patient). The univariate analyses of the variables tested for survival included age, sex, underlying diseases, sites of primary tumors, KPS, ECOG, time from onset to diagnosis, laboratory examinations, sites and sizes of primary tumor, AJCC stage (T classification, N classification, M classification), pathological grading (differentiation, mitosis, necrosis and FNCLCC), metabolic metrics (SUVmax and SUVavg of the primary tumor, the MTV and TLG of primary tumor and whole body), and treatment modalities. Univariate and multivariate Cox proportional hazards model, which yields hazard ratios (HR), were used to select potential predictors and assess the potential independent effect of the variables. Survival was analyzed by the Kaplan-Meier method. The log-rank method was used to identify optimal cut points for continuous variables.
Results
Clinical and laboratory characteristics
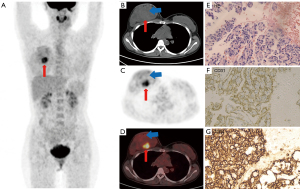
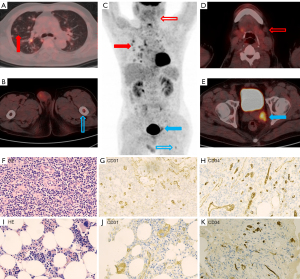
Four patients had secondary angiosarcoma, including one patient with a history of trauma at tumor sites, one patient with radiotherapy at tumor sites of breast cancer and two patients with hepatitis B cirrhosis. A female patient with primary angiosarcoma of right atrium had a family history of her mother having angiosarcoma of the breast. One-third of the patients (n=6) had a history of hypertension. Cutaneous angiosarcomas (26.3%, n=5) were the most common sites of primary tumors, including the head and neck (n=2), chest (n=1), upper arm (n=1) and abdominal wall (n=1). In 14 patients (73.7%), tumors had a visceral or deep soft tissue origin, including the right atrium (n=4), liver (n=4), bone (n=2), artery (n=2), spleen (n=1) and breast (n=1) (Figure 1). Two rare cases were located in the primary artery, one in the left pulmonary artery and the other in the left internal iliac artery (Figure 2). The median primary tumor length was 5.7 cm, with an interquartile range from 1.4 to 12.5 cm. The median KPS score was 80, with an interquartile range from 30 to 90. The KPS score is low in elderly patients. The ECOG performance status grade was 1 in 10 patients, 2 in 5 patients, 3 in 2 patients and 4 in 2 patients. The median time from the onset to diagnosis was 5 weeks, with an interquartile range from 1 to 60 weeks. The median and interquartile ranges of laboratory testing are summarized in Table 1.
The median (interquartile range) scores for the Hb levels were 110 (53–148 g/dL). Seven patients had anemia of varying degrees of severity (4 cases of mild anemia, 2 cases of moderate anemia and 1 case of severe anemia). CRP increased in nearly half of the patients (10/19), with a median (interquartile range) of 17.9 (2.7–76.1 mg/L). The median survival time was 2.5 months (interquartile range, 1–24 months).
Eighteen patients died from multiple organ failure because of primary tumor progression. Three patients were treated only for vital support without surgery or chemotherapy because of unstable vital signs at the time of the diagnosis of angiosarcoma.
Histologic features and TNM stage
Histologic features and TNM stage are summarized in Table 2. Differentiation grade 2 (n=13), mitotic activity grade 2 (n=14) and necrosis grade 1 (n=11) were the most common histological parameter classifications, and FNCLCC grades 2–3 dominated in tumor histologic grading (17/19). Lymph node metastases (n=10) and/or distant metastases (n=11) of angiosarcoma were common. These patients were advanced with AJCC stage IV at the time of diagnosis. Distant metastases (n=11) included single organ metastasis (n=3) and multiple organ metastases (n=8). Bone or bone marrow (10/19) and lung (8/19) were the most common distant metastatic organs. Seven patients had metastases to bone (or bone marrow) and lung at the same time on 18F-FDG PET/CT (Figure 2A). Bone metastases were characterized by osteolytic bone destruction with mild and moderate increases in FDG metabolism (SUVmax 1.4–8.2). Four cases of bone marrow metastases without obvious bone destruction were diagnosed by bone marrow biopsy of the right iliac bone because of anemia, including 2 patients with occult bone marrow hypermetabolism (Figure 2B,2C) and 2 patients with normal bone marrow metabolism on 18F-FDG PET/CT. Additionally, 2 patients with bone marrow metastases showed myelofibrosis. Lung metastases were characterized by multiple margin blurred nodular and patchy shadows with mild FDG metabolism (SUVmax 0.4–4.7) on 18F-FDG PET/CT and was often misdiagnosed as inflammation. Other false-negative lesions were also found in 18F-FDG PET/CT such as left submandibular low 18F-FDG uptake lymph nodes in a patient with advanced angiosarcoma (Figure 2D). The pathology showed metastases finally (Figure 2F-2H).
One patient with splenic angiosarcoma with AJCC stage I showed no signs of recurrence after radical surgery in the 9-month follow-up. Two patients had angiosarcoma with AJCC stage II (one case in the bone and the other in the right atrium) and AJCC stage IIIB (both in the liver with hepatitis B cirrhosis, misdiagnosed as hepatocellular carcinoma).
Survival analysis
Kaplan-Meier overall survival curves were constructed for all the patients (n=19). Data from Kaplan-Meier survival analysis for the low Hb level groups and normal Hb level groups (Figure 3A), high Fer level groups and normal Fer level groups (Figure 3B), bone metastases groups and non-bone metastases groups (Figure 3C) and lung metastases groups and non-lung metastases groups (Figure 3D) are presented. Patients with bone metastases, low Hb levels and high Fer levels had significantly poorer overall survival than those with non-bone metastases, normal Hb and normal Fer groups by log-rank test, with P values of 0.027, 0.030 and 0.015, respectively. No significant difference was observed between the lung metastases groups and non-lung metastases groups (log-rank P=0.173). Patients with distant metastases (n=11) were divided into single organ metastasis group and multiple organ metastases group (Figure 3E). Patients with multiple metastases had significantly poorer overall survival than those with single metastasis (log-rank P=0.008). In the univariate survival analysis, KPS, Hb levels, bone metastases, pTLG40% and wMTV2.5 were significant predictors of overall survival (Table 4). Thus, high KPS, low Hb levels, bone metastases, high pTLG40% and wMTV2.5 were associated with worse outcomes. In multivariate survival analysis, only wMTV2.5 was a significant independent prognostic factor (Table 5). For wMTV2.5, 870.3 cm3 was the best cut-off point to discriminate between a good and poor prognosis (log-rank P=0.001) (Figure 3F), with a high hazard ratio of 6.78 by univariate analysis using the Cox proportional hazards model (P=0.007).
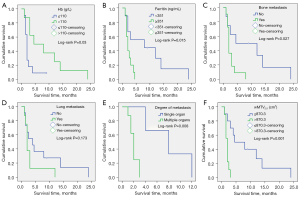
Table 4
| Variables (group) | HR | 95% CI | P value |
|---|---|---|---|
| Age (1 year-increase) | 0.990 | 0.970–1.020 | 0.690 |
| Sex (male vs. female) | 1.604 | 0.608–4.237 | 0.340 |
| KPS (10 unit-increase) | 0.967 | 0.936–0.999 | 0.044 |
| ECOG PS (PS1 vs. PS2/3/4) | 0.709 | 0.264–1.907 | 0.496 |
| Time from onset to diagnose (1 month-increase) | 0.975 | 0.042–1.008 | 0.131 |
| White-cell count (0.1*109/L-increase) | 0.996 | 0.845–1.174 | 0.962 |
| Hemoglobin (0.1 g/dL-increase) | 0.971 | 0.947–0.995 | 0.019 |
| Platelet count (1*109/L-increase) | 1.004 | 0.997–1.101 | 0.249 |
| C-reactive protein (0.1 mg/L-increase) | 1.019 | 0.996–1.042 | 0.100 |
| Lactate dehydrogenase (1 U/L-increase) | 1.002 | 0.999–1.005 | 0.292 |
| Ferritin (1 U/L-increase) | 1.000 | 1.000–1.001 | 0.109 |
| Glutamic-pyruvic transaminase (1 U/L-increase) | 1.002 | 0.997–1.006 | 0.416 |
| Creatinine (1 U/L-increase) | 1.003 | 0.993–1.014 | 0.523 |
| Surgical therapy (yes vs. no) | 1.620 | 0.575–4.560 | 0.361 |
| Sites of primary tumors (cutaneous vs. deep) | 0.440 | 0.134–1.452 | 0.178 |
| Size of primary tumors (0.1 cm-increase) | 0.869 | 0.735–1.028 | 0.101 |
| Ki67 (10%-increase) | 0.995 | 0.969–1.021 | 0.684 |
| Differentiation (G3 vs. G1 and G2) | 1.205 | 0.335–4.332 | 0.775 |
| Mitotic activity (G1 vs. G2 and G3) | 0.181 | 0.024–1.386 | 0.100 |
| Necrosis (G2 vs. G0 and G1) | 1.498 | 0.546–4.109 | 0.433 |
| FNCLCC (G3 vs. G1 and G2) | 1.465 | 0.524–4.100 | 0.567 |
| T classification (T1 and T2 vs. T3 and T4) | 2.942 | 0.886–9.775 | 0.078 |
| N classification (N1 vs. N0) | 1.737 | 0.549–5.078 | 0.313 |
| M classification (M1 vs. M0) | 0.890 | 0.328–2.421 | 0.820 |
| Bone metastasis (yes vs. no) | 0.295 | 0.089–0.098 | 0.047 |
| Lung metastasis (yes vs. no) | 0.522 | 0.191–1.430 | 0.206 |
| AJCC stage (I and II vs. III and IV) | 0.896 | 0.251–3.191 | 0.865 |
| *pSUVmax of (1-unit increase) | 0.953 | 0.895–1.016 | 0.140 |
| pSUVavg (1-unit increase) | 0.861 | 0.732–1.012 | 0.069 |
| pMTV40% (1-unit increase) | 1.000 | 0.997–1.002 | 0.762 |
| pMTV2.5 (1-unit increase) | 1.000 | 1.000–1.001 | 0.590 |
| pTLG40% (1-cm3 increase) | 1.000 | 0.999–1.000 | 0.040 |
| pTLG2.5 (1-cm3 increase) | 1.000 | 1.000–1.000 | 0.286 |
| #wMTV40% (1-unit increase) | 1.001 | 1.000–1.001 | 0.076 |
| wMTV2.5 (1-unit increase) | 1.000 | 1.000–1.001 | 0.034 |
| wTLG40% (1-cm3 increase) | 1.000 | 1.000–1.000 | 0.595 |
| wTLG2.5 (1-cm3 increase) | 1.000 | 1.000–1.000 | 0.969 |
*, P indicates primary tumor; #, w indicates whole-body tumor. HR, hazard ratio; CI, confidence interval; KPS, Karnofsky Performance Status; ECOG, Eastern Cooperative Oncology Group; FNCLCC, Federation Nationale des Centres de Lutte Contre le Cancer; AJCC, American Joint Committee on Cancer; SUVmax, maximum standardized uptake value; SUVavg, average standardized uptake value; MTV2.5, metabolic tumor volume using SUVmax cut-off value of 2.5; MTV40%, metabolic tumor volume using 40% of SUVmax as threshold; TLG2.5, total lesion glycolysis using SUVmax cut-off value of 2.5; TLG40%, total lesion glycolysis using 40% of SUVmax as threshold.
Table 5
| Variables (group) | HR | 95% CI | P value |
|---|---|---|---|
| KPS (10 unit-increase) | 0.969 | 0.933–1.007 | 0.114 |
| hemoglobin (0.1 g/dL-increase) | 0.984 | 0.952–1.017 | 0.336 |
| *pTLG40% (1-cm3 increase) | 1.000 | 0.999–1.000 | 0.231 |
| #wMTV2.5 (1-unit increase) | 1.001 | 1.000–1.001 | 0.035 |
| Bone metastasis (yes vs. no) | 0.746 | 0.336–4.061 | 1.220 |
*, P indicates primary tumor; #, w indicates whole-body tumor. HR, hazard ratio CI Confidence interval; KPS, Karnofsky Performance Status; TLG40%, total lesion glycolysis using 40% of SUVmax as threshold; MTV2.5, metabolic tumor volume using SUVmax cut-off value of 2.5.
Discussion
The etiology and pathogenesis of angiosarcoma remain unclear. Approximately 3% of angiosarcoma cases are genetically induced (19). In our study, a female patient with primary angiosarcoma of the right atrium had a family history of her mother who having angiosarcoma of the breast. Some familial genetic factors may exist but have not been detected. Extrinsic risk factors include toxic substances (e.g., thorium oxide colloids, arsenic, and long-term use of anabolic steroids or estrogen) (20), medical history (e.g., chronic venous ulcers and Stewart-Treves syndrome), foreign bodies (e.g., vascular graft materials and surgical sponges) and ionizing radiation (in particular, radiation whose direct tumorigenic effects and ischemic changes resulting from long-term cellular repair can cause tissue damage) (21). Two patients with hepatitis B cirrhosis developed liver angiosarcoma rather than hepatocarcinoma in this study, suggesting that HBV infection is also an important risk factor for hepatic angiosarcoma.
Angiosarcoma has a poor prognosis. Previous literature suggests that many factors leading to the prognosis of angiosarcoma patients include age, tumor size, metastasis, positive surgical margin, higher pathological tissue grade, deeper tumor site, physical status score and treatment pattern (19). A study of primary angiosarcoma of the heart in the United States showed that only 1/18 patient achieved R0 resection with a lower overall survival and indicated that the site of angiosarcoma was also a prognostic factor (22). In our prognostic evaluation, the patient’s age, KPS, ECOG, tumor size, pathological grade, time of diagnosis, Ki67, treatment pattern and tumor location (deep and superficial, cardiac or non-cardiac) were not significant. The possible reasons for these results include three points: (I) the sample of 19 cases was too small; (II) angiosarcoma occurred in various regions and metastasized to distant areas easily; (III) pathological manifestations were varied. The rarity and clinical and pathological diversity of angiosarcoma make it difficult to study, diagnose and ultimately formulate appropriate treatment strategies. Systemic therapy, including radiotherapy, chemotherapy, targeted therapy and immunotherapy, is important to treat angiosarcoma, but no consensus exists on clinical treatment guidelines leading to differences in treatment.
However, there are no laboratory tests for early detection of angiosarcoma specifically and role of laboratory examination in assessing the prognosis of patients with angiosarcoma was unclear. Laboratory studies are usually unremarkable unless the mass effect causes the compression of critical organs and laboratory abnormalities or the disease is fairly advanced to cause subtle laboratory abnormalities (e.g., anemia of chronic disease and elevated sedimentation rate) (23). In our study, anemia was the most common laboratory abnormality, similar to other studies (2,22,23). Previous articles have not suggested a specific answer to this hypothesis. Possible explanations for anemia included anemia of chronic disease, bleeding, hemolysis, sequestration of erythroid elements, a tumor-associated factor or obliteration of the normal bone marrow hematopoietic elements. Surprisingly, we found significant fibrosis in bone marrow biopsies in 2 patients. Therefore, we speculated that bone marrow fibrosis caused by tumor metastasis to the bone marrow may be the main cause of anemia. We also found that half of the patients had a CRP increase, possibly due to secondary inflammation caused by tumor growth. However, few previous articles had assessed the prognosis of angiosarcoma using laboratory examination. Patients with bone metastases, low Hb levels and high Fer levels, which may represent existing internal causality, had a significantly poorer overall survival than those with non-bone metastases, normal Hb levels and normal Fer levels by the log-rank test in our study.
TNM staging of 18F-FDG PET/CT did not predict the prognosis of angiosarcoma in our study. However, further analysis suggested that the prognosis of patients with multiple organ metastases was significantly worse than that of patients with single organ metastasis. 18F-FDG PET/CT found some hidden metastases and performed more accurate TNM staging. Therefore, in clinical and 18F-PET/CT evaluations of angiosarcoma, recommending using further grading of M stage with M1a (single organ metastasis) and M1b (multiple organ metastases) may be more accurate to assess the prognosis of patients. False-negative lesions were also found, such as left submandibular low 18F-FDG uptake lymph nodes. Therefore, developing new tracers is urgent to improve the specificity and accuracy of imaging and the accuracy of prognosis judgment.
SUVmax is a useful indicator in the prognosis of patients with soft tissue sarcoma (22,24,25). However, SUVmax is a semiquantitative index and is influenced by biological and technological factors such as injection activity and scan duration (26). Therefore, multi-quantitative parameters and tumor description methods are necessary to predict the prognosis of angiosarcoma. Kato et al. (27) suggested that higher PSUVmax, MTV, whole-body TLG, primary TBR, and whole-body TLG ratio correlated significantly with a poorer overall survival in a study of 16 cases of angiosarcoma by 18F-FDG PET/CT before treatment. In our study and multivariate survival analysis, only wMTV2.5 was shown to be a significant independent prognostic factor. The best cut-off point to discriminate between good and poor prognoses of wMTV2.5 was 870.3 cm3, which was significantly higher than the data (41.1 cm3) suggested in a previous article by Kato (27). MTV and TLG have been well used as prognostic parameters in soft tissue sarcoma because they can reflect the load of tumors and biological behavior of tumors but have not yet obtained a more recognized grouping threshold. First, in our paper, the common fixed boundary value (at an early SUV of 2.5) was more meaningful in predicting the prognosis of angiosarcoma than the percentage boundary value (fixed thresholds of 40% of SUVmax), and we will continue to explore its optimal threshold in the following study. Second, MTV was more meaningful in predicting the prognosis of angiosarcoma than TLG. The reason may be that the MTV represents the tumor volume, likely indicating that the heterogeneity of the tumor increases and degree of differentiation worsens with the growth of the tumor. TLG reflects tumor load. However, angiosarcoma and its metastatic lesions are often associated with necrosis and hand-drawn ROIs of SUVavg measurement inhomogeneity. Therefore, TLG may not be as good as MTV in some negative or hypometabolic metastatic lesions. Finally, limited sample size was still the primary reason for our differences from previous studies. Knowledge of the interobserver variability of quantitative parameters is important in sarcomas because these tumors are frequently large and demonstrate heterogeneous 18F-FDG uptake.
We recognized the following limitations of our study. First, the study was performed at a single-center and the sample size (n=19) was relatively small to achieve appropriate sampling of rare malignant neoplasms; multicenter analysis will be designed in a future study to achieve a better representation of angiosarcoma patient population. Second, we could not control for the effects of treatment modalities on OS. Therefore, effective and appropriate treatment strategies for angiosarcoma must be developed. Third, this was a retrospective study, and the use of 18F-FDG PET/CT in the assessment of patients produced a potential bias toward an advanced tumor cohort.
Conclusions
Compared to the conventional imaging, the systemic 18F-FDG PET/CT with high sensitivity and specificity has significant advantages in the evaluation of angiosarcoma, particularly in detecting occult metastases such as bone marrow, subcutaneous tissue, liver, and even hydrothorax and ascitic fluid. Furthermore, 18F-FDG PET/CT with laboratory testing of Hb and Fer also play important roles in angiosarcoma. MTV2.5 of the whole body is a significant independent metabolic prognostic factor for poor overall survival in patients with angiosarcoma.
Acknowledgments
We gratefully acknowledge our colleagues for their comments on this study.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-21-563/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-21-563/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This retrospective study was approved by the Ethics Committee of the First Affiliated Hospital, Zhejiang University School of Medicine (No. IIT2020639A) and the requirement to obtain written informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Naka N, Ohsawa M, Tomita Y, Kanno H, Uchida A, Myoui A, Aozasa K. Prognostic factors in angiosarcoma: a multivariate analysis of 55 cases. J Surg Oncol 1996;61:170-6. [Crossref] [PubMed]
- Naka N, Ohsawa M, Tomita Y, Kanno H, Uchida A, Aozasa K. Angiosarcoma in Japan. A review of 99 cases. Cancer 1995;75:989-96. [Crossref] [PubMed]
- Morgan MB, Swann M, Somach S, Eng W, Smoller B. Cutaneous angiosarcoma: a case series with prognostic correlation. J Am Acad Dermatol 2004;50:867-74. [Crossref] [PubMed]
- Lydiatt WM, Shaha AR, Shah JP. Angiosarcoma of the head and neck. Am J Surg 1994;168:451-4. [Crossref] [PubMed]
- Fury MG, Antonescu CR, Van Zee KJ, Brennan MF, Maki RG. A 14-year retrospective review of angiosarcoma: clinical characteristics, prognostic factors, and treatment outcomes with surgery and chemotherapy. Cancer J 2005;11:241-7. [Crossref] [PubMed]
- Chen KT, Kirkegaard DD, Bocian JJ. Angiosarcoma of the breast. Cancer 1980;46:368-71. [Crossref] [PubMed]
- Asmane I, Litique V, Heymann S, Marcellin L, Métivier AC, Duclos B, Bergerat JP, Kurtz JE. Adriamycin, cisplatin, ifosfamide and paclitaxel combination as front-line chemotherapy for locally advanced and metastatic angiosarcoma. Analysis of three case reports and review of the literature. Anticancer Res 2008;28:3041-5. [PubMed]
- Mark RJ, Poen JC, Tran LM, Fu YS, Juillard GF. Angiosarcoma. A report of 67 patients and a review of the literature. Cancer 1996;77:2400-6. [Crossref] [PubMed]
- Lahat G, Dhuka AR, Hallevi H, Xiao L, Zou C, Smith KD, Phung TL, Pollock RE, Benjamin R, Hunt KK, Lazar AJ, Lev D. Angiosarcoma: clinical and molecular insights. Ann Surg 2010;251:1098-106. [Crossref] [PubMed]
- Zacarias Föhrding L, Macher A, Braunstein S, Knoefel WT, Topp SA. Small intestine bleeding due to multifocal angiosarcoma. World J Gastroenterol 2012;18:6494-500. [Crossref] [PubMed]
- Penel N, Marréaud S, Robin YM, Hohenberger P. Angiosarcoma: state of the art and perspectives. Crit Rev Oncol Hematol 2011;80:257-63. [Crossref] [PubMed]
- Tan H, Gu Y, Yu H, Hu P, Zhang Y, Mao W, Shi H. Total-Body PET/CT: Current Applications and Future Perspectives. AJR Am J Roentgenol 2020;215:325-37. [Crossref] [PubMed]
- Czernin J, Phelps ME. Positron emission tomography scanning: current and future applications. Annu Rev Med 2002;53:89-112. [Crossref] [PubMed]
- Eary JF, Conrad EU. Imaging in sarcoma. J Nucl Med 2011;52:1903-13. [Crossref] [PubMed]
- Hicks RJ, Toner GC, Choong PF. Clinical applications of molecular imaging in sarcoma evaluation. Cancer Imaging 2005;5:66-72. [Crossref] [PubMed]
- Eary JF, O'Sullivan F, Powitan Y, Chandhury KR, Vernon C, Bruckner JD, Conrad EU. Sarcoma tumor FDG uptake measured by PET and patient outcome: a retrospective analysis. Eur J Nucl Med Mol Imaging 2002;29:1149-54. [Crossref] [PubMed]
- Macpherson RE, Pratap S, Tyrrell H, Khonsari M, Wilson S, Gibbons M, Whitwell D, Giele H, Critchley P, Cogswell L, Trent S, Athanasou N, Bradley KM, Hassan AB. Retrospective audit of 957 consecutive 18F-FDG PET-CT scans compared to CT and MRI in 493 patients with different histological subtypes of bone and soft tissue sarcoma. Clin Sarcoma Res 2018;8:9. [Crossref] [PubMed]
- Benz MR, Evilevitch V, Allen-Auerbach MS, Eilber FC, Phelps ME, Czernin J, Weber WA. Treatment monitoring by 18F-FDG PET/CT in patients with sarcomas: interobserver variability of quantitative parameters in treatment-induced changes in histopathologically responding and nonresponding tumors. J Nucl Med 2008;49:1038-46. [Crossref] [PubMed]
- Qureshi SS, Rekhi B, Pungavkar S. Congenital angiosarcoma of the arm in a pediatric patient: a therapeutic dilemma. J Clin Oncol 2012;30:e112-4. [Crossref] [PubMed]
- Al-Enezi M, Brassard A. Chronic venous ulceration with associated angiosarcoma. J Dermatol Case Rep 2009;3:8-10. [Crossref] [PubMed]
- Tamaoki M, Nio Y, Tamaoki M, Sakamoto M, Uesugi K, Sakamoto T, Imai S, Maruyama R. Radiation-Associated Angiosarcoma That Developed in the Irradiated Residual Breast after Breast-Conserving Surgery for Breast Cancer-A Case Report and Review of the Literature. Gan To Kagaku Ryoho 2020;47:77-81. [PubMed]
- Look Hong NJ, Pandalai PK, Hornick JL, Shekar PS, Harmon DC, Chen YL, Butrynski JE, Baldini EH, Raut CP. Cardiac angiosarcoma management and outcomes: 20-year single-institution experience. Ann Surg Oncol 2012;19:2707-15. [Crossref] [PubMed]
- Neuhauser TS, Derringer GA, Thompson LD, Fanburg-Smith JC, Miettinen M, Saaristo A, Abbondanzo SL. Splenic angiosarcoma: a clinicopathologic and immunophenotypic study of 28 cases. Mod Pathol 2000;13:978-87. [Crossref] [PubMed]
- Hawkins DS, Schuetze SM, Butrynski JE, Rajendran JG, Vernon CB, Conrad EU 3rd, Eary JF. 18FFluorodeoxyglucose positron emission tomography predicts outcome for Ewing sarcoma family of tumors. J Clin Oncol 2005;23:8828-34. [Crossref] [PubMed]
- Schuetze SM, Rubin BP, Vernon C, Hawkins DS, Bruckner JD, Conrad EU 3rd, Eary JF. Use of positron emission tomography in localized extremity soft tissue sarcoma treated with neoadjuvant chemotherapy. Cancer 2005;103:339-48. [Crossref] [PubMed]
- Bodet-Milin C, Eugène T, Gastinne T, Frampas E, Le Gouill S, Kraeber-Bodéré F. FDG-PET in Follicular Lymphoma Management. J Oncol 2012;2012:370272. [Crossref] [PubMed]
- Kato A, Nakamoto Y, Ishimori T, Saga T, Togashi K. Prognostic Value of Quantitative Parameters of 18F-FDG PET/CT for Patients With Angiosarcoma. AJR Am J Roentgenol 2020;214:649-57. [Crossref] [PubMed]


