
Clinical and magnetic resonance imaging features of 14 patients with trilateral retinoblastoma
Introduction
Retinoblastoma is the most common intraocular childhood malignancy, with a prevalence of 1 in 18,000 children aged <5 years (1-5). It is initiated by a mutation of the retinoblastoma 1 (RB1) gene, which was the first described tumor-suppressor gene (2). Trilateral retinoblastoma (TRB) has been estimated to occur in 0.5–6% of patients with bilateral retinoblastoma (6-8); TRB refers to the development of a primary intracranial primitive neuroectodermal tumor in a patient with intraocular RB and was first described in 1971 by Jacobiec et al. (1,7,9-14). With rare exceptions, TRB is located in the pineal gland or the suprasellar or parasellar region, and the presence of a second midline tumor in the suprasellar or parasellar region is referred to as quadrilateral retinoblastoma (QRB). An associated midline intracranial tumor is not a metastasis, and it represents multifocal disease. However, the origin of intracranial tumors in RB patients is still uncertain (15). It has been hypothesized that intracranial tumors originate from ectopic foci of retinal cells in the third ventricle floor or from pineal photoreceptors, which are functionally and morphologically similar to retinal photoreceptors. The retina and pineal gland’s common photoreceptor origin may account for this susceptibility to coexistent disease (16).
The reported survival rate for patients with unilateral RB is approximately 93% at 5 years, and that for bilateral RB is approximately 92% at 5 years (15). TRB’s prognosis is much poorer, especially when the tumor spreads to the subarachnoid space (9,15). Yamanaka et al. conducted a systematic review of 72 TRB studies and found that the median survival for patients with a pineal tumor was 9.4 months, with a 5-year survival rate of 15.5%. The median survival was 12 months for patients with sellar tumors, and the 5-year survival rate was 17.8% (1,17). However, we have that TRB studies have mostly been based on case reports, and only a small number of articles have analyzed the survival or treatment of TRB patients as a single independent study.
This study retrospectively analyzed the clinical and imaging features of 14 participants with T/Q RB diagnosed in Xinhua Hospital Affiliated to Shanghai Jiaotong University School of Medicine from January 2012 to December 2019, aiming to understand the clinical features and magnetic resonance imaging (MRI) findings better and to improve the survival of TRB patients.
Methods
Participant population
We included 14 patients with T/Q RB among 1,392 patients with RB, who were initially diagnosed or underwent long-term follow-up at our hospital between January 2012 and December 2019. All of the participants’ parents had denied any history of RB. The Ethics Committee approved this study of Xinhua Hospital Affiliated to Shanghai Jiaotong University School of Medicine (XHEC-D-2020-124) and was conducted following the Declaration of Helsinki and International Ethical Guidelines for Health-related Research Involving Humans. Informed consent was provided by each subject or their responsible guardian.
Imaging protocols
The MRI was performed under sedation using a 3.0-T MR scanner (Signa HDx; GE Medical Systems, Milwaukee, WI, USA) with an 8-channel head coil. Axial unenhanced T1-weighted spin-echo images with a repetition time (TR)/echo time (TE) of 400–560/9–14 ms and T2-weighted fast spin-echo images (TR/TE of 3,000–3,700/80–110) with fat saturation were obtained. Images were obtained in ≥2 planes with a 3-mm slice thickness and a 0.5-mm interslice gap.
Diffusion-weighted imaging (DWI) images were acquired in the axial plane before administration of contrast medium using a single-shot echo-planar imaging sequence [TR/TE effective range of 3,200–5,000/70–100 ms; slice thickness of 3 mm; a gap of 0.5 mm; the field of view (FOV), 18–24 cm; matrix, 128×128]. The b-values corresponding to the diffusion-sensitizing gradient were 0 and 1,000 s/mm2 and was applied in 3 orthogonal directions.
After intravenous administration of 0.1 mmol/kg gadopentetate dimeglumine, we obtained the axial contrast-enhanced T1-weighted images (T1WI) with fat saturation of all participants (TR/TE of 400–575/13–15 ms; slice thickness of 2 mm; a gap of 0.5 mm; the field of view, 18–24 cm; matrix, 256×256). Oblique sagittal contrast-enhanced T1WI with fat saturation, parallel to the optic nerve, were also obtained using the same parameters. Axial contrast-enhanced T1-weighted images of the head without fat saturation were obtained with 5-mm section thicknesses and 1-mm intersection gaps.
Treatment
Chemotherapy was administered to 8 participants, and 6 participants had given up treatment due to treatment costs and poor prognostic expectations. Among the 8 participants who received chemotherapy, 6 received vincristine, cisplatin, cyclophosphamide, and etoposide; and 2 received high-dose chemotherapy and autologous hematopoietic stem cell rescue.
Results
Clinical characteristics
The incidence of TRB and QRB in our hospital was 1% (14/1,392) in all RB patients and 3.5% (14/403) in all bilateral RB patients. The clinical characteristics of the 14 participants are summarized in Tables 1,2, and Table S1. Briefly, there were 7 participants with pineal TRB (participants 1, 2, 8–11, and 13), 5 with suprasellar TRB (participants 3–5, 12, and 14), and 2 with QRB (participants 6 and 7). The age at first diagnosis of intraocular RB was 12.7±8.8 months. A total of 11 participants had TRB/QRB at the initial diagnosis of intraocular RB, at the age of 16.6±14.8 months; and 3 had a later onset at 37±19.1 months. The time from diagnosis of intraocular disease to later-onset TRB disease was 18.3±8.0 months. Only 2 participants with intraocular RB, at the ages of 6 and 26 months, had quadrilateral RB at initial diagnosis. The mean age at suprasellar TRB diagnosis was 9.1±5.6 months, compared with 22.2±18.4 months for pineal TRB.
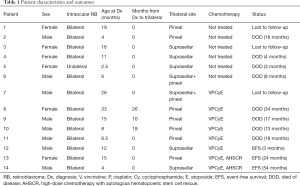
Full table
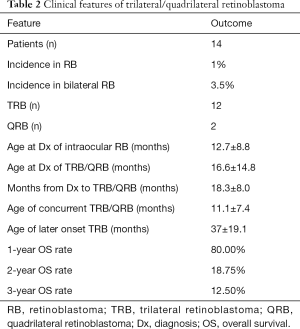
Full table
MRI characteristics of intraocular lesions
For the 11 patients who had intraocular RB associated with concurrent TRB/QRB, the affected 20 eyes (one was a unilateral lesion, and one eye was detected by ophthalmoscopy; participants 5 and 6, respectively) were classified according to the International Classification of Retinoblastoma (ICRB) as group A (n=1), group B (n=2), group C (n=2), group D (n=8), and group E (n=7). In group A (participant 6), the lesion was too small to be detected by MRI imaging and was detected only by ophthalmoscopy. The eyeball MRI in the other 4 groups showed glaucoma in 5 eyes, eyeball atrophy in 1 eye, and subretinal hemorrhage in 2 eyes. Intraocular tumors showed medium signal intensity in T1WI, heterogeneous low signal intensity in T2WI, intense enhancement in contrast-enhanced T1WI, and significant diffusion restriction in DWI with an apparent diffusion coefficient (ADC) of (0.619±0.22)×10−3 mm2/s (Table S1).
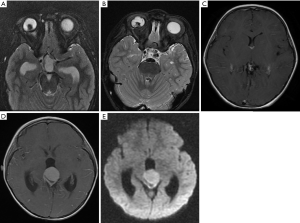
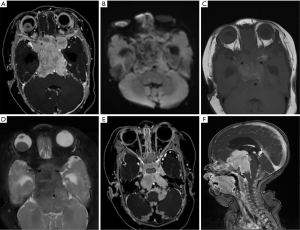
The 3 participants with later-onset pineal TRB were initially diagnosed with bilateral RB and underwent chemotherapeutic treatment. After progression-free survival (PFS) of 10–26 months, MRI images revealed that a pineal tumor had emerged, but the bilateral intraocular lesions remained stable (Figure 1).
MRI characteristics of intracranial lesions
The intracranial lesions of 7 participants were located in the pineal region (participants 1, 2, 8–11, and 13), 5 in the suprasellar area (participants 3–5, 12, and 14), and 2 in the pineal and suprasellar regions concurrently (participants 6 and 7). The MRI results of these lesions showed circumscribed round or oval masses with an average length of 17.4±10.4 mm. The intracranial lesions demonstrated homogeneous medium signal intensity or heterogeneously mixed signal intensity in T1WI/T2WI, owing to intratumoral hemorrhage or calcification, and intense enhancement in contrast-enhanced T1WI. The lesions were also investigated with DWI, and the results showed significant diffusion restriction with an ADC of (0.680±0.206)×10−3 mm2/s (Table S1).
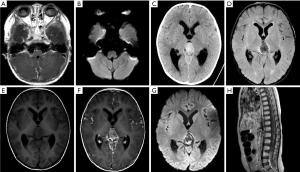
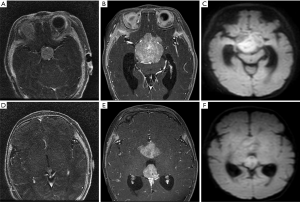
Leptomeningeal and cerebrospinal dissemination was found in 1 pineal TRB and 1 suprasellar TRB participant (participants 1 and 5; Figures 2 and 3; respectively).
Treatment outcome and overall survival (OS)
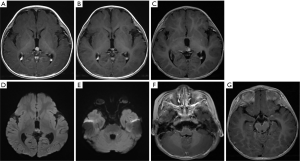
Among the 14 participants, 6 had given up any treatment due to high treatment costs, coupled with poor prognostic expectations (participants 1–6). Of these 6, 2 who had given up treatment had leptomeningeal and cerebrospinal dissemination at the time of origin; 1 was lost to follow-up (participant 1; Figure 2), and 1 died due to disease progression after 3 months (participant 5; Figure 3). The 3 remaining participants died 4–18 months after follow-up due to disease progression (participants 2, 4, and 6), including a QRB participant (participant 6; Figure 4), and 1 was lost to follow-up (participant 3).
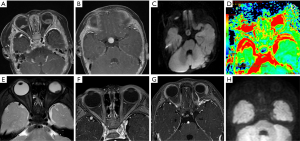
The remaining 8 participants received active chemotherapy (participants 7–14). Vincristine, cisplatin, cyclophosphamide, and etoposide was received by 6 participants (participants 7–12), and 2 simultaneously received high-dose autologous hematopoietic stem cell rescue (participants 13 and 14). Among these 8 participants, contact with 1 case of QRB was lost during the follow-up (participant 7). In the other 5 participants (participants 8–12), including 3 cases of later-onset pineal TRB, the intracranial tumors had significantly shrunk after standardized chemotherapy, and the intraocular lesions reached a stable stage (approximately 8–19 months). However, except for 1 participant (participant 12) who had undergone treatment and was followed up for only 3 months, these 4 participants died of tumor recurrence and cerebrospinal fluid dissemination during the continuous period (3–15 months) of follow-up (Figure 5). For the 2 participants who had received autologous hematopoietic stem cell rescue in addition to high-dose chemotherapy, the intraocular and intracranial lesions had all significantly shrunk, and they were all alive without recurrence for 24 and 54 months (participant 13 and 14, respectively, Figure 6). The 1-, 2-, and 3-year OS rates were 80%, 18.75%, and 12.5% in our study. There were no participant deaths due to chemotherapy.
Discussion
As the most common intraocular childhood malignancy, RB has a prevalence of 1 in 18,000 (1-4,9,18-20). It is caused by germinal and/or somatic mutations inactivating both alleles of RB1 on chromosome 13q14 (16). TRB/QRB is rare, with an incidence of 3% of all cases and 8–10% of hereditary cases (6-8,16). In our hospital, 12 participants with TRB, and 2 with QRB were found among 1,392 intraocular RB patients, and the incidence of TRB was 0.86% of all cases (12/1,392). The incidence of QRB was even lower than that of TRB; in the past 8 years, the incidence of QRB was 0.14% (2/1,392) in our hospital.
It has been reported in the literature that 95% of patients with TRB are aged <5 years. The average age at the initial diagnosis of intraocular tumors is 23–48 months, while the age at diagnosis of intracranial lesions is comparatively later, with an interval of approximately 21 months (14,21,22). The average age at first diagnosis of suprasellar TRB is earlier than that of pineal TRB by up to 12 months (1,11,22). In our study, all 14 participants were aged <3 years at first diagnosis, and intracranial lesions were also diagnosed at <3 years of age in 13 participants; only 1 patient was close to 5 years of age. Pineal and suprasellar TRB can occur simultaneously or can occur after the diagnosis of the intraocular tumor, and concurrent pineal or suprasellar TRB is always observed at a lower age, which may be because non-pineal TRB develops earlier than pineal disease and is, therefore, more likely to be diagnosed concurrently with RB (1,12,17). In our study, the participants with concurrent TRB/QRB were <30 months old, whereas it was 37 months of age for later-onset TRB participants.
Children with hereditary RB usually have multifocal, bilateral RB and/or a family history or constitutional RB1 mutation, whereas children with the somatic form generally have unilateral, unifocal disease, and negative family history (17). Approximately 89% of TRB patients have bilateral intraocular RB, and 11% have unilateral RB (1,8,14,23,24). In our study, 13 participants (92.9%) had bilateral RB, and 1 patient (7.1%) had unilateral RB; all declared a negative family history. It should be noted that 1 participant had been misdiagnosed with unilateral RB associated with suprasellar and pineal tumors by MRI, and the “normal” eye by MRI was diagnosed with punctate RB classified as Group A by ophthalmoscopy. Previous reports have demonstrated that all multifocal, bilateral RBs are hereditary and progress to TRB. Some researchers have recently shown that up to 15% of sporadic unilateral RB cases may be hereditary, and these patients may also be at risk for the development of a second primary tumor at the intracranial midline (8,25). In this study, a 2.5-month-old girl was diagnosed with unilateral RB and a concomitant suprasellar tumor and showed extensive intracranial cerebrospinal fluid dissemination. Therefore, all unilateral/bilateral RB patients must undergo routine MRI combined with ophthalmoscopy to prevent missed diagnoses of intraocular or intracranial tumors. In our hospital, we recommend a long term and scheduled a follow-up for all RB patients, especially those with TRB, as follows: once every 3 months in the first 2 years, once every 6 months after 3–5 years, and once a year after 5 years. The follow-up duration should be ≥5 years. During the follow-up period, the frequency should increase if the disease progresses. Concurrently, we optimized the orbital MRI scanning package for suspected or confirmed RB with a package including orbital T1WI, T2WI, DWI, orbital contrast-enhanced T1WI, and additional contrast-enhanced T1WI of the head, which is useful for the exclusion of pineal or suprasellar tumor and/or leptomeningeal dissemination.
The RB’s intracranial lesion mostly occurs in the pineal region and 25% in the suprasellar area (11,22). In our study, 50% (7/14) of intracranial tumors were located in the pineal region, 35.7% (5/14) in the suprasellar region, and 14.3% (2/14) in the pineal and suprasellar regions concurrently, which was classified as QRB. Similar to previously reported cases (7-9,11,14-16,21,26), intracranial lesions in our study showed a circumscribed round or oval mass in the pineal and/or suprasellar region. The lesions always demonstrated homogeneous medium signal intensity or heterogeneously mixed signal intensity in T1WI/T2WI owing to intratumoral hemorrhage or calcification, intense enhancement in contrast-enhanced T1WI, and significant diffusion restriction in DWI with a low ADC of (0.680±0.206)×10−3 mm2/s, which was similar to the ADC value of pineal TRB reported by Farouk Sait et al. (27). The incidence of TRB is low, and the ADC value of intracranial lesions in our study can be used as a reference for diagnosis or tumor monitoring.
Although the 5-year survival rate for patients with unilateral or bilateral RB is >92%, RB has a poor prognosis, with a 5-year survival of 15.7%, especially when the tumor spreads to the subarachnoid space (9,15,17). Patients with concurrent TRB have higher 5-year survival than those whose disease is diagnosed >3 months after TRB (47% vs. 5.8%, respectively) (1,28,29). The difference may be due to the longer time interval for detecting the later-onset intracranial lesions, which allows the increase of tumor volume, cerebrospinal fluid spread, and death rate. Limited by follow-up time, we can only calculate up to the 3-year OS rate for our participants. The 1-, 2-, and 3-year OS rates were 80%, 18.75%, and 12.5%, respectively. Due to the small number of cases, it was impossible to calculate the 5-year survival rate of concurrent and later-onset TRB. Although TRB’s 5-year survival rate is low, early detection of intracranial disease and increased use of high-dose chemotherapy, especially with stem cell rescue, can improve survival (1,17). In a previous study, all but one of the 22 long-term survivors received chemotherapy, and almost half underwent high-dose chemotherapy with stem cell rescue (1). In our study, 8 participants received chemotherapy. However, only the 2 participants who had simultaneously received high-dose chemotherapy and autologous hematopoietic stem cell rescue survived the whole follow-up period (24 and 54 months), which further demonstrated the great clinical benefit of autologous hematopoietic stem cell rescue.
Proton beam radiation therapy (PBRT) is a highly conformal type of radiation therapy and has gained increasing importance in oncology, especially for the treatment of pediatric tumors. The main benefit of protons over photons radiotherapy is the reduction of dose burden and, consequently, reducing the risk of long-term side effects. Several clinical studies have shown that PBRT for RB was effective even in patients suffering recurrence or showing resistance to chemotherapy, while few radiation-associated malignancies were noted (30-32). Therefore, PBRT seems to be an alternative disease-curing option for TRB patients, which may also reduce the risk of visual impairment and secondary malignancies in the foreseeable future.
In conclusion, TRB is rare and has a high mortality risk. Pineal or suprasellar TRB can occur simultaneously, or one can appear later. The intraocular and intracranial tumors showed similar MRI features with slightly different ADC values. Regular MRI scans and ophthalmoscopy for each RB patient should be used for early diagnosis and comprehensive assessment. High-dose chemotherapy with stem cell rescue can significantly improve patient survival.
Acknowledgments
Funding: This study was supported by the Science and Technology Commission of Shanghai Municipality (16411968300).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/qims-20-605). The authors have no conflicts of interest to declare.
Ethical Statement: The study was conducted in accordance with the Declaration of Helsinki and International Ethical Guidelines for Health-related Research Involving Humans. This study was approved by the Ethics Committee of Xinhua Hospital Affiliated to Shanghai Jiaotong University School of Medicine (XHEC-D-2020-124). Informed consent was provided by each participant or their responsible guardian.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- de Jong MC, Kors WA, de Graaf P, Castelijns JA, Kivela T, Moll AC. Trilateral retinoblastoma: a systematic review and meta-analysis. Lancet Oncol 2014;15:1157-67. [Crossref] [PubMed]
- Dimaras H, Kimani K, Dimba EA, Gronsdahl P, White A, Chan HS, Gallie BL. Retinoblastoma. Lancet 2012;379:1436-46. [Crossref] [PubMed]
- Chawla B, Sharma S, Sen S, Azad R, Bajaj MS, Kashyap S, Pushker N, Ghose S. Correlation between clinical features, magnetic resonance imaging, and histopathologic findings in retinoblastoma: a prospective study. Ophthalmology 2012;119:850-6. [Crossref] [PubMed]
- Rauschecker AM, Patel CV, Yeom KW, Eisenhut CA, Gawande RS, O’Brien JM, Ebrahimi KB, Daldrup-Link HE. High-resolution MR imaging of the orbit in patients with retinoblastoma. Radiographics 2012;32:1307-26. [Crossref] [PubMed]
- Cui Y, Luo R, Wang R, Liu H, Zhang C, Zhang Z, Wang D. Correlation between conventional MR imaging combined with diffusion-weighted imaging and histopathologic findings in eyes primarily enucleated for advanced retinoblastoma: a retrospective study. Eur Radiol 2018;28:620-9. [Crossref] [PubMed]
- Dunkel IJ, Jubran RF, Gururangan S, Chantada GL, Finlay JL, Goldman S, Khakoo Y, O’Brien JM, Orjuela M, Rodriguez-Galindo C, Souweidane MM, Abramson DH. Trilateral retinoblastoma: potentially curable with intensive chemotherapy. Pediatr Blood Cancer 2010;54:384-7. [Crossref] [PubMed]
- Provenzale JM, Weber AL, Klintworth GK, McLendon RE. Radiologic-pathologic correlation. Bilateral retinoblastoma with coexistent pinealoblastoma (trilateral retinoblastoma). AJNR Am J Neuroradiol 1995;16:157-65. [PubMed]
- Andrade GC, Pinto NP, Motono M, Chojniak MM, Chojniak R, Bezerra SM. Trilateral retinoblastoma with unilateral eye involvement. Rev Assoc Med Bras (1992) 2015;61:308-10. [Crossref] [PubMed]
- Provenzale JM, Gururangan S, Klintworth G. Trilateral retinoblastoma: clinical and radiologic progression. AJR Am J Roentgenol 2004;183:505-11. [Crossref] [PubMed]
- Richter S, Vandezande K, Chen N, Zhang K, Sutherland J, Anderson J, Han L, Panton R, Branco P, Gallie B. Sensitive and efficient detection of RB1 gene mutations enhances care for families with retinoblastoma. Am J Hum Genet 2003;72:253-69. [Crossref] [PubMed]
- Paulino AC. Trilateral retinoblastoma: is the location of the intracranial tumor important? Cancer 1999;86:135-41. [Crossref] [PubMed]
- Kivela T. Trilateral retinoblastoma: a meta-analysis of hereditary retinoblastoma associated with primary ectopic intracranial retinoblastoma. J Clin Oncol 1999;17:1829-37. [Crossref] [PubMed]
- Amare P, Jose J, Chitalkar P, Kurkure P, Pai S, Nair C, Advani S. Trilateral retinoblastoma with an RB1 deletion inherited from a carrier mother: a case report. Cancer Genet Cytogenet 1999;111:28-31. [Crossref] [PubMed]
- Marcus DM, Brooks SE, Leff G, McCormick R, Thompson T, Anfinson S, Lasudry J, Albert DM. Trilateral retinoblastoma: insights into histogenesis and management. Surv Ophthalmol 1998;43:59-70. [Crossref] [PubMed]
- James SH, Halliday WC, Branson HM. Best cases from the AFIP: Trilateral retinoblastoma. Radiographics 2010;30:833-7. [Crossref] [PubMed]
- D’Elia G, Grotta S, Del Bufalo F, De Ioris MA, Surace C, Sirleto P, Romanzo A, Cozza R, Locatelli F, Angioni A. Two novel cases of trilateral retinoblastoma: genetics and review of the literature. Cancer Genet 2013;206:398-401. [Crossref] [PubMed]
- Yamanaka R, Hayano A, Takashima Y. Trilateral retinoblastoma: A systematic review of 211 cases. Neurosurg Rev 2019;42:39-48. [Crossref] [PubMed]
- Corson TW, Gallie BL. One hit, two hits, three hits, more? Genomic changes in the development of retinoblastoma. Genes Chromosomes Cancer 2007;46:617-34. [Crossref] [PubMed]
- Burkhart DL, Sage J. Cellular mechanisms of tumour suppression by the retinoblastoma gene. Nat Rev Cancer 2008;8:671-82. [Crossref] [PubMed]
- Dimaras H, Khetan V, Halliday W, Orlic M, Prigoda NL, Piovesan B, Marrano P, Corson TW, Eagle RC Jr, Squire JA, Gallie BL. Loss of RB1 induces non-proliferative retinoma: increasing genomic instability correlates with progression to retinoblastoma. Hum Mol Genet 2008;17:1363-72. [Crossref] [PubMed]
- de Jong MC, Kors WA, de Graaf P, Castelijns JA, Moll AC, Kivela T. The Incidence of Trilateral Retinoblastoma: A Systematic Review and Meta-Analysis. Am J Ophthalmol 2015;160:1116-26 e5.
- Wright KD, Qaddoumi I, Patay Z, Gajjar A, Wilson MW, Rodriguez-Galindo C. Successful treatment of early detected trilateral retinoblastoma using standard infant brain tumor therapy. Pediatr Blood Cancer 2010;55:570-2. [Crossref] [PubMed]
- de Jong MC, de Graaf P, Brisse HJ, Galluzzi P, Goricke SL, Moll AC, Munier FL, Popovic MB, Moulin AP, Binaghi S, Castelijns JA, Maeder P. European Retinoblastoma Imaging C. The potential of 3T high-resolution magnetic resonance imaging for diagnosis, staging, and follow-up of retinoblastoma. Surv Ophthalmol 2015;60:346-55. [Crossref] [PubMed]
- de Jong MC, Moll AC, Goricke S, van der Valk P, Kors WA, Castelijns JA, de Graaf P. From a Suspicious Cystic Pineal Gland to Pineoblastoma in a Patient with Familial Unilateral Retinoblastoma. Ophthalmic Genet 2016;37:116-8. [PubMed]
- Ibarra MS, O’Brien JM. Is screening for primitive neuroectodermal tumors in patients with unilateral retinoblastoma necessary? J AAPOS 2000;4:54-6. [Crossref] [PubMed]
- Shah I, Baig A, Razzaq A, Faruqi A, Ali A, Khan FQ. Trilateral retinoblastoma with unilateral eye involvement. J Pak Med Assoc 2013;63:910-2. [PubMed]
- Farouk Sait S, Haque S, Karimi S, Rebeiz KJ, Francis JH, Marr BP, Abramson DH, Souweidane MM, Dunkel IJ. A Potential Role For Apparent Diffusion Coefficient in the Diagnosis of Trilateral Retinoblastoma. J Pediatr Hematol Oncol 2020;42:238-43. [PubMed]
- Kivela T. The epidemiological challenge of the most frequent eye cancer: retinoblastoma, an issue of birth and death. Br J Ophthalmol 2009;93:1129-31. [Crossref] [PubMed]
- Kaliki S, Srinivasan V, Gupta A, Mishra DK, Naik MN. Clinical features predictive of high-risk retinoblastoma in 403 Asian Indian patients: a case-control study. Ophthalmology 2015;122:1165-72. [Crossref] [PubMed]
- Jung EH, Kim JH, Kim JY, Jo DH, Yu YS. Outcomes of Proton Beam Radiation Therapy for Retinoblastoma With Vitreous Seeds. J Pediatr Hematol Oncol 2018;40:569-73. [Crossref] [PubMed]
- Thomas H, Timmermann B. Paediatric proton therapy. Br J Radiol 2020;93:20190601. [Crossref] [PubMed]
- Chang JW, Yu YS, Kim JY, Shin DH, Choi J, Kim JH, Kim SJ. The clinical outcomes of proton beam radiation therapy for retinoblastomas that were resistant to chemotherapy and focal treatment. Korean J Ophthalmol 2011;25:387-93. [Crossref] [PubMed]


