
The characteristic computed tomography findings of pulmonary B-cell non-Hodgkin’s lymphoma and their role in predicting patient survival
Introduction
Extranodal non-Hodgkin’s lymphoma (NHL) with primary lung involvement comprises less than 3% of all extranodal lymphomas (1). Primary pulmonary lymphoma (PPL), which originates from the pulmonary parenchyma or bronchi with or without hilar lymph node involvement, is an extremely rare primary lung tumor and lymphoma (2). Secondary pulmonary lymphoma (SPL), which occurs more frequently than PPL, is characterized by secondary involvement of the lung with systemic lymphoma.
More than 95% of PPLs originate from B-cells, with the most common pathological type being mucosa-associated lymphoid tissue (MALT) lymphoma (2). Among SPLs, the most common subtype is diffuse large B-cell lymphoma (DLBCL). The primary subtype of SPL is strongly related to clinical behavior. More aggressive subtypes exhibit more extensive findings at presentation and rapidly progress (3). Finally, MALT the most common subtype of PPL has a more favorable clinical outcome than SPL (4,5).
Owing to its capacity to evaluate both nodal and extranodal involvement, computed tomography (CT) covering the chest, abdomen, and pelvis was considered to be a basic diagnostic investigative tool for pulmonary lymphoma in the National Comprehensive Cancer Network Guidelines of 2015 (6). CT also has the advantage of accurately staging disease and follow-up treatment responses (7-9).
Previous studies have validated the use of CT findings in both the diagnosis of PPL and in differentiating it from SPL (3,10,11). However, to our knowledge, few studies have concentrated on the prognostic role of CT features in patients with pulmonary B-cell NHL. Therefore, this study aimed to evaluate the value of characteristic CT findings in predicting the survival of patients with pulmonary B-cell NHL.
Methods
Study population
This study retrospectively enrolled 84 patients who were histopathologically confirmed with pulmonary B-cell NHL lymphoma in Shanghai Jiao Tong University School of Medicine Affiliated Ruijin Hospital between April 2004 and October 2018. Enrolled patients met the following criteria: (I) histopathologically confirmed with pulmonary B-cell NHL; (II) underwent chest CT scan at initial diagnosis; (III) received systemic chemotherapy; (IV) with complete clinical and follow-up data. Patient follow-up data were obtained through out-patient visits or telephone interviews. The study received approval from the Ethics Committee of Ruijin Hospital Affiliated to the Shanghai Jiao Tong University School of Medicine. Due to the retrospective nature of the study, the requirement for informed consent was waived.
Analysis of the initial and follow-up data revealed that all patients were treated with rituximab, either alone (n=5) or combined with CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone, n=69), COP (cyclophosphamide, vincristine, and prednisone, n=8), or FC (fludarabine and cyclophosphamide, n=2). Two patients with primary MALT and one with secondary DLBCL also received radiotherapy, and one patient with secondary DLBCL was also treated with lenalidomide. The 45 males and 39 females included in this study had a median age of 59 years, with an age range of 15–81 years. Among them, 36 were diagnosed with PPL (MALT, n=29, DLBCL, n=7), and 48 with SPL (DLBCL, n=33, MALT, n=6, follicular lymphoma, n=3, Burkitt lymphoma, n=2, mantle cell lymphoma, n=2, small lymphocytic lymphoma, n=2).
Image analysis
The initial chest CT scans were performed by using one of the following scanners: LightSpeed 16 (GE Healthcare, Chicago, IL, USA), LightSpeed VCT 64 (GE Healthcare, Chicago, IL, USA), SOMATOM Definition Flash (Siemens Healthcare, Erlangen, Germany), or Aquilion ONE (Toshiba Medical Systems, Otawara, Japan). Scans covered the region from the top of the thoracic cage to the level of bilateral adrenal glands and 49 patients underwent a contrast-enhanced CT scan (non-ionic contrast medium, 60–80 mL). The scanning protocols were as follows: 120 kVp; 150–300 mAs; slice thickness, 5.0–7.5 mm. All images were viewed with both lung window (window width, 1,500 HU; window level, −500 HU) and mediastinal window (window width, 350 HU; window level, 50 HU).
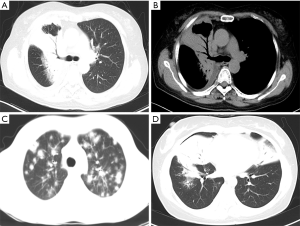
A series of imaging features defined by radiologists demonstrated the characteristics of the lung, lymph nodes, and pleura. These features included the number of lung lesions (single or multiple), air bronchogram (12) (Figure 1A,B; air-filled bronchi through the consolidation or nodular/mass lesions, the halo sign (13) (Figure 1C; a solid nodule surrounded by a halo of ground-glass attenuation), cross-lobe sign (11) (Figure 1D; a consolidative lesion invading the interlobar fissure distributed along multiple lung lobes), hilar/mediastinal lymphadenopathy (LAP) (14) (shorter diameter >10 mm), and pleural involvement (pleural thickening including pleural nodularity or diffuse thickening and/or pleural effusion). The scans of patients in this study were reviewed by two experienced radiologists (ZC Pan and Q Song, with 5 and 24 years in diagnostic imaging, respectively). The observers were blinded to the clinical and pathological diagnoses of the patients and disagreements were resolved by consensus.
Statistical analysis
All statistical analyses were performed with Statistical Package for the Social Sciences (SPSS) 25.0 software (SPSS Inc., Chicago, USA). Differences in the characteristic CT findings and clinicopathological features of patients were compared with Chi-squared or Fisher’s exact tests.
Overall survival (OS) was measured from the date of diagnosis to the date of death or the date of the last follow-up (30 April, 2019). Progression-free survival (PFS) was calculated from the date when treatment began to the date of first disease progression, relapse, or death, or the date of last follow-up. The data of patients with no progression or who were still alive at the date of the last follow-up were right censored. For univariate analysis, the Kaplan-Meier method was used to estimate survival. Imaging features that appeared to be significant in the univariate analyses were incorporated into multivariate analyses to determine which variables were independently associated with survival. Cox regression was used to estimate the hazard ratio (HR) and its confidence interval (CI). A P value <0.05 was considered to be statistically significant.
Results
Clinicopathological features
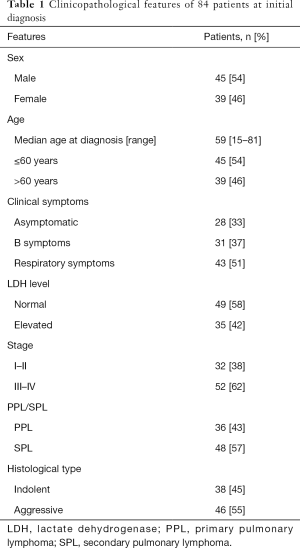
The patients’ clinicopathological features are summarized in Table 1. Twenty-eight patients who underwent routine radiographic detection of pulmonary lesions presented with no clinical symptoms. B symptoms were present in 31 patients, while 43 patients had non-specific respiratory symptoms, such as cough, chest pain, and hemoptysis at initial diagnosis. Forty-nine (58%) patients had normal serum lactate dehydrogenase (LDH) levels and 52 (62%) presented with advanced-stage disease (stages III–IV). Indolent and aggressive histological types accounted for 45% and 55% of all cases, respectively.
Full table
Differences in characteristic CT findings and clinicopathological features
To compare CT findings between patients with various clinicopathological features, the patients were divided into groups according to CT characteristics based on whether single or multiple lung lesions were present, and whether air bronchogram, halo sign, cross-lobe sign, pleural involvement, and hilar/mediastinal LAP were present or absent. The patient clinicopathological features assessed included sex, age, clinical symptoms, LDH level, PPL or SPL, histological type, and stage. Patients were staged according to the Ann Arbor classification into the early (I and II) or advanced (III and IV) stage (15). PPL was staged according to the Ann Arbor stages modified by Ferraro et al. (5) as follows: stage IE: unilateral or bilateral lung presentation; stage IIE: lung presentation and hilar and/or mediastinal lymph node involvement; stage IIEW: lung presentation and adjacent chest wall or diaphragm involvement; stage III: lung presentation and involvement of lymph nodes below diaphragm; and stage IV: diffused involvement of one or more extralymphatic organs or tissues.
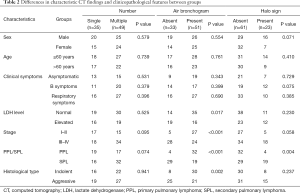
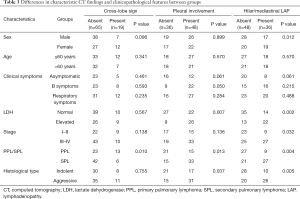
The differences in characteristic CT findings and clinicopathological features between groups are shown in Tables 2,3.
Full table
Full table
Comparisons of sex, age, clinical symptoms, LDH level, stage, PPL or SPL, and histological type between the single and multiple lung lesion groups showed no significant differences (all P>0.05).
The presence of air bronchogram in patients with early-stage disease, PPL, and the indolent histological type was higher than that among those with advanced-stage disease, SPL, and the aggressive histological type (P<0.001, P<0.001, and P=0.002, respectively). Thirty-five patients with air bronchogram had normal LDH levels, while elevated levels were present in 16 patients (P=0.017). In terms of sex, age, and clinical symptoms, no significant differences were found between the two groups (all P>0.05).
The halo sign was observed more in the SPL group (19/48, 40%) than in the PPL group (4/36, 11%; P=0.004), and cross-lobe sign was observed in 13 patients (36%) with PPL and 6 patients (13%) with SPL (P=0.010). Other clinicopathological features showed no significant differences between patients with or without the halo and cross-lobe signs (all P>0.05).
Twenty-six patients with pleural involvement and 22 with hilar/mediastinal LAP had an elevated LDH level (P=0.007, and P=0.002, respectively). The presence of pleural involvement and hilar/mediastinal LAP in patients with SPL and the aggressive histological type were higher than those in patients with PPL and the indolent histological type (P=0.013 and P=0.037; P=0.004 and P=0.005, respectively). Advanced-stage patients presented with hilar/mediastinal LAP more frequently than early-stage patients (P=0.032).
The role of characteristic CT findings in predicting survival
At a median follow-up of 26 months (range, 2–154 months), relapse or progression of disease occurred in 37 patients, with an average time of 33 months (range, 2–154 months), and death occurred in 21 cases, with an average time of 41 months (range, 2–154 months). The median PFS was 24 months (range, 2–154 months), the median OS was 26 months (range, 2–154 months), and the estimated 2-year PFS and OS rates of patients with pulmonary B-cell NHLs were 61% and 76%, respectively.
In the univariate analyses, the number of lung lesions, cross-lobe sign, pleural involvement, and hilar/mediastinal LAP were significantly correlated with PFS (P=0.040, P=0.012, P<0.001, and P=0.023, respectively), and the halo sign and pleural involvement were independent prognostic factors for OS (P=0.010 and P=0.007).
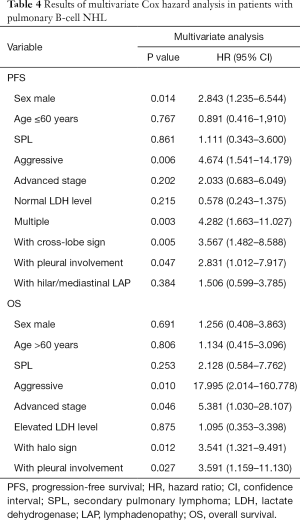
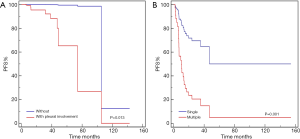
In the multivariate analyses (Table 4, Figures 2-4), the number of lung lesions, cross-lobe sign, and pleural involvement were found to be independent prognostic factors for PFS (P=0.003, P=0.005, and P=0.047, respectively), and the halo sign and pleural involvement were significantly correlated with OS (P=0.012 and P=0.027). In the multivariate analyses for PPL, pleural involvement was shown to be an independent prognostic factor for PFS (P=0.013), and the number of lung lesions was significantly correlated with PFS in patients with aggressive pulmonary B-cell NHLs (P=0.001).
Full table
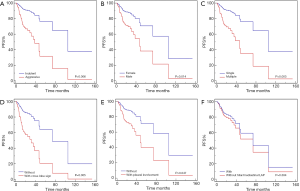
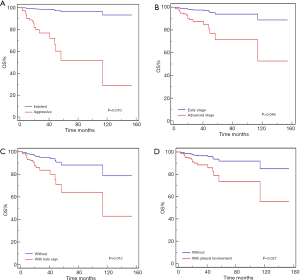
The outcomes of patients with the aggressive histological type were significantly worse than those of patients with the indolent histological type (2-year PFS: 40% versus 90%, 2-year OS: 57% versus 100%, all P<0.001). A 2-year OS of 61%, OS was found to be lower in patients at an advanced stage than in patients at an early stage (P=0.002). Furthermore, the 2-year PFS of male patients was 51%, which was lower than that of female patients (72%; P<0.001).
No other clinicopathological features (age, serum LDH level, PPL, and SPL) were found to contribute to the survival outcome.
Discussion
The results of this study show that air bronchogram occurred more frequently in patients with early-stage disease, PPL, and the indolent histological type, while the halo sign was observed more frequently in those with SPL and the cross-lobe sign more frequently in patients with PPL. Pleural involvement and hilar/mediastinal LAP were observed more frequently in patients with SPL and the aggressive histological type. The survival analyses revealed that the number of lung lesions, cross-lobe sign, and pleural involvement were independent prognostic factors for PFS, while the halo sign and pleural involvement were significantly correlated with OS.
Air bronchogram is regarded as a remarkable CT finding for pulmonary lymphoma. Chen et al. (11) reported this sign to be more commonly observed in patients with primary pulmonary MALT lymphoma than in those with non-MALT lymphoma, due to differences in biological invasiveness. In our study, air bronchogram presented more frequently in patients with PPL than in those with SPL, which was in line with previous studies. We speculate that PPL, of which MALT is the most common subtype, infiltrates along the peribronchovascular interstitium without invading the vascular wall, while SPL, which includes more aggressive subtypes of lymphoma, develops rapidly and can invade the pulmonary vessels at an early stage.
The halo sign is an area of ground-glass attenuation surrounding a pulmonary nodule (13). It is an early and specific sign for diagnosing invasive pulmonary aspergillosis, which is characterized pathologically by foci of infarction surrounded by alveolar hemorrhage (16). In our study, this sign was found more frequently in patients with SPL than in the PPL group. In pulmonary lymphoma, this sign represents tumor-cell infiltration of the surrounding interstitium, which is related to the invasiveness of the disease. Cross-lobe sign occurred more frequently in patients with PPL, and there was no difference in its distribution between primary pulmonary MALT lymphoma and non-MALT lymphoma, which was also in accordance with the findings of a previous study (11). A previous study found that pleural involvement, which is determined by pleural thickening and/or pleural effusion, rarely occurred in patients with PPL11. In our study, the rate of pleural involvement was higher in patients with SPL and the aggressive histological type. Compared with PPL, SPL is the secondary involvement of systemic lymphoma and more frequent. Malignant lymphoid cells from direct mediastinal node extension or from lymphatic or hematogenous dissemination from distant sites reach the pulmonary parenchyma (3). This may explain why hilar/mediastinal LAP was more commonly observed in patients with SPL in our study.
The presence of multiple lesions has been found to indicate a poor prognosis in several different malignancies in previous studies (17-19). Tirtei et al. (17), for instance, reported that osteosarcoma patients with a single pulmonary metastasis displayed better 5-year post-relapse survival than those with multiple metastases. Studying the survival outcomes of patients with primary melanoma, Rowe et al. (18) found that patients with multiple lesions were more at risk of death from melanoma. Our study showed that patients with multiple lung lesions had a worse PFS than those with single lesions, which had not been reported previously in pulmonary lymphoma. We speculate that the higher burden of tumors may result in a worse outcome for patients.
In our study, air bronchogram was not found to be significantly correlated with OS or PFS; however, previous studies (20,21) have suggested that it could be an independent prognostic factor for other malignancies. Onoda et al. (20) found that air bronchogram was a favorable prognostic factor for pulmonary pleomorphic carcinoma. They speculated that air bronchogram may indicate a less aggressive tumor, as it shows that the intratumoral bronchi have remained intact, without destruction by tumor invasion or expansion. Supporting this, Yoshino et al. (21) reported that patients without air bronchogram for stage I pulmonary adenocarcinoma had a worse prognosis than those with it.
Patients with the halo sign had a shorter OS than patients without it in this study. The presence of this sign is related to disease invasiveness; however, there are no reports of its prognostic role in patients with pulmonary lymphoma. Greene et al. (22) found that the initiation of antifungal treatment based on the identification of a halo sign on chest CT was related to a significantly better treatment response and improved survival, although Mucha et al. (23) suggested that a thoracic halo sign in patients with post-transplant lymphoproliferative disease might reflect a worse prognosis.
In the present study, patients with cross-lobe sign tended to relapse or progress and this sign was shown to be an independent prognostic factor for PFS. We speculate that infiltration of the lesion through the interlobar fissure to the adjacent lobe is associated with high interstitial invasiveness. However, to date, few reports have focused on the prognostic role of cross-lobe sign, and more studies on its role in predicting the survival of patients with B-cell lymphoma with lung involvement are needed.
Patients with pleural involvement have poor clinical outcomes (24-27). Our study revealed that pleural involvement is an independent prognostic factor for PFS and OS in both patients with pulmonary B-cell NHLs and the PPL subgroup. El-Galaly et al. (24) reported that pleural involvement in patients with DLBCL was associated with an inferior OS and PFS. Chang et al. (25) studied the clinical outcomes of patients with lymphoblastic lymphoma. They found that the presence of pleural effusion was a poor prognostic factor for OS and PFS, and suggested that it might reflect tumor burden. Other studies (26,27) have also demonstrated poor prognosis in patients with pleural effusion at presentation.
In our study, hilar/mediastinal LAP was shown to be related to PFS in the univariate analysis, but it was not an independent prognostic factor in the multivariate analysis. Hu et al. (28) studied prognostic factors in 22 Chinese patients with PPL and found that hilar/mediastinal LAP was an independent prognostic factor in the Cox-regression analysis. However, Kurtin et al. (29) reported that lymph node involvement was not associated with an adverse outcome in patients with pulmonary MALT lymphoma. The prognostic role of regional LAP in several malignancies has been considered in previous studies (30,31) and reviews (32). Cahoon et al. (32) indicated that understaging of internal thoracic LAP in patients with breast cancer significantly impacted patient outcomes.
Disease stage was an independent prognostic factor for OS in our study, and an advanced stage indicated a worse prognosis. However, the role of stage classification in predicting survival is controversial. Olszewski et al. (33) reported that advanced-stage patients with marginal zone lymphoma had poor outcomes. Other reports (34-36) suggested that an advanced stage of disease might be associated with poor prognosis in patients with lymphoma of various pathological types. However, while Ueda et al.’s study involving patients with advanced extranodal MALT lymphoma (37) revealed a shorter PFS in univariate analysis, subsequent multivariate analysis failed to confirm this finding.
The current study also confirmed that histological type (indolent or aggressive) was significantly correlated with OS and PFS, and found that male patients had a worse outcome than females, which has not been reported previously.
There are some limitations to this study. The first of these is the small sample size and the retrospective study design. Secondly, a detailed pathological study was absent to be correlated to the characteristic CT findings. Therefore, further investigation using larger samples with a detailed radiological-pathological correlation study is needed to confirm our results. Thirdly, our study used different CT equipment. Finally, different pathological subtypes of pulmonary B-cell NHLs were involved, and each subtype may have different clinicopathological features and treatment response. However, this problem was unavoidable, due to the limitations associated with a retrospective study. Despite these limitations, to the best of our knowledge, the present study is the first large series of pulmonary B-cell NHLs investigated through an analysis of characteristic CT findings and their possible role in predicting survival.
Conclusions
In conclusion, the halo sign and pleural involvement are independent prognostic factors for OS, and the number of lung lesions, cross-lobe sign, and pleural involvement are correlated with PFS.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/qims-20-1139). The authors have no conflicts of interest to declare.
Ethical Statement: The Institutional Review Board of the hospital approved this retrospective study and waived the requirement for informed patient consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lal A, Bhurgri Y, Vaziri I, Rizvi NB, Sadaf A, Sartajuddin S, Islam M, Kumar P, Adil S, Kakepoto GN, Masood N, Khurshed M, Alidina A. Extranodal non-Hodgkin's lymphomas-a retrospective review of clinico-pathologic features and outcomes in comparison with nodal non-Hodgkin's lymphomas. Asian Pac J Cancer Prev 2008;9:453-8. [PubMed]
- Piña-Oviedo S, Weissferdt A, Kalhor N, Moran CA. Primary Pulmonary Lymphomas. Adv Anat Pathol. 2015;22:355-75. [Crossref] [PubMed]
- Bligh MP, Borgaonkar JN, Burrell SC, MacDonald DA, Manos D. Spectrum of CT Findings in Thoracic Extranodal Non-Hodgkin Lymphoma. Radiographics 2017;37:439-61. [Crossref] [PubMed]
- Sirajuddin A, Raparia K, Lewis VA, Franks TJ, Dhand S, Galvin JR, White CS. Primary Pulmonary Lymphoid Lesions: Radiologic and Pathologic Findings. Radiographics 2016;36:53-70. [Crossref] [PubMed]
- Ferraro P, Trastek VF, Adlakha H, Deschamps C, Allen MS, Pairolero PC. Primary non-Hodgkin's lymphoma of the lung. Ann Thorac Surg 2000;69:993-7. [Crossref] [PubMed]
- National Comprehensive Cancer Network (NCCN) guideline in Hodgkin and non-Hodgkin lymphoma of oncology (version 2, 2015). Available online: https://www.nccn.org. Accessed 26 April 2016.
- Castellino RA, Hilton S, O'Brien JP, Portlock CS. Non-Hodgkin lymphoma: contribution of chest CT in the initial staging evaluation. Radiology 1996;199:129-32. [Crossref] [PubMed]
- Gómez León N, Delgado-Bolton RC, Del Campo Del Val L, Cabezas B, Arranz R, García M, Cannata J, González Ortega S, Pérez Sáez MÁ, López-Botet B, Rodríguez-Vigil B, Mateo M, Colletti PM, Rubello D, Carreras JL. Multicenter Comparison of Contrast-Enhanced FDG PET/CT and 64-Slice Multi-Detector-Row CT for Initial Staging and Response Evaluation at the End of Treatment in Patients with Lymphoma. Clin Nucl Med 2017;42:595-602. [Crossref] [PubMed]
- Kulkarni NM, Pinho DF, Narayanan S, Kambadakone AR, Abramson JS, Sahani DV. Imaging for Oncologic Response Assessment in Lymphoma. AJR Am J Roentgenol 2017;208:18-31. [Crossref] [PubMed]
- Dong Y, Zeng M, Zhang B, Han L, Liu E, Lian Z, Liu J, Liang C, Zhang S. Significance of imaging and clinical features in the differentiation between primary and secondary pulmonary lymphoma. Oncol Lett 2017;14:6224-30. [Crossref] [PubMed]
- Chen Y, Chen A, Jiang H, Zhang Y, Zhu L, Xia C, Yu H. HRCT in primary pulmonary lymphoma: can CT imaging phenotypes differentiate histological subtypes between mucosa-associated lymphoid tissue (MALT) lymphoma and non-MALT lymphoma? J Thorac Dis 2018;10:6040-9. [Crossref] [PubMed]
- Algın O, Gökalp G, Topal U. Signs in chest imaging. Diagn Interv Radiol 2011;17:18-29. [PubMed]
- Primack SL, Hartman TE, Lee KS, Müller NL. Pulmonary nodules and the CT halo sign. Radiology 1994;190:513-5. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Lister TA, Crowther D, Sutcliffe SB, Glatstein E, Canellos GP, Young RC, Rosenberg SA, Coltman CA, Tubiana M. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin's disease: Cotswolds meeting. J Clin Oncol 1989;7:1630-6. [Crossref] [PubMed]
- Lee YR, Choi YW, Lee KJ, Jeon SC, Park CK, Heo JN. CT halo sign: the spectrum of pulmonary diseases. Br J Radiol 2005;78:862-5. [Crossref] [PubMed]
- Tirtei E, Asaftei SD, Manicone R, Cesari M, Paioli A, Rocca M, Ferrari S, Fagioli F. Survival after Second and Subsequent Recurrences in Osteosarcoma: A Retrospective Multicenter Analysis. Tumori 2018;104:202-6. [Crossref] [PubMed]
- Rowe CJ, Law MH, Palmer JM, MacGregor S, Hayward NK, Khosrotehrani K. Survival outcomes in patients with multiple primary melanomas. J Eur Acad Dermatol Venereol 2015;29:2120-7. [Crossref] [PubMed]
- Paulsson AK, Holmes JA, Peiffer AM, Miller LD, Liu W, Xu J, Hinson WH, Lesser GJ, Laxton AW, Tatter SB, Debinski W, Chan MD. Comparison of clinical outcomes and genomic characteristics of single focus and multifocal glioblastoma. J Neurooncol 2014;119:429-35. [Crossref] [PubMed]
- Onoda H, Kimura T, Tao H, Okabe K, Matsumoto T, Ikeda E. Air bronchogram in pleomorphic carcinoma of the lung is associated with favorable prognosis. Thorac Cancer 2018;9:718-25. [Crossref] [PubMed]
- Yoshino I, Nakanishi R, Kodate M, Osaki T, Hanagiri T, Takenoyama M, Yamashita T, Imoto H, Taga S, Yasumoto K. Pleural retraction and intra-tumoral air-bronchogram as prognostic factors for stage I pulmonary adenocarcinoma following complete resection. Int Surg 2000;85:105-12. [PubMed]
- Greene RE, Schlamm HT, Oestmann JW, Stark P, Durand C, Lortholary O, Wingard JR, Herbrecht R, Ribaud P, Patterson TF, Troke PF, Denning DW, Bennett JE, de Pauw BE, Rubin RH. Imaging findings in acute invasive pulmonary aspergillosis: clinical significance of the halo sign. Clin Infect Dis 2007;44:373-9. [Crossref] [PubMed]
- Mucha K, Foroncewicz B, Palczewski P, Sułkowska K, Ziarkiewicz-Wróblewska B, Orłowski T, Gołębiowski M, Pączek L. Pulmonary post-transplant lymphoproliferative disorder with a CT halo sign. Ann Transplant 2013;18:482-7. [Crossref] [PubMed]
- El-Galaly TC, Villa D, Alzahrani M, Hansen JW, Sehn LH, Wilson D, de Nully Brown P, Loft A, Iyer V, Johnsen HE, Savage KJ, Connors JM, Hutchings M. Outcome prediction by extranodal involvement, IPI, R-IPI, and NCCN-IPI in the PET/CT and rituximab era: A Danish-Canadian study of 443 patients with diffuse-large B-cell lymphoma. Am J Hematol 2015;90:1041-6. [Crossref] [PubMed]
- Chang MH, Kim SJ, Kim K, Oh SY, Lee DH, Huh J, Ko YH, Choi CW, Yang DH, Won JH, Kim WS, Suh C. Clinical features and treatment outcomes of adult B- and T-lymphoblastic lymphoma: results of multicentre analysis in Korea. Leuk Lymphoma 2009;50:1119-25. [Crossref] [PubMed]
- McCarten KM, Metzger ML, Drachtman RA, Pei Q, Friedman DL, Schwartz CL, Kelly KM. Significance of pleural effusion at diagnosis in pediatric Hodgkin lymphoma: a report from Children's Oncology Group protocol AHOD0031. Pediatr Radiol 2018;48:1736-44. [Crossref] [PubMed]
- Kirn D, Mauch P, Shaffer K, Pinkus G, Shipp MA, Kaplan WD, Tung N, Wheeler C, Beard CJ, Canellos GP, et al. Large-cell and immunoblastic lymphoma of the mediastinum: prognostic features and treatment outcome in 57 patients. J Clin Oncol 1993;11:1336-43. [Crossref] [PubMed]
- Hu YH, Hsiao LT, Yang CF, Chiou TJ, Liu JH, Gau JP, Yen CC, Chou TY, Hsu WH, Chen PM, Tzeng CH. Prognostic factors of Chinese patients with primary pulmonary non-Hodgkin's lymphoma: the single-institute experience in Taiwan. Ann Hematol 2009;88:839-46. [Crossref] [PubMed]
- Kurtin PJ, Myers JL, Adlakha H, Strickler JG, Lohse C, Pankratz VS, Inwards DJ. Pathologic and clinical features of primary pulmonary extranodal marginal zone B-cell lymphoma of MALT type. Am J Surg Pathol 2001;25:997-1008. [Crossref] [PubMed]
- Dell'Oglio P, Larcher A, Muttin F, Di Trapani E, Trevisani F, Ripa F, Carenzi C, Briganti A, Salonia A, Montorsi F, Bertini R, Capitanio U. Lymph node dissection should not be dismissed in case of localized renal cell carcinoma in the presence of larger diseases. Urol Oncol 2017;35:662.e9-662.e15. [Crossref] [PubMed]
- McIntosh LJ, O'Neill AC, Bhanusupriya S, Matalon SA, Van den Abbeele AD, Ramaiya NH, Shinagare AB. Prognostic significance of supradiaphragmatic lymph nodes at initial presentation in patients with stage III high-grade serous ovarian cancer. Abdom Radiol (NY) 2017;42:2513-20. [Crossref] [PubMed]
- Cahoon AR, Smith BD, Yang WT. Internal Thoracic Lymphadenopathy in Breast Cancer. Radiographics 2017;37:1024-36. [Crossref] [PubMed]
- Olszewski AJ, Castillo JJ. Survival of patients with marginal zone lymphoma: analysis of the Surveillance, Epidemiology, and End Results database. Cancer 2013;119:629-38. [Crossref] [PubMed]
- Hwang JP, Lim I, Byun BH, Kim BI, Choi CW, Lim SM. Prognostic value of SUVmax measured by pretreatment 18F-FDG PET/CT in patients with primary gastric lymphoma. Nucl Med Commun 2016;37:1267-72. [Crossref] [PubMed]
- Han X, Duan M, Hu L, Zhou D, Zhang W. Plasmablastic lymphoma: Review of 60 Chinese cases and prognosis analysis. Medicine (Baltimore) 2017;96:e5981. [Crossref] [PubMed]
- Lu CH, Lee KF, Chen CC, Chen YY, Huang CE, Tsai PS, Tsou HY, Chou HJ, Chen MF, Chen PT, Lee KD, Lung J. Clinical characteristics and treatment outcome in a Taiwanese population of patients with Epstein-Barr virus-positive diffuse large B-cell lymphoma. Jpn J Clin Oncol 2014;44:1164-71. [Crossref] [PubMed]
- Ueda K, Terui Y, Yokoyama M, Sakajiri S, Nishimura N, Tsuyama N, Takeuchi K, Hatake K. Non-gastric advanced mucosa-associated lymphoid tissue (MALT) lymphoma has worse prognosis than gastric MALT lymphoma even when treated with rituximab-containing chemotherapy. Leuk Lymphoma 2013;54:1928-33. [Crossref] [PubMed]


