
The effect of ApoE ε 4 on clinical and structural MRI markers in prodromal Alzheimer’s disease
Introduction
Alzheimer’s disease (AD) is a common progressive neurodegenerative disorder characterized by distinct brain pathological changes, including amyloid β (Aβ) accumulation, neurofibrillary tangle deposition, synaptic dysfunction, and neuronal death with gross brain atrophy (1,2). Mild cognitive impairment (MCI) is conceptualized as an intermediate state (3) between healthy aging and clinical dementia. Individuals with MCI are often at an increased risk of developing dementia (4). MCI is regarded as a prodromal stage of AD (5) and a better therapeutic time window for AD clinical trials. Furthermore, diagnoses of AD and MCI based on clinical criteria have limited sensitivity and specificity compared with autopsy (6). Approximately 50% of subjects with clinically diagnosed MCI show amyloid-negative (Aβ-) which is insufficient to represent prodromal AD (7). Prodromal AD requires Aβ plaques and fibrillar tau.
The ε 4 allele of apolipoprotein E (ApoE) is the most significant genetic risk factor for sporadic AD (8,9). Some studies have demonstrated that carriers of the ApoE ε 4 allele who have AD dementia or amnestic MCI predominantly show memory impairment and medial temporal lobe atrophy, particularly involving the hippocampus compared with non-carriers (10-16). However, previous research on ApoE ε 4 of AD has been limited by the absence of biomarker-based confirmation of AD diagnosis, which makes it possible that subjects have been included in research with false-positive diagnoses of AD.
In 2018, the National Institute on Aging and Alzheimer’s Association (NIA-AA) proposed a new research framework focusing on diagnoses of AD with biomarkers for living persons (17). The scheme [which is labeled AT (N)] is based on grouping biomarkers into 3 categories: β amyloid deposition (A), pathologic tau (T), and neurodegeneration (N) (17). Aβ biomarkers can determine whether an individual is in the Alzheimer’s continuum or not. Pathologic tau biomarkers can determine whether someone in the Alzheimer’s continuum has AD, as deposits of both Aβ and paired helical filament (PHF) tau are required to fulfill neuropathologic criteria for AD (18,19). With AT (N) biomarkers playing a vital role in AD research, we focused on biologically diagnosed prodromal AD. Our main goal was, therefore, to evaluate the impacts of ApoE ε 4 on clinical cognition, cerebrospinal fluid (CSF) biomarkers, and neuroimaging regions in prodromal AD. We hypothesized that ApoE ε 4 carriers would be associated with differences in cognitive profiles and cerebral atrophy patterns.
Methods
Alzheimer’s Disease Neuroimaging Initiative (ADNI) and study participants
Data were downloaded from the ADNI in August 2016 (adni.loni.usc.edu). The ADNI database was launched in 2003 as a public-private partnership, led by its principal investigator, Michael W. Weiner, MD. The primary goal of ADNI was to identify the optimal combinations of serial magnetic resonance imaging (MRI), positron emission tomography (PET), CSF, and neuropsychological assessment to test the progression of MCI and early AD. For more up-to-date information, see www.adni-info.org. For the present study, we selected 780 ADNI-GO/2 participants meeting the criteria for MCI who had Mini-Mental State Examination (MMSE) scores of 24–30, a clinical dementia rating score of 0.5, subjective memory complaint and objective memory loss, and the absence of other neuropsychiatric disorders (20). More specifically, all selected participants had undergone lumbar puncture, 18F-fluorodeoxyglucose PET (FDG-PET), Florbetapir-PET-AV45, structural MRI scanning, and neuropsychological assessments. We excluded MCI cases attributed to non-AD, such as medication and demyelination disease, unknown or uncertain etiology, aging, small vessel disease, stress, depression, and subjects without complete information. In total, we included only 178 A+T+MCI participants with positive biomarkers of Aβ plaques and fibrillar tau, as described above.
Ethics approval and consent to participate
The ADNI study ethical approval was given by the institutional review boards of all participating institutions. All participants or authorized representatives provided written informed consent.
Neuropsychological assessment
Neuropsychological tests were performed by certified raters using standardized ADNI protocols (www.adni-info.org). The Rey Auditory Verbal Learning Test (RAVLT) (21) and the ADNI composite scores for memory (ADNI-MEM) (22) were used to measure memory. For evaluation of executive function, Trail Making Test (TMT) parts A and B (23) were used. For language function, Category Fluency Tests (24) and the Boston Naming Test (BNT) were conducted; to assess global cognition, the MMSE (21), Clinical Dementia Rating Sum of Boxes (CDR-SB), Alzheimer’s Disease Assessment Scale Cognitive subscale (ADAS-Cog) consisting of 11 (ADAS-Cog11) and 13 items (ADAS-Cog13), Montreal Cognitive Assessment (MoCA) (25), and the Functional Assessment Questionnaire (FAQ) were used. Clock tests were included to evaluate visuospatial ability.
ApoE genotyping
ApoE genotypes were determined using standard polymerase chain reaction methods, which have been described previously (26). Individuals with 1 or 2 copies of allele 4 were designated as ε 4-carriers (ε 4 +); individuals with no allele 4 were designated as non-carriers (ε 4 −). ApoE ε 4 genotyping methods are described at http://www.adni-info.org.
CSF data
As previously mentioned, CSF Aβ1-42, total tau (t-Tau), and p-Tau were measured using the multiplex xMAPLuminex platform (Luminex) with Innogenetics (INNO-BIA AlzBio3) immunoassay kit-based reagents (27). The CSF data used in this article were obtained from the ADNI files “UPENNBIOMK5-8.csv”. Further details of ADNI methods for CSF acquisition, measurements, and quality control procedures are available online (http://adni.loni.usc.edu/). To select subjects with fibrillar tau (T+), we set the CSF cut-off point at 23 pg/mL for p-Tau181p, as previous studies described (27).
FDG data
FDG-PET was determined as a sum of mean glucose metabolism averaged across 5 regions of interest (ROIs) (i.e., right and left angular gyri, bilateral posterior cingulate, right and left inferior temporal gyri) (28). In addition to the composite FDG-PET, we also considered measurements for separate FDG-ROIs labeled as an “AD signature meta-ROI” (i.e., right and left angular, right and left temporal, bilateral cingulum post) (29). The FDG data used in this study were obtained from the ADNI file “UCBERKELEYFDG_07_30_15.csv”.
Florbetapir-PET-AV45
To calculate the amyloid burden, we analyzed SUVR means of Florbetapir-PET-AV45 (anterior and posterior cingulate, precuneus, prefrontal, orbitofrontal, parietal, temporal cortices) (30). Previous studies have proven that PET quantitation might be preferable for accurate selection and therapeutic monitoring of individuals in clinical trials (31,32). Participants were classified as Aβ-positive (A+) or Aβ-negative (A−) according to the SUVR cutoff of 1.1 for amyloid positivity. Data on cortical amyloid burden were obtained from the ADNI file “UCBERKELEYAV45_06_15_16.csv”. Further details of ADNI methods for image acquisition and processing can be found at www.adni-info.org/methods.
Structural MRI
All ADNI MRI scans in this study were acquired at multiple sites using GE, Philips, and Siemens. All 3 available T MRI scans were collected for each subject using a sagittal MPRAGE sequence, and the raw DICOM images were obtained from the public ADNI site. Parameter values may vary depending on the scanning site, which can be downloaded at http://www.loni.ucla.edu/ADNI/Research/Cores. All MRI scans were processed, with little to no manual intervention using the segmentation module of the FreeSurfer software, as described in previous reports (33-35).
In the present study, we used ROIs analysis to calculate differences between ApoE ε 4-carriers and non-carriers. In total, 107 ROIs were automatically segmented according to the label on the Jacob atlas defined by FreeSurfer (36), and then non-carriers and ε 4-carriers groups were compared. At last, 7 ROIs that could significantly influence MCI progression were selected,. The 7 ROIs included the average cortical thickness of the right rostral anterior cingulate and bilateral entorhinal, subcortical volume of the left amygdala, bilateral hippocampus, and left ventral diencephalon (DC). The structural MRI neuroimaging data were downloaded from the ADNI file “UCSFFSL_11_02_15” and “UCSFFSX51_11_02_15_V2”.
Statistical analysis
Continuous variables are shown as mean ± SD, and categorical variables are represented as frequencies (percentages). Comparisons between the two groups on continuous variables were performed with independent-samples t-tests, and categorical variables were analyzed with chi-squared tests. Wilcoxon rank-sum test was used when the variance did not satisfy the normality or homogeneity.
A multivariate analysis of variance was separately used to assess differences in cognitive scores and ROIs of structural MRI between study groups. The effects of age, gender, and years of education were adjusted for all analyses of covariance (ANCOVAs). ROIs of structural MRI and cognitive measures that statistically differed between both groups were further explored with linear regression models, adjusted for age, gender, education, and ApoE ε 4 status.
Furthermore, longitudinal associations between ApoE ε 4 status and cognitive measures were assessed by linear mixed models using the lme4 package of R software. Separate models were fitted for ApoE ε 4 status in relation to each dependent cognitive assessment. The longitudinal effect of ApoE ε 4 status on cognitive performance was estimated by the two-way interaction between time and ApoE ε 4 status, with adjustment for confounders (age, gender, and education).
All statistical analyses were conducted with IBM SPSS and R software. The analysis of variance with P<0.05 was considered statistically significant.
Results
Subject demographics and sample characteristics
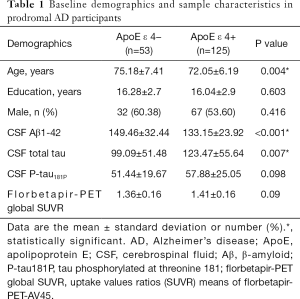
Demographics and sample characteristics of the study population, including age, gender, educational level, SUVR means of Florbetapir-PET-AV45, and CSF biomarker values (Aβ1-42, t-Tau, and p-Tau) are presented in Table 1.
Full table
ApoE ε 4 (+) prodromal AD participants were slightly younger (72.05±6.19 versus 75.18±7.41, P=0.004), with significantly lower levels of CSF Aβ 1-42 (P<0.001) and higher levels of t-Tau (P=0.007), compared to ApoE ε 4 (−) prodromal AD subjects. The CSF concentration of p-Tau was numerically higher in ApoE ε 4 (+) prodromal AD participants, but the difference was not significant. There were no significant differences in gender, year of education, and amyloid burden measured by Florbetapir-PET-AV45.
Effects of ApoE ε 4 on FDG data
There were no differences in glucose metabolism measured by FDG-PET between ApoE ε 4 carriers and non-carriers, adjusted for age, sex, and year of education. FDG-PET in regional brain regions (the right and left angular gyri, bilateral posterior cingulate, and right and left inferior temporal gyri) also did not differ by ApoE ε 4 status (data not shown).
Effects of ApoE ε 4 on cognition
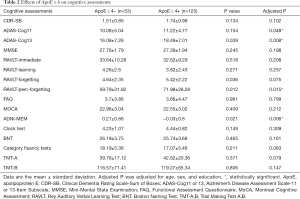
ApoE ε 4 status showed significant differences in cognitive assessments, as highlighted in Table 2.
Full table
We found that ApoE ε 4 (+) prodromal AD participants had worse results on global cognition and memory-related tasks. ApoE ε 4 was not associated with differences in executive function, visuospatial ability, and language domains.
More specifically, ApoE ε 4 (+) prodromal AD participants had higher mean RAVLT-perc-forgetting score (71.98±28.28 versus 59.79±31.82, P=0.015), lower mean ADNI-MEM score (−0.03±0.6 versus 0.21±0.66, P=0.006), higher mean ADAS-Cog11 score (11.22±4.77 versus 10.08±5.04, P=0.048), and higher mean ADAS-Cog13 score (18.49±7.01 versus 16.08±7.26, P=0.008). These analyses were adjusted for age, sex, and year of education.
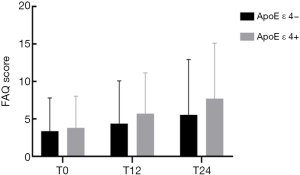
Longitudinal FAQ data were available in a subset of the subjects (Figure 1). Significant interactions between ApoE ε 4 allele status and time were identified for the FAQ score. Possession of the ApoE ε 4 allele was accompanied by an added annual increase of 1.5796 points on the FAQ score.
Effects of ApoE ε 4 on brain structure
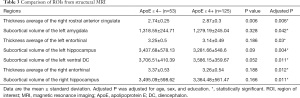
We evaluated differences in 107 ROIs obtained from structural MRI between the ApoE ε 4 carriers and non-carriers, and 7 of them had significant differences. ApoE ε 4 (+) prodromal AD participants showed thinner cortical thickness in the bilateral entorhinal, along with smaller subcortical volume of the left amygdala, bilateral hippocampus and left ventral DC, while ApoE ε 4 (−) prodromal AD participants showed thinner cortical thickness in the right rostral anterior cingulate (2.74±0.25 versus 2.87±0.3, P=0.006). Details of these findings are provided in Table 3. These analyses were adjusted for age, sex, and year of education.
Full table
Regional atrophy-cognition relationships
To better understand how cognitive differences relate to underlying neuroanatomy, linear regression models were created. We evaluated independent effects of ROIs in structural MRI (cortical thickness average of the right rostral anterior cingulate and bilateral entorhinal; the subcortical volume of the left amygdala, bilateral hippocampus, and left ventral DC) on cognitive assessments (ADAS-Cog11, ADAS-Cog13, RAVLT-perc-forgetting, and ADNI-MEM). The ApoE ε 4 status, age, sex, and years of education were included in models to avoid the effect of ApoE ε 4 group differences driving these correlations. These results are shown in Table 4. The results indicate that the subcortical volumes of the left amygdala and bilateral hippocampus, along with the cortical thickness average of the bilateral entorhinal, were highly correlated with global cognition (ADAS-Cog11, ADAS-Cog13) and memory measures (RAVLT-perc-forgetting, ADNI-MEM). The cortical thickness average of the bilateral entorhinal had the most significant effect on global cognition and memory performance.
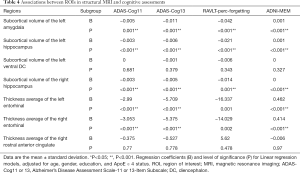
Full table
Discussion
Our goal was to provide a comprehensive evaluation of the effects of ApoE ε 4 carrier status on CSF biomarkers (Aβ 1-42, t-Tau, and p-Tau levels), glucose metabolism measured by FDG–PET, clinical cognitive performances, and neurodegeneration (atrophy on structural MRI) in prodromal AD participants. The major findings regarding effects the of ApoE ε 4 genotype in MCI individuals with Aβ plaques and fibrillar tau biomarkers were as follows: (I) ApoE ε 4 (+) prodromal AD participants had lower CSF Aβ 1-42 levels and higher t-Tau levels, compared with ApoE ε 4 (−) participants; (II) ApoE ε 4 (+) prodromal AD participants had worse global cognition and memory performance, more rapid progression of global cognitive decline, and thinner cortical thickness in the bilateral entorhinal, along with smaller subcortical volume of the left amygdala, bilateral hippocampus, and left ventral DC, compared to ApoE ε 4 (−) prodromal AD subjects; (III) significant associations among memory, global cognition, and cortical thickness in bilateral entorhinal were found. In line with previous findings on ApoE ε 4 (37-39), our results indicate a significant ApoE ε 4-dependent heterogeneity in AD, with differences in clinical characteristics, biochemistry, and brain structure. Importantly, we showed that specific MRI regions have correlated cognitive domains which will be helpful for precise assessment in prodromal AD.
The most important advance relative to prior studies was that we conducted the current study in MCI participants with Aβ plaques and fibrillar tau biomarkers based on the AT (N) system from the 2018 NIA-AA research framework. Although many previous studies have detected the effects of ApoE ɛ 4 in AD, their diagnostic criteria were based on clinical classification, which ignored the pathologic changes of AD at autopsy (40,41). Around 10–30% of clinically diagnosed AD patients did not show any AD neuropathologic changes at autopsy (40). In the current study, only MCI participants with Aβ plaques and fibrillar tau biomarkers were included in order to significantly mitigate the concern of misdiagnosis that might occur in samples defined on the clinical basis. In this way, it was hoped that the conclusion made concerning the clinical characteristics of the MCI individuals would be more accurate.
Previous research has shown that the ApoE ε 4 genotype plays an important role in Aβ metabolism (42). In the present study, we demonstrated that prodromal AD participants carrying the ApoE ε 4 allele had lower CSF Aβ1-42 levels than those without the ε 4 allele, despite have an amyloid burden with similar levels to those measured by Florbetapir-PET-AV45. ApoE ε 4-associated cerebral Aβ deposition might be illustrated by the ApoE ε 4 allele being associated with enhanced Aβ deposition into plaques and the differentially regulated clearance of Aβ from the brain (43-46).
Many studies have associated the presence of the ApoE ε 4 allele with both reduced cognitive performance and accelerated cognitive decline in AD, MCI, and cognitively healthy subjects (16,47,48). Memory deficits were often considered to be a major cognitive impairment in AD, and the ApoE ε 4 genotype might modify the clinical phenotype of AD. In the present study, we found that prodromal AD participants carrying the ApoE ε 4 allele had inferior performance in global cognition and memory. This finding confirms a previous study on clinically diagnosed MCI subjects, seemingly resembling subjects in the early stages of AD in terms of cognition and memory (16). Furthermore, longitudinal studies revealed that the ApoE ε 4 genotype is related to a more rapid decline in global cognition.
Cerebral atrophy is one of the gross pathologic features of AD. Structural imaging based on MRI has been widely used for the prediction of patients with AD or MCI. Medial temporal lobe substructures, which include the entorhinal cortex, amygdala, and hippocampus, are the earliest regions of the brain to show AD-related neurodegeneration. Atrophy later spreads to the temporal lobe, and the parietal and frontal cortices. Several studies have shown important associations between brain atrophy measured using MRI and cognitive impairments in people with MCI and AD. MR volumetric studies have indicated that the memory impairment in subjects with MCI and AD have demonstrated a close relation to the degeneration of the medial temporal lobe, especially in the entorhinal cortex and hippocampus (49-51). By studying the relationship between structural MRI and cognitive impairment, we found that the bilateral entorhinal region was most significantly associated with cognitive function. An earlier study showed extended associations between verbal episodic memory and diencephalon shrinkage in AD, while our results also showed a smaller subcortical volume of left ventral DC in ApoE ε 4 (+) prodromal AD participants. Yet, an association between the left ventral DC and memory has not been observed, at least in the present prodromal AD participants (52). Thus, our results confirm the complexity of the relationship between the ApoE ε 4 genotype and MRI markers of neurodegeneration, and future studies should further clarify the effects of ApoE ε 4 and other candidate gene variants on MRI biomarkers, which in turn may help elucidate the roles of genetic factors in the neuropathology of AD.
There are several potential limitations to this study. First, only baseline MRI scans were analyzed in the current study. Future investigation on the association of longitudinal measures of cognitive decline and brain atrophy is warranted. Second, a small sample size had amyloid PET scans, and this might have affected the accuracy. Third, we specifically limited our study to those subjects who had already been clinically diagnosed as MCI. Thus, the study group might not be generalizable to all settings or populations. Finally, there was insufficient β-amyloid plaque density in some AD clinical phenotypes. The cause of their dementia is not investigated as a part of this research, but may be clarified in later research. Further studies are needed to support the present findings with larger sample sizes.
Conclusions
In summary, we found that ApoE ε 4 (+) prodromal AD participants had more memory and global cognitive impairment, more rapid memory decline, and greater brain atrophy. The findings highlight the heterogeneity in prodromal AD and the need for molecular diagnostics of the disease. ApoE ε 4 genotypes seem to be an important contributor to the heterogeneity of AD, and this may have implications on therapeutic targets for AD prevention.
Acknowledgments
Funding: The study was funded by the Shandong Provincial Key Research and Development Project (2018GSF118235). Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and the Department of Defense (DOD) (award number W81XWH-12-2-0012). The ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd. and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Ng 4Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: All procedures performed in studies involving the ADNI participants were conducted following the Helsinki declaration. The ADNI study was approved by the institutional review boards of all of the participating institutions. Informed written consent was obtained from all participants and their legal representatives at each site before the collection of clinical, genetic, and imaging data.
References
- Tiraboschi P, Hansen LA, Thal LJ, Corey-Bloom J. The importance of neuritic plaques and tangles to the development and evolution of AD. Neurology 2004;62:1984-9. [Crossref] [PubMed]
- Jack CR Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, Shaw LM, Vemuri P, Wiste HJ, Weigand SD, Lesnick TG, Pankratz VS, Donohue MC, Trojanowski JQ. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol 2013;12:207-16. [Crossref] [PubMed]
- Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med 2004;256:183-94. [Crossref] [PubMed]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999;56:303-8. [Crossref] [PubMed]
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:270-9. [Crossref] [PubMed]
- Beach TG, Monsell SE, Phillips LE, Kukull W. Accuracy of the clinical diagnosis of Alzheimer disease at National Institute on Aging Alzheimer Disease Centers, 2005-2010. J Neuropathol Exp Neurol 2012;71:266-73. [Crossref] [PubMed]
- Wolk DA, Price JC, Saxton JA, Snitz BE, James JA, Lopez OL, Aizenstein HJ, Cohen AD, Weissfeld LA, Mathis CA, Klunk WE, De-Kosky ST. Amyloid imaging in mild cognitive impairment subtypes. Ann Neurol 2009;65:557-68. [Crossref] [PubMed]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science 1993;261:921-3. [Crossref] [PubMed]
- Bertram L, Lill CM, Tanzi RE. The genetics of Alzheimer disease: back to the future. Neuron 2010;68:270-81. [Crossref] [PubMed]
- Agosta F, Vossel KA, Miller BL, Migliaccio R, Bonasera SJ, Filippi M, Boxer AL, Karydas A, Possin KL, Gorno-Tempini ML. Apolipoprotein E epsilon4 is associated with disease-specific effects on brain atrophy in Alzheimer's disease and frontotemporal dementia. Proc Natl Acad Sci U S A 2009;106:2018-22. [Crossref] [PubMed]
- Troyer AK, Murphy KJ, Anderson ND, Craik FI, Moscovitch M, Maione A, Gao F. Associative recognition in mild cognitive impairment: relationship to hippocampal volume and apolipoprotein E. Neuropsychologia 2012;50:3721-8. [Crossref] [PubMed]
- Hashimoto M, Yasuda M, Tanimukai S, Matsui M, Hirono N, Kazui H, Mori E. Apolipoprotein E epsilon 4 and the pattern of regional brain atrophy in Alzheimer's disease. Neurology 2001;57:1461-6. [Crossref] [PubMed]
- Geroldi C, Pihlajamaki M, Laakso MP, DeCarli C, Beltramello A, Bianchetti A, Soininen H, Trabucchi M, Frisoni GB. APOE-epsilon4 is associated with less frontal and more medial temporal lobe atrophy in AD. Neurology 1999;53:1825-32. [Crossref] [PubMed]
- Lehtovirta M, Laakso MP, Soininen H, Helisalmi S, Mannermaa A, Helkala EL, Partanen K, Ryynanen M, Vainio P, Hartikainen P, et al. Volumes of hippocampus, amygdala and frontal lobe in Alzheimer patients with different apolipoprotein E genotypes. Neuroscience 1995;67:65-72. [Crossref] [PubMed]
- Pievani M, Rasser PE, Galluzzi S, Benussi L, Ghidoni R, Sabattoli F, Bonetti M, Binetti G, Thompson PM, Frisoni GB. Mapping the effect of APOE epsilon4 on gray matter loss in Alzheimer's disease in vivo. Neuroimage 2009;45:1090-8. [Crossref] [PubMed]
- Farlow MR, He Y, Tekin S, Xu J, Lane R, Charles HC. Impact of APOE in mild cognitive impairment. Neurology 2004;63:1898-901. [Crossref] [PubMed]
- Jack CR Jr, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Sperling R. Contributors. NIA-AA Research Framework: Toward a biological definition of Alzheimer's disease. Alzheimers Dement 2018;14:535-62. [Crossref] [PubMed]
- Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Thies B, Trojanowski JQ, Vinters HV, Montine TJ. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease. Alzheimers Dement 2012;8:1-13. [Crossref] [PubMed]
- Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Trojanowski JQ, Vinters HV, Hyman BT. National Institute on A, Alzheimer's A. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol 2012;123:1-11. [Crossref] [PubMed]
- Bondi MW, Edmonds EC, Jak AJ, Clark LR, Delano-Wood L, McDonald CR, Nation DA, Libon DJ, Au R, Galasko D, Salmon DP. Neuropsychological criteria for mild cognitive impairment improves diagnostic precision, biomarker associations, and progression rates. J Alzheimers Dis 2014;42:275-89. [Crossref] [PubMed]
- Wolk DA, Dickerson BC. Alzheimer's Disease Neuroimaging I. Fractionating verbal episodic memory in Alzheimer's disease. Neuroimage 2011;54:1530-9. [Crossref] [PubMed]
- Crane PK, Carle A, Gibbons LE, Insel P, Mackin RS, Gross A, Jones RN, Mukherjee S, Curtis SM, Harvey D, Weiner M, Mungas D. Alzheimer's Disease Neuroimaging I. Development and assessment of a composite score for memory in the Alzheimer's Disease Neuroimaging Initiative (ADNI). Brain Imaging Behav 2012;6:502-16. [Crossref] [PubMed]
- Reitan RM. The relation of the trail making test to organic brain damage. J Consult Psychol 1955;19:393-4. [Crossref] [PubMed]
- Butters N, Granholm E, Salmon DP, Grant I, Wolfe J. Episodic and semantic memory: a comparison of amnesic and demented patients. J Clin Exp Neuropsychol 1987;9:479-97. [Crossref] [PubMed]
- Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695-9. [Crossref] [PubMed]
- Saykin AJ, Shen L, Foroud TM, Potkin SG, Swaminathan S, Kim S, Risacher SL, Nho K, Huentelman MJ, Craig DW, Thompson PM, Stein JL, Moore JH, Farrer LA, Green RC, Bertram L, Jack CR Jr, Weiner MW. Alzheimer's Disease Neuroimaging I. Alzheimer's Disease Neuroimaging Initiative biomarkers as quantitative phenotypes: Genetics core aims, progress, and plans. Alzheimers Dement 2010;6:265-73. [Crossref] [PubMed]
- Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, Blennow K, Soares H, Simon A, Lewczuk P, Dean R, Siemers E, Potter W, Lee VM, Trojanowski JQ. Alzheimer's Disease Neuroimaging I. Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Ann Neurol 2009;65:403-13. [Crossref] [PubMed]
- Landau SM, Harvey D, Madison CM, Koeppe RA, Reiman EM, Foster NL, Weiner MW, Jagust WJ. Alzheimer's Disease Neuroimaging I. Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol Aging 2011;32:1207-18. [Crossref] [PubMed]
- Jagust WJ, Bandy D, Chen K, Foster NL, Landau SM, Mathis CA, Price JC, Reiman EM, Skovronsky D, Koeppe RA. Alzheimer's Disease Neuroimaging I. The Alzheimer's Disease Neuroimaging Initiative positron emission tomography core. Alzheimers Dement 2010;6:221-9. [Crossref] [PubMed]
- Landau SM, Mintun MA, Joshi AD, Koeppe RA, Petersen RC, Aisen PS, Weiner MW, Jagust WJ. Alzheimer's Disease Neuroimaging I. Amyloid deposition, hypometabolism, and longitudinal cognitive decline. Ann Neurol 2012;72:578-86. [Crossref] [PubMed]
- Ben Bouallègue F, Mariano-Goulart D, Payoux P. Alzheimer's Disease Neuroimaging I. Comparison of CSF markers and semi-quantitative amyloid PET in Alzheimer's disease diagnosis and in cognitive impairment prognosis using the ADNI-2 database. Alzheimers Res Ther 2017;9:32. [Crossref] [PubMed]
- Spallazzi M, Barocco F, Michelini G, Morelli N, Scarlattei M, Baldari G, Ruffini L, Caffarra P. The Incremental Diagnostic Value of [18F]Florbetaben PET and the Pivotal Role of the Neuropsychological Assessment in Clinical Practice. J Alzheimers Dis 2019;67:1235-44. [Crossref] [PubMed]
- Risacher SL, Kim S, Shen L, Nho K, Foroud T, Green RC, Petersen RC, Jack CR Jr, Aisen PS, Koeppe RA, Jagust WJ, Shaw LM, Trojanowski JQ, Weiner MW, Saykin AJ. Alzheimer's Disease Neuroimaging Initiative d. The role of apolipoprotein E (APOE) genotype in early mild cognitive impairment (E-MCI). Front Aging Neurosci 2013;5:11. [Crossref] [PubMed]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 1999;9:179-94. [Crossref] [PubMed]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage 1999;9:195-207. [Crossref] [PubMed]
- Bryant C, Giovanello KS, Ibrahim JG, Chang J, Shen D, Peterson BS, Zhu H. Alzheimer's Disease Neuroimaging I. Mapping the genetic variation of regional brain volumes as explained by all common SNPs from the ADNI study. PLoS One 2013;8:e71723. [Crossref] [PubMed]
- Wolk DA, Dickerson BC. Alzheimer's Disease Neuroimaging I. Apolipoprotein E (APOE) genotype has dissociable effects on memory and attentional-executive network function in Alzheimer's disease. Proc Natl Acad Sci U S A 2010;107:10256-61. [Crossref] [PubMed]
- Ossenkoppele R, Cohn-Sheehy BI, La Joie R, Vogel JW, Moller C, Lehmann M, van Berckel BN, Seeley WW, Pijnenburg YA, Gorno-Tempini ML, Kramer JH, Barkhof F, Rosen HJ, van der Flier WM, Jagust WJ, Miller BL, Scheltens P, Rabinovici GD. Atrophy patterns in early clinical stages across distinct phenotypes of Alzheimer's disease. Hum Brain Mapp 2015;36:4421-37. [Crossref] [PubMed]
- Smits LL, Pijnenburg YA, van der Vlies AE, Koedam EL, Bouwman FH, Reuling IE, Scheltens P, van der Flier WM. Early onset APOE E4-negative Alzheimer's disease patients show faster cognitive decline on non-memory domains. Eur Neuropsychopharmacol 2015;25:1010-7. [Crossref] [PubMed]
- Nelson PT, Head E, Schmitt FA, Davis PR, Neltner JH, Jicha GA, Abner EL, Smith CD, Van Eldik LJ, Kryscio RJ, Scheff SW. Alzheimer's disease is not "brain aging": neuropathological, genetic, and epidemiological human studies. Acta Neuropathol 2011;121:571-87. [Crossref] [PubMed]
- Barnes LL, Leurgans S, Aggarwal NT, Shah RC, Arvanitakis Z, James BD, Buchman AS, Bennett DA, Schneider JA. Mixed pathology is more likely in black than white decedents with Alzheimer dementia. Neurology 2015;85:528-34. [Crossref] [PubMed]
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergstrom M, Savitcheva I, Huang GF, Estrada S, Ausen B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, Langstrom B. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol 2004;55:306-19. [Crossref] [PubMed]
- Holtzman DM, Bales KR, Tenkova T, Fagan AM, Parsadanian M, Sartorius LJ, Mackey B, Olney J, McKeel D, Wozniak D, Paul SM. Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A 2000;97:2892-7. [Crossref] [PubMed]
- Holtzman DM. Role of apoe/Abeta interactions in the pathogenesis of Alzheimer's disease and cerebral amyloid angiopathy. J Mol Neurosci 2001;17:147-55. [Crossref] [PubMed]
- Castellano JM, Kim J, Stewart FR, Jiang H, DeMattos RB, Patterson BW, Fagan AM, Morris JC, Mawuenyega KG, Cruchaga C, Goate AM, Bales KR, Paul SM, Bateman RJ, Holtzman DM. Human apoE isoforms differentially regulate brain amyloid-beta peptide clearance. Sci Transl Med 2011;3:89ra57. [Crossref] [PubMed]
- Wildsmith KR, Holley M, Savage JC, Skerrett R, Landreth GE. Evidence for impaired amyloid beta clearance in Alzheimer's disease. Alzheimers Res Ther 2013;5:33. [Crossref] [PubMed]
- El Haj M, Antoine P, Amouyel P, Lambert JC, Pasquier F, Kapogiannis D, Apolipoprotein E. APOE) epsilon4 and episodic memory decline in Alzheimer's disease: A review. Ageing Res Rev 2016;27:15-22. [Crossref] [PubMed]
- O'Donoghue MC, Murphy SE, Zamboni G, Nobre AC, Mackay CE. APOE genotype and cognition in healthy individuals at risk of Alzheimer's disease: A review. Cortex 2018;104:103-23. [Crossref] [PubMed]
- Mori E, Yoneda Y, Yamashita H, Hirono N, Ikeda M, Yamadori A. Medial temporal structures relate to memory impairment in Alzheimer's disease: an MRI volumetric study. J Neurol Neurosurg Psychiatry 1997;63:214-21. [Crossref] [PubMed]
- Scahill RI, Schott JM, Stevens JM, Rossor MN, Fox NC. Mapping the evolution of regional atrophy in Alzheimer's disease: unbiased analysis of fluid-registered serial MRI. Proc Natl Acad Sci U S A 2002;99:4703-7. [Crossref] [PubMed]
- Wei H, Kong M, Zhang C, Guan L, Ba M. for Alzheimer's Disease Neuroimaging I. The structural MRI markers and cognitive decline in prodromal Alzheimer's disease: a 2-year longitudinal study. Quant Imaging Med Surg 2018;8:1004-19. [Crossref] [PubMed]
- Stout JC, Bondi MW, Jernigan TL, Archibald SL, Delis DC, Salmon DP. Regional cerebral volume loss associated with verbal learning and memory in dementia of the Alzheimer type. Neuropsychology 1999;13:188-97. [Crossref] [PubMed]


