Synchronous Kimura lesions at two different sites–a diagnostic dilemma!
Introduction
Kimura disease (KD) is a rare chronic inflammatory disease of unknown etiology, which usually manifests with unilateral soft tissue swellings with no malignant potential in the head and neck region, including salivary glands and lymph nodes and is associated with peripheral blood eosinophilia and raised IgE levels (1). It most commonly occurs in young male adults in their second and third decades of life and is considered to be an endemic disease of Asia, which should be borne in mind in the differential diagnosis of head and neck swellings (2). Histopathology is confirmatory and surgical excision is the treatment of choice though recurrences are common (3). We present this patient who had two asymptomatic synchronous swellings of the right preauricular region and right thigh, which led to a clinical diagnostic dilemma. Both lesions were excised and on follow-up after 2 years the patient has been disease free.
Case presentation
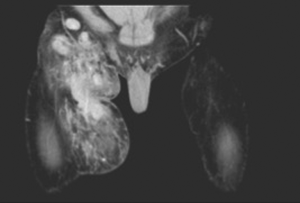
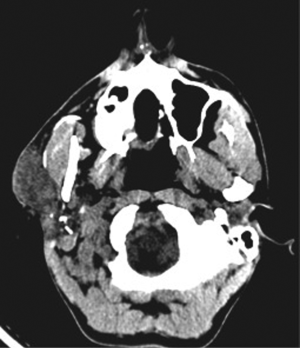
A 35-year-old male presented with complaints of asymptomatic swellings that appeared simultaneously in the right preauricular region and right thigh of 2-year duration and had gradually increased in size. On examination, there was a firm, mobile, non-tender, 4 cm × 3 cm irregular swelling in the right preauricular region with no cervical lymph nodes. The thigh swelling was 5 cm × 4 cm in size, firm, intramuscular with restricted mobility, non-tender but warm with doubtful pulsatile (vascular) nature. Hence a CT angiogram of right thigh was done which showed a diffusely vascular intramuscular swelling with a few lymph nodes within the swelling (Figure 1). CT scan of right parotid region revealed a neoplasm (Figure 2) of which fine needle aspiration cytology (FNAC) was inconclusive. In view of the above findings, a right superficial parotidectomy and excision of the right thigh swelling were done. Intraoperatively, the parotid gland was enlarged with multiple dense adhesions to the facial nerve. Multiple lymph nodes were seen, frozen section of which showed no evidence of malignancy. The thigh swelling was vascular but easily excisable. Postoperative period was uneventful, except for a mild right sided facial nerve palsy which was managed with physiotherapy. The final histopathological report of both the swellings revealed features suggestive of KD (Figure 3). The diagnosis was also confirmed by the laboratory correlation of raised IgE and peripheral eosinophilia. Patient was reassured and the benign nature of the disease was explained. He has been kept on close follow-up and after 2 years has no recurrence and the peripheral eosinophilia has decreased.
Discussion
KD is a rare, chronic, benign inflammatory disorder of unknown etiology mimicking neoplastic disease and is characterized by multiple subcutaneous nodules and masses, primarily in the cervical region, accompanied by peripheral eosinophilia and lymphadenopathy (1). It is considered as an endemic disease of Asia, though recent studies have shown that despite its rarity, if clinically suspected, should be included in the differential diagnosis of neck masses for people of any racial group (2,3). The peak age of onset is around the third decade of life with a marked male preponderance with male-female ratios ranging from 3.5:1 to 9:1 in epidemiological studies. A detailed literature review revealed that synchronous Kimura lesions at 2 different sites have been described only in 3 patients. A total of 4 cases (isolated KD) have been reported from Asia (4-7).
The etiology of KD is still unclear. Theories that have been put forward include an allergic immunogenic response to an unknown antigenic stimulus (parasitic, viral, fungal or toxin), aberrant autoimmune inflammatory reactions or even trauma. Candida albicans has been implicated as the probable antigenic stimulus, but no clear-cut evidence is available (6,7). The role of an antigen triggering IgE mediated type 1 eosinophilic reaction or abnormal T-cell activation causing release of eosinophilotrophic cytokines (IL 4, IL 5, IL 13, TNFα) has also been postulated (8). Moreover, its association with immunologically mediated renal disease and occasionally with atopic disorders (bronchial asthma, allergic rhinitis and atopic dermatitis), and the role of corticosteroids in treatment of KD and those conditions further support the case of an allergic or autoimmune process being the likely cause (9,10).
The clinical presentation of KD is often with a solitary enlarged painless lymph node or generalized lymphadenopathy (67–100%) (5,6). It may also present as multiple subcutaneous nodules or masses in the head and neck region, predominantly in preauricular and submandibular areas (43%) (11). It is often accompanied by salivary adenopathy in the form of hyperplasia of the parotid and submandibular salivary glands (12). Other sites of involvement include the groin (15%), extremities (12%) and trunk (3%). Unusual sites of involvement include the epiglottis, lacrimal glands, tympanic membranes, paranasal sinuses, parapharyngeal spaces, colon, spermatic cord and the median nerve (9,11,13-15). While systemic symptoms are uncommon, renal involvement is often seen (10–60%). Clinically relevant proteinuria is seen in 12–16% of cases, while 10–12% suffer from nephrotic syndrome (16).
Differential diagnosis includes angiolymphoid hyperplasia with eosinophilia (ALHE), Hodgkin’s disease, angioimmunoblastic T-cell lymphoma, Langerhans cell histiocytosis, florid follicular hyperplasia, Castleman disease, dermatopathic lymphadenopathy, allergic granulomatosis of Churg-Strauss, drug reaction, parasitic lymphadenitis and other inflammatory and neoplastic conditions causing cervical lymphadenopathy (1). KD is most often confused with ALHE due to their similar clinical presentations. However, regional lymphadenopathy, serum eosinophilia and raised IgE levels are not commonly seen in cases of ALHE (9,17).
Peripheral eosinophilia and elevated IgE levels are the most consistent laboratory findings in patients with KD (1). Some studies have shown that the serum eosinophil count is closely correlated to the size of the neck masses (18). Imaging studies can help in staging the extent and progression of disease, lymph node involvement and to rule out other causes of neck masses. CT & MR imaging in cases of KD of the neck shows multiple ill-defined enhancing masses within and around the parotid gland with associated regional lymphadenopathy (19).
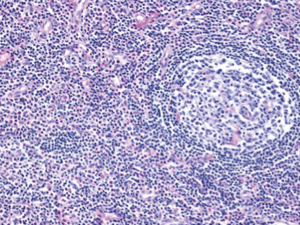
Histological diagnosis is often confirmatory, with the diagnosis established on lymph node or salivary gland specimen (1,3). Characteristic histological features of lymph nodes in KD include: follicular hyperplasia with reactive germinal centers and well-formed mantle zones, prominent eosinophilic microabscesses, eosinophilic folliculolysis along with proliferation of postcapillary venules. Germinal center necrosis, proteinaceous deposits and eosinophilic infiltrates in the germinal centers; vascularization of germinal centers and sclerotic areas are also seen (9,19,20). Immunochemistry findings described are IgE reticular network in germinal centers and IgE coated non-degranulated mast cells (2,3).
The treatment of KD is primarily surgical excision but is associated with up to 25% risk of recurrence and due to the lack of malignant potential, conservative surgery is always preferred (1,21). Radiotherapy in combination with an immunosuppressant may be preferred in patients with large head and neck masses, when surgery may be difficult, given the high risk of neurologic deficit and the need for subsequent facial reconstruction (6,9). Management of recurrence is usually done with surgical re-resection, oral steroids and local radiotherapy with 25–30 Gy radiation (22).
For patients with renal involvement, oral steroids (prednisolone) are the treatment of choice, though relapse rates are high. Intralesional corticosteroids may be effective for localized disease (5). Other modalities of treatment that have been attempted successfully include use of cyclosporine, azathioprine, leflunomide, cetrizine, pranlukast, pentoxifylline, IV immunoglobulin, photodynamic therapy, all-trans-retinoic acid and imatinib (3,23-27).
Conclusions
In summary, KD is a rare immunological complex characterized by subcutaneous or lymph nodal masses, salivary gland enlargement and peripheral eosinophilia. Surgical excision often results in cure with recurrences being somewhat common. Systemic corticosteroids, immunosuppressive agents and radiation therapy are reserved for patients with renal involvement or recurrent disease.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Faras F, Abo-Alhassan F, Al-Sebeih K, Bastaki J. Kimura disease manifesting as synchronous bilateral parotid swelling in a young middle-eastern patient. Case Rep Surg 2014;2014:648607.
- Chen H, Thompson LD, Aguilera NS, Abbondanzo SL. Kimura disease: a clinicopathologic study of 21 cases. Am J Surg Pathol 2004;28:505-13. [Crossref] [PubMed]
- Sun QF, Xu DZ, Pan SH, Ding JG, Xue ZQ, Miao CS, Cao GJ, Jin DJ. Kimura disease: review of the literature. Intern Med J 2008;38:668-72. [Crossref] [PubMed]
- Kawada A. Kimura’s disease. Demonstration of the disease and its differential diagnosis. Hautarzt 1976;27:309-17. [PubMed]
- Wang DY, Mao JH, Zhang Y, Gu WZ, Zhao SA, Chen YF, Liu AM. Kimura disease: a case report and review of the Chinese literature. Nephron Clin Pract 2009;111:c55-61. [Crossref] [PubMed]
- Tseng CF, Lin HC, Huang SC, Su CY. Kimura’s disease presenting as bilateral parotid masses. Eur Arch Otorhinolaryngol 2005;262:8-10. [Crossref] [PubMed]
- Mitsui M, Ogino S, Ochi K, Ohashi T. Three cases of eosinophilic lymphfolliculoid granuloma of the soft tissue originating from the parotid gland. Acta Otolaryngol Suppl 1996;522:130-2. [PubMed]
- Tsukadaira A, Kitano K, Okubo Y, Horie S, Ito M, Momose T, Takashi S, Itoh S, Kiyosawa K, Sekiguchi M. A case of pathophysiologic study in Kimura’s disease: measurement of cytokines and surface analysis of eosinophils. Ann Allergy Asthma Immunol 1998;81:423-7. [Crossref] [PubMed]
- Khoo BP, Chan R. Kimura disease: 2 case reports and a literature review. Cutis 2002;70:57-61. [PubMed]
- Rajpoot DK, Pahl M, Clark J. Nephrotic syndrome associated with Kimura disease. Pediatr Nephrol 2000;14:486-8. [Crossref] [PubMed]
- Mrówka-Kata K, Kata D, Kyrcz-Krzemień S, Helbig G. Kikuchi-Fujimoto and Kimura diseases: the selected, rare causes of neck lymphadenopathy. Eur Arch Otorhinolaryngol 2010;267:5-11. [Crossref] [PubMed]
- Armstrong WB, Allison G, Pena F, Kim JK. Kimura’s disease: two case reports and a literature review. Ann Otol Rhinol Laryngol 1998;107:1066-71. [Crossref] [PubMed]
- Yamamoto T, Minamiguchi S, Watanabe Y, Tsuji J, Asato R, Manabe T, Haga H. Kimura disease of the epiglottis: a case report and review of literature. Head Neck Pathol 2014;8:198-203. [Crossref] [PubMed]
- Yoganathan P, Meyer DR, Farber MG. Bilateral lacrimal gland involvement with Kimura disease in an African American male. Arch Ophthalmol 2004;122:917-9. [Crossref] [PubMed]
- Isomoto H, Shikuwa S, Mizuta Y, Kohno S. Clinical Challenges and Images in GI: image 3. Colonic involvement in Kimura disease. Gastroenterology 2008;135:743,1020.
- Dixit MP, Scott KM, Bracamonte E, Dixit NM, Schumacher MJ, Hutter J, Nagle R. Kimura disease with advanced renal damage with anti-tubular basement membrane antibody. Pediatr Nephrol 2004;19:1404-7. [Crossref] [PubMed]
- Chun SI, Ji HG. Kimura’s disease and angiolymphoid hyperplasia with eosinophilia: clinical and histopathologic differences. J Am Acad Dermatol 1992;27:954-8. [Crossref] [PubMed]
- Sakamoto M, Komura A, Nishimura S. Hematoserological analysis of Kimura’s disease for optimal treatment. Otolaryngol Head Neck Surg 2005;132:159-60. [Crossref] [PubMed]
- Park SW, Kim HJ, Sung KJ, Lee JH, Park IS. Kimura disease: CT and MR imaging findings. AJNR Am J Neuroradiol 2012;33:784-8. [Crossref] [PubMed]
- Kapoor NS, O’Neill JP, Katabi N, Wong RJ, Shah JP. Kimura disease: diagnostic challenges and clinical management. Am J Otolaryngol 2012;33:259-62. [Crossref] [PubMed]
- Takeishi M, Makino Y, Nishioka H, Miyawaki T, Kurihara K. Kimura disease: diagnostic imaging findings and surgical treatment. J Craniofac Surg 2007;18:1062-7. [Crossref] [PubMed]
- Hareyama M, Oouchi A, Nagakura H, Asakura K, Saito A, Satoh M, Tamakawa M, Akiba H, Sakata K, Yoshida S, Koito K, Imai K, Kataura A, Morita K. Radiotherapy for Kimura’s disease: the optimum dosage. Int J Radiat Oncol Biol Phys 1998;40:647-51. [Crossref] [PubMed]
- Sato S, Kawashima H, Kuboshima S, Watanabe K, Kashiwagi Y, Takekuma K, Hoshika A. Combined treatment of steroids and cyclosporine in Kimura disease. Pediatrics 2006;118:e921-3. [Crossref] [PubMed]
- Dai L, Wei XN, Zheng DH, Mo YQ, Pessler F, Zhang BY. Effective treatment of Kimura’s disease with leflunomide in combination with glucocorticoids. Clin Rheumatol 2011;30:859-65. [Crossref] [PubMed]
- Ben-Chetrit E, Amir G, Shalit M. Cetirizine: An effective agent in Kimura’s disease. Arthritis Rheum 2005;53:117-8. [Crossref] [PubMed]
- Hernandez-Bautista V, Yamazaki-Nakashimada MA, Vazquez-García R, Stamatelos-Albarrán D, Carrasco-Daza D, Rodríguez-Lozano AL. Treatment of Kimura disease with intravenous immunoglobulin. Pediatrics 2011;128:e1633-5.
- Boulanger E, Gachot B, Verkarre V, Valensi F, Brousse N, Hermine O. all-trans-Retinoic acid in the treatment of Kimura’s disease. Am J Hematol 2002;71:66. [Crossref] [PubMed]