
Usefulness of computed tomography during arteriography in a selective arterial calcium agent injection test for pancreatic gastrinoma: a case description
Introduction
Pancreatic gastrinoma is a rare pancreatic neuroendocrine tumor with an incidence of about 0.5–21.5 cases per million people worldwide. The most common sites are the duodenum (75%), pancreas (20%), and other organs (5%). Gastrinomas are malignant in about 60–90% of cases (1-5). Malignant gastrinomas originating beneath the duodenal mucosa often metastasize to the liver, even if the tumor diameter is ≤1 cm. Therefore, in patients with gastrinoma and Zollinger–Ellison syndrome, it is important to determine the number and site(s) of gastrinoma(s), as well as the presence or absence of liver metastases. However, the detection sensitivity of computed tomography (CT) and magnetic resonance imaging is about 10–50%, that of angiography is 20–50%, and that of somatostatin receptor scintigraphy is 30–70%.
Tumor size is strongly correlated with the detection sensitivity of any examination modality, and tumors of <1 cm in diameter are often difficult to detect (1,2). Therefore, the selective arterial calcium agent injection (SACI) test was developed as an effective method to detect small gastrinomas (2,6,7). In the SACI test, calcium gluconate is injected via a microcatheter into selected arterial branches to stimulate gastrin secretion from the gastrinoma located in the corresponding arterial territory. Generally, calcium gluconate is selectively injected into the gastroduodenal artery (GDA), superior mesenteric artery (SMA), distal or proximal portion of the splenic artery (distal SpA, proximal SpA), and the proper hepatic artery (PHA). The subsequent increase in the serum gastrin level is monitored using blood samples taken from the hepatic vein before and at 30, 60, 90, and 120 s after the injection. The location of the gastrinoma is determined based on a significant increase in serum gastrin levels in response to calcium gluconate (maximum serum gastrin level after calcium injection/serum gastrin level before calcium agent injection; with a median change of about 4.21 times baseline) (8). However, there are two limitations of the SACI test. First, the distribution of calcium varies between individual patients due to anatomical differences in the pancreatic arterial branches and the arterial territories. Second, there are many false positives following the injection of calcium gluconate into the GDA (median change of 4.15 times the baseline value) because calcium gluconate may stimulate normal gastrin-secreting cells in the gastric prepylorus and duodenal bulbus (8,9). It is possible to visualize the distribution of calcium following its injection by performing CT during arteriography (CTA). Here, we report a case in which the SACI test was combined with CTA for the diagnosis of a gastrinoma located in the pancreatic head and to exclude the presence of liver metastases.
Case presentations
A 49-year-old man with a history of repeated diarrhea, gastrointestinal ulcers, and hypergastrinemia was referred for testing. Contrast-enhanced CT revealed a 13-mm-diameter nodule located in the pancreatic head that showed gradual enhancement. Somatostatin receptor scintigraphy showed increased 111In uptake in a region consistent with the nodule in the pancreatic head. Thus, imaging suggested a gastrinoma located in the pancreatic head. He was diagnosed with a gastrin-positive neuroendocrine tumor following endoscopic ultrasonographic fine needle aspiration. An SACI test was performed to confirm whether the case had a solitary gastrinoma in the pancreatic head, and to determine the appropriate treatment plan. Because the patient had severe symptoms of gastrointestinal ulcers, proton pump inhibitors could not be discontinued during the SACI test.
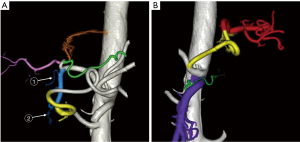
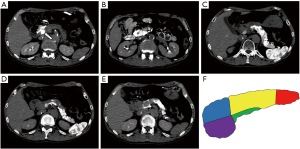
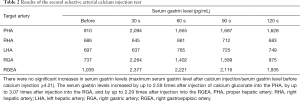
To evaluate the vascular anatomy, CT during aortography was performed and three-dimensional images of the arteries were reconstructed (Figure 1). The GDA branched from the common hepatic artery, and the posterior superior pancreatic duodenal artery (PSPDA) and anterior superior pancreaticoduodenal artery (ASPDA) both branched from the GDA. In addition, the right hepatic artery (RHA) and the left hepatic artery (LHA) branched from the PHA, and the right gastric artery (RGA) branched from the LHA (Figure 1A). The great pancreatic artery branched from the proximal SpA, and the caudal pancreatic artery branched from the distal SpA. The inferior pancreaticoduodenal artery, the dorsal pancreatic artery, and the transverse pancreatic artery (TPA) branched from the SMA (Figure 1B).The first conventional SACI test was performed by inserting a microcatheter into the GDA, PHA, proximal SpA, distal SpA, SMA, and dorsal pancreatic artery + TPA, and the territories supplied by each artery were visually evaluated by CTA. CTA from the GDA revealed a 13-mm-diameter nodule in the pancreatic head that was considered to be a primary gastrinoma (Figure 2A). CTA of the other arteries did not reveal any obvious pancreatic tumors (Figure 2B,C,D,E). Calcium gluconate (0.025 mEq/kg) was selectively injected into each artery. To help stabilize the gastrin level before each test, the injection of calcium gluconate into the GDA, which supplied the tumor, was performed last. The injections were performed in the following order: proximal SpA, distal SpA, PHA, SMA, TPA, and GDA. Each injection of calcium gluconate was performed at an interval of ≥5 min. Blood samples were collected from the hepatic vein before and at 30, 60, 90, and 120 s after each injection to measure the serum gastrin level. In the first SACI test, there were significant increases in serum gastrin levels following injection of calcium gluconate into the GDA and PHA (Table 1). The increase in the serum gastrin level from the GDA was considered to be due to stimulation of a primary gastrinoma in the pancreatic head. The increase in the serum gastrin level from PHA was consistent with the presence of a gastrinoma in the liver, liver metastasis, and stimulation of normal gastrin-secreting cells in the stomach and duodenum. Therefore, an additional SACI test was performed two weeks later. CTA during the second SACI test revealed that the RGA and the right gastroepiploic artery (RGEA) supplied the gastric prepylorus and duodenal bulbus (Figure 3). Therefore, a microcatheter was super-selectively inserted into the following arteries in the order of PHA, RHA, LHA, RGA, and RGEA. Then, calcium gluconate (0.025 mEq/kg) was injected into each artery as in the first SACI test. In this test, injection of calcium gluconate via the PHA, RGA, and RGEA increased serum gastrin levels to the same extent. However, stimulation of the RHA and LHA did not elicit significant increases in serum gastrin levels (Table 2). These findings indicate that the increase in serum gastrin level from PHA in the first and second SACI tests was a false positive due to stimulation of normal gastrin-secreting cells in the stomach and duodenum. The patient was therefore diagnosed with a gastrinoma located in the pancreatic head, and he underwent pancreaticoduodenectomy. The pathological diagnosis confirmed that the pancreatic head tumor was a gastrinoma. His preoperative diarrhea improved immediately after surgery, and his serum gastrin level had decreased to 199 pg/mL at 17 days after surgery.

Full table

Full table
Discussion
Determining the location of a gastrinoma or insulinoma is an important factor when determining the treatment plan, and the SACI test is an effective diagnostic method for this purpose (8,10). The SACI test is effective for detecting small lesions, but it has a high false positive rate (8). False positives are due to the distribution of calcium gluconate into the stomach and duodenum (8), as observed in our case. However, this limitation can be solved by mapping the vascular anatomy, and evaluating the region of each organ supplied by each artery. Therefore, in our case, we visualized and mapped the arteries suppling the pancreas, stomach, duodenum, and liver by performing CTA. Effective localization of a gastrinoma using the SACI test is dependent on super-selective stimulation through appropriate arteries. In particular, the serum gastrin level increases when calcium gluconate flows into the stomach and duodenum, even in the absence of a gastrinoma. The RGA and RGEA supply the gastric prepylorus and duodenal bulbus. When diagnosing the presence of liver metastasis, calcium gluconate should be injected into an artery that the RGA or RGEA does not branch from, or it should be injected directly into the hepatic artery. The RGA is a branch of the common hepatic artery (CHA) and PHA in about 40% of individuals, of the LHA in 40.5%, the RHA in 5.5%, and the GDA in 8% (11). Because the RGA branches from the hepatic artery in about 50% of individuals, it is important to evaluate the artery that the RGA branches from before performing the SACI test, and to perform the SACI test with super-selective injection of calcium gluconate into specific arteries. In terms of the vascular anatomy of the RGEA, the GDA forms the RGEA after the ASPDA branches from the GDA. One way to improve the accuracy of the SACI test is to specifically inject calcium gluconate into the PSPDA and ASPDA, or into the GDA and RGEA. If the injections into the GDA and RGEA yield similar increases in serum gastrin levels, these changes are likely to be due to stimulation of normal gastrin-producing cells. In addition, by evaluating the territory supplied by each artery, the region into which calcium gluconate flows can be accurately determined. In this way, the lesion’s location can be accurately determined. Accurate diagnosis of localization is important in performing surgical treatment.
There are some limitations to the report. In particular, no clear cut-off value has been reported for the changes in serum gastrin levels due to stimulation of normal gastrin-producing cells. In other words, if there are high gastrin levels after injection of calcium gluconate into the RGA or RGEA, the possibility of a submucosal gastrinoma in the stomach or duodenum cannot be eliminated. One solution is to perform the SACI test by super-selective injection of calcium gluconate into both the RGA and RGEA, as in our case. If the serum gastrin levels increase similarly after injection into the RGA and RGEA, it is possible that the increase is due to stimulation of normal gastrin-producing cells.
In conclusion, it may be possible to improve the accuracy of SACI tests for localization of a gastrinoma by injecting calcium gluconate more selectively than in conventional SACI tests. Comprehensive evaluation of the vascular anatomy and the territory supplied by each artery using CTA is very helpful for localization of a gastrinoma.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/qims-20-1224). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Falconi M, Eriksson B, Kaltsas G, Bartsch DK, Capdevila J, Caplin M, Kos-Kudla B, Kwekkeboom D, Rindi G, Klöppel G, Reed N, Kianmanesh R, Jensen RT. Vienna Consensus Conference participants. ENETS Consensus Guidelines Update for the Management of Patients with Functional Pancreatic Neuroendocrine Tumors and Non-Functional Pancreatic Neuroendocrine Tumors. Neuroendocrinology 2016;103:153-71. [Crossref] [PubMed]
- Falconi M, Bartsch DK, Eriksson B, Klöppel G, Lopes JM, O'Connor JM, Salazar R, Taal BG, Vullierme MP, O'Toole D. Barcelona Consensus Conference participants. ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms of the digestive system: well-differentiated pancreatic non-functioning tumors. Neuroendocrinology 2012;95:120-34. [Crossref] [PubMed]
- Jensen RT, Cadiot G, Brandi ML, de Herder WW, Kaltsas G, Komminoth P, Scoazec JY, Salazar R, Sauvanet A, Kianmanesh R. Barcelona Consensus Conference participants. ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms: functional pancreatic endocrine tumor syndromes. Neuroendocrinology 2012;95:98-119. [Crossref] [PubMed]
- Pavel M, Baudin E, Couvelard A, Krenning E, Öberg K, Steinmüller T, Anlauf M, Wiedenmann B, Salazar R. Barcelona Consensus Conference participants. ENETS Consensus Guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology 2012;95:157-76. [Crossref] [PubMed]
- Lopez CL, Falconi M, Waldmann J, Boninsegna L, Fendrich V, Goretzki PK, Langer P, Kann PH, Partelli S, Bartsch DK. Partial pancreaticoduodenectomy can provide cure for duodenal gastrinoma associated with multiple endocrine neoplasia type 1. Ann Surg 2013;257:308-14. [Crossref] [PubMed]
- Imamura M, Komoto I, Ota S, Hiratsuka T, Kosugi S, Doi R, Awane M, Inoue N. Biochemically curative surgery for gastrinoma in multiple endocrine neoplasia type 1 patients. World J Gastroenterol 2011;17:1343-53. [Crossref] [PubMed]
- Okada K, Sudo T, Miyamoto K, Yokoyama Y, Sakashita Y, Hashimoto Y, Kobayashi H, Otsuka H, Sakoda T, Shimamoto F. The Selective Arterial Calcium Injection Test is a Valid Diagnostic Method for Invisible Gastrinoma with Duodenal Ulcer Stenosis: A Case Report. Hiroshima J Med Sci 2016;65:13-7. [PubMed]
- Hayashi R, Minami I, Sasahara Y, Izumiyama H, Yoshimoto T, Kishino M, Kudo A, Tateishi U, Tanabe M, Yamada T. Diagnostic accuracy of selective arterial calcium injection test for localization of gastrinoma. Endocr J 2020;67:305-15. [Crossref] [PubMed]
- Ray JM, Squires PE, Curtis SB, Meloche MR, Buchan AM. Expression of the calcium-sensing receptor on human antral gastrin cells in culture. J Clin Invest 1997;99:2328-33. [Crossref] [PubMed]
- Turner JJ, Wren AM, Jackson JE, Thakker RV, Meeran K. Localization of gastrinomas by selective intra-arterial calcium injection. Clin Endocrinol (Oxf) 2002;57:821-5. [Crossref] [PubMed]
- Michels NA. Blood supply and anatomy of the upper abdominal organs. With a descriptive atlas. Pitman Medical Publishing Co. Ltd, 1955:139-54.


