
Left ventricular deformation in patients with lymphedema before and after the use of medical compression stockings—detailed analysis from the three-dimensional speckle-tracking echocardiographic MAGYAR-Path Study
Introduction
Lymphedema (LE) is a chronic condition, which refers to tissue swelling due to excess interstitial fluid accumulation or impaired lymphatic conduit (1). LE has several stages from a temporary state (stage 1) progressing into swollen limbs with loss of function (stage 2) to an advanced state when pitting edema is transformed into non-pitting edema with fibrosis and adipose tissue enlargement (stage 3) (2). From treatment strategies, the most common lymphatic therapy applies graduated medical compression stocking (MCS) for decongestion (1). MSC use in LE limbs significantly increases the returning flow to the left ventricle (LV) (3).
LE-related fluid retention affects LV mechanics, which could be analyzed in detail by recent three-dimensional speckle-tracking echocardiography (3DSTE) (4-7). In a recent study, moderate effects of MCS use on LV rotational mechanics could be demonstrated in LE patients after the use of compression stockings (8). However, LV strains, quantitative features of LV deformation were never investigated in relation with MCS use in LE patients (9). Due to the strong relationship between LV deformation and rotational mechanics, the question has rightly arisen as to whether changes in LV deformation could be detected, as well. Therefore, it was purposed to examine LV strains in LE patients before and one hour after the use of MCS and to compare their results to those of matched normal subjects.
Methods
Patient population
The study consisted of 26 LE subjects, however, 4 cases had to be excluded due to inferior quality of images. The remaining population of LE patients comprised 22 patients (mean age: 47.1±12.3 years, one male). Lymphedema was defined according to recent guidelines (2). Their findings were compared to that of 27 age- and gender-matched healthy controls (mean age: 43.3±9.2 years, one male). A subject was considered to be healthy, if physical examination, electrocardiography and routine two-dimensional Doppler echocardiography showed results within the normal ranges. None of the lymphedema patients and matched controls had any known disorder, other pathological states or conditions or typical cardiovascular symptoms. The presented findings is a part of the MAGYAR-Path Study (Motion Analysis of the heart and Great vessels bY three-dimensionAl speckle-tRacking echocardiography in Pathological cases) aiming to assess LV deformation in various pathophysiological conditions among others (‘Magyar’ means ‘Hungarian’ in Hungarian language). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Institutional and Regional Human Biomedical Research Committee of University of Szeged (Hungary) approved the study on the following registration number: 71/2011 (and updated versions). Informed consent was taken from all the subjects.
Two-dimensional Doppler echocardiography
In all LE patients and matched controls, complete two-dimensional echocardiographic analysis was performed extended with Doppler measurements (2DE) using a Toshiba ArtidaTM echocardiographic tool (Toshiba Medical Systems, Tokyo, Japan) attached to a 1–5 MHz PST-30BT phased-array transducer. Measurements were performed before and one hour after the use of MCS in all LE cases by expert in echocardiography (Kormányos Á). Chamber quantifications and Doppler analysis followed recent guidelines. Colour Doppler echocardiography was used for visual grading of potential valvular regurgitations and pulsed Doppler was used for quantification of potential valvular stenoses (9).
Three-dimensional speckle-tracking echocardiography
At each stages of the protocol, 3DSTE-derived data acquisitions were performed for later offline analysis (4-7). Using the same Toshiba ArtidaTM echocardiographic tool, the transducer was changed to a PST-25SX matrix-array one with 3D capability. Full volume 3D echocardiographic datasets were digitally acquired from the apical window during a breath-hold in subjects who were in sinus rhythm during 6 cardiac cycles for optimal images. Offline data analysis was performed later by vendor-provided 3D Wall Motion Tracking software version 2.7 (Ultra Extend, Toshiba Medical Systems, Tochigi, Japan). Using automatically selected apical four- (AP4CH), two-chamber (AP2CH) and 3 cross-sectional views at different levels of the LV, mitral annular septal and lateral edges and LV apex were defined by the operator, then a 3D LV cast was created following a sequential analysis and automatic contour detection (Figure 1). Several LV strains were determined using this virtual LV model:
- Radial strain (RS) to represent myocardial thickening and thinning;
- Longitudinal strain (LS) to represent myocardial lengthening and shortening;
- Circumferential strain (CS) to represent myocardial widening and narrowing;
- Area strain (AS) to represent combined LS and CS;
- 3D strain (3DS) to represent combined RS, LS and CS.
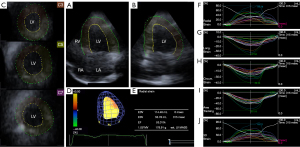
Together with LV volumes, global, mean segmental and regional LV strains were measured featuring the whole LV, calculated as an average of 16 segmental strains and measured from basal, midventricular and apical LV segmental strains, respectively (4-7,10-12).
Experimental protocol
Firstly, 2DE was performed in all subjects extended with 3DSTE data acquisition. After that LE patients, their MCSs were pulled on and wore them for sixty minutes. The second 3DSTE was performed exactly at the end of the application of the elastic compression with the patient still wearing the compression stockings. None of the patients with LE did any kind of physical exercise or consumed food or beverage until the second 3DSTE, they were sitting with straight legs or standing, as it was comfortable. Temperature of the examination room and relative humidity were at 21–22 ℃ and 45–50%, respectively. Each compression stocking was used for the first time by each patient and physiotherapists were at the disposal of the subjects. All cases were informed about the protocol of the study before the procedure.
Experimental compression garments
Bauerfeind black VenoTrain CuraFlow flat-kitted ccl 2 (23–32 mmHg) pantyhose produced by Bauerfeind (Zeulenroda, Germany) made of 73% polyamide and 27% elastane was used. Pressure between the skin and the compression material was measured by a Picopress device (Microlab Elettronica, Nicolò, Italy) (13) at B1 point (14) in standing position, which proved to be 27.3±4.2 mmHg in this group of patients.
Statistical analysis
Frequency/percentage and mean ± standard deviation formats were used for categorical and continuous data, respectively. Statistical significance was considered to be in the presence of a value of P<0.05. Fisher’s exact test was used for analysis of categorical variables. Normality of distribution was assessed for continuous variables with Shapiro-Wilks test: unpaired and paired Student’s t-tests were performed for normally distributed datasets and Mann-Whitney-Wilcoxon test for non-normally distributed datasets. One-way analysis of variance (ANOVA) test with Bonferroni correction was used, where appropriate. Confounders were examined by a multivariable regression analysis. SPSS software was used during statistical analyses (SPSS Inc., Chicago, IL, USA, version 22).
Results
Demographic data
From the remaining 22 LE subjects, two patients had recurrent erysipelas-related bilateral secondary lower limb lymphedema, four patients were undergoing bilateral inguinal lymphadenectomy due to malignant melanoma, fifteen patients had gynaecological cancer treatment-related lymphedema and one patient had idiopathic bilateral lymphedema. None of them showed significant venous hemodynamic or morphological changes or had any known cardiovascular diseases. Clinical data of LE patients and controls are demonstrated in Table 1.
Table 1
| Data | Controls (n=27) | Lymphedema patients (n=22) | P value |
|---|---|---|---|
| Risk factors | |||
| Age (years) | 43.3±9.2 | 47.1±12.3 | 0.20 |
| Male gender | 1 [4] | 1 [5] | 1.00 |
| Hypertension | 0 [0] | 0 [0] | 1.00 |
| Hypercholesterolemia | 0 [0] | 0 [0] | 1.00 |
| Diabetes mellitus | 0 [0] | 0 [0] | 1.00 |
| Body mass index (kg/m2) | 24.1±1.9 | 27.3±2.8 | 0.10 |
| Two-dimensional echocardiography | |||
| LA diameter (mm) | 34.4±4.3 | 38.0±4.8 | <0.01 |
| LV end-diastolic diameter (mm) | 45.8±4.3 | 48.0±4.4 | 0.07 |
| LV end-diastolic volume (mL) | 94.5±17.1 | 109.5±21.8 | 0.01 |
| LV end-diastolic volume index (mL/m2) | 54.6±8.1 | 57.6±12.0 | 0.45 |
| LV end-systolic diameter (mm) | 32.8±11.4 | 29.8±3.5 | 0.20 |
| LV end-systolic volume (mL) | 33.5±9.7 | 35.5±9.6 | 0.50 |
| LV end-systolic volume index (mL/m2) | 19.4±4.8 | 18.7±5.0 | 0.86 |
| Interventricular septum (mm) | 8.8±1.3 | 8.0±0.7 | 0.01 |
| LV posterior wall (mm) | 9.3±2.0 | 8.2±0.9 | 0.02 |
| E (cm/s) | 76.7±17.7 | 78.2±18.1 | 0.80 |
| A (cm/s) | 65.6±15.5 | 74.0±13.6 | 0.06 |
| E/A | 1.21±0.33 | 1.09±0.31 | 0.20 |
| LV ejection fraction (%) | 64.4±4.4 | 68.2±4.4 | 0.004 |
| Indexed LV ejection fraction (%) | 64.5±3.2 | 67.5±2.5 | 0.05 |
Data are presented as mean ± standard deviation or number [frequency]. A, late transmitral flow velocity; E, early transmitral flow velocity; LA, left atrium; LV, left ventricle.
Two-dimensional echocardiographic data
Although the interventricular septum and the LV posterior wall were thicker in controls, increased LA diameter, LV end-diastolic volume (EDV) and LV ejection fraction (EF) were seen in LE patients as compared to matched controls. Although indexed LV volumes did not differ between the groups, calculated LV-EF proved to be increased. No LE patients and no healthy subjects showed ≥ grade 2 valvular insufficiency and/or significant valvular stenosis in any valves (Table 1).
Three-dimensional speckle-tracking echocardiography
While LV-EDV and LV-ESV and their indexed counterpart did not differ between LE patients and controls before the use of MCS, LV-EF (and indexed LV-EF) proved to be higher in LE patients. Following the use of MCS, tendentious increase in LV-EDV was seen which was accompanied with preserved LV-EF. 3DSTE-derived global LV-CS and LV-AS and mean segmental LV-CS were increased in LE patients before the use of MCS as compared to controls. One hour after the MCS use, none of global and mean segmental LV strains showed changes, but tendentious reduction was seen in LV-CS (Table 2). With LV segmental analysis, midventricular LV-RS, LV-CS and LV-AS proved to be significantly increased, while basal LV-LS and midventricular LV-3DS were decreased in LE patients as compared to controls. With the exception of midventricular LV-3DS, which tended to grow, no changes in regional LV strains could be detected after one-hour use of MCS as compared to data collected at rest (Table 3).
Table 2
| Data | Controls (n=27) | Lymphedema patients at rest (n=22) | Lymphedema patients following 1-hour MCS use (n=22) |
|---|---|---|---|
| 3DSTE-derived LV volumes and ejection fraction | |||
| EDV (mL) | 75.5±13.3 | 73.2±16.4 | 80.1±9.8 |
| EDV index (mL/m2) | 43.6±6.5 | 38.5±8.5 | 42.2±5.0 |
| ESV (mL) | 33.0±7.1 | 29.4±8.8 | 33.0±5.1 |
| ESV index (mL/m2) | 19.8±3.6 | 15.5±4.7 | 17.3±2.6 |
| EF (%) | 56.1±6.7 | 60.3±5.7* | 58.7±4.2 |
| Indexed EF (%) | 54.6±3.5 | 59.7±2.9* | 59.0±2.5* |
| 3DSTE-derived LV global strains | |||
| Radial (%) | 24.2±10.8 | 26.6±8.7 | 25.4±14.3 |
| Circumferential (%) | −25.9±5.4 | −30.4±5.0* | −28.4±4.3 |
| Longitudinal (%) | −17.0±2.5 | −17.0±2.7 | −17.9±2.2 |
| 3D (%) | 26.4±10.6 | 29.0±9.3 | 27.5±14.1 |
| Area (%) | −39.5±5.4 | −42.6±5.1* | −42.1±3.4 |
| 3DSTE-derived LV mean segmental strains | |||
| Radial (%) | 26.8±10.1 | 28.8±8.8 | 27.8±13.6 |
| Circumferential (%) | −27.1±5.4 | −31.2±4.9* | −29.1±4.3 |
| Longitudinal (%) | −17.8±2.4 | −17.8±2.6 | −18.6±2.2 |
| 3D (%) | 28.9±10.0 | 31.3±9.4 | 29.7±13.5 |
| Area (%) | −40.7±5.2 | −43.6±4.9 | −42.8±3.4 |
Data are presented as mean ± standard deviation. *, P<0.05 vs. Controls. LV, left ventricular; 3D, three-dimensional; STE, speckle-tracking echocardiography; MCS, medical compression stocking; EDV, end-diastolic volume; ESV, end-systolic volume; EF, ejection fraction.
Table 3
| Data | Controls (n=27) | Lymphedema patients at rest (n=22) | Lymphedema patients following 1-hour MCS use (n=22) |
|---|---|---|---|
| RSbasal (%) | 32.5±15.9 | 28.6±11.9 | 28.0±18.0 |
| RSmid (%) | 27.5±10.4 | 34.1±8.7* | 30.5±12.7 |
| RSapex (%) | 17.2±7.3 | 21.3±12.3 | 23.4±14.2 |
| CSbasal %) | −24.4±5.7 | −26.3±6.6 | −25.0±6.3 |
| CSmid (%) | −26.5±6.1 | −32.0±5.3* | −29.4±4.8 |
| CSapex (%) | −32.1±9.8 | −37.3±9.3 | −34.7±8.3 |
| LSbasal (%) | −22.0±4.8 | −18.5±4.3* | −20.4±4.6 |
| LSmid (%) | −14.5±2.8 | −16.4±4.4 | −16.4±3.2* |
| LSapex (%) | −16.5±5.4 | −18.5±5.1 | −19.0±3.5 |
| 3DSbasal (%) | 35.1±15.4 | 36.1±8.8 | 30.7±17.5 |
| 3DSmid (%) | 28.8±10.5 | 23.4±12.4* | 31.9±12.2* |
| 3DSapex (%) | 18.8±7.6 | 29.0±9.3 | 25.2±14.9 |
| ASbasal (%) | −40.3±6.9 | −39.5±6.9 | −40.1±6.9 |
| ASmid (%) | −37.7±6.4 | −43.6±5.9* | −41.7±4.5 |
| ASapex (%) | −44.5±10.9 | −49.8±10.1 | −48.6±8.3 |
Data are presented as mean ± standard deviation. *, P<0.05 vs. Controls. LV, left ventricular; MCS, medical compression stocking; RS, radial strain; CS, circumferential strain; mid, midventricular; LS, longitudinal strain; 3DS, three-dimensional strain; AS, area strain.
Multiple regression analysis
Several confounders including age, gender and body mass index were analyzed, but none of them proved to be a significant confounder during multivariable regression analysis.
Discussion
To the best of the authors’ knowledge, this is the first study in which LV deformation and its changes after 1-hour use of MCS were investigated in LE patients. Moreover, one of the most recent echocardiographic tools, 3DSTE was used for the analysis, which makes one of the most detailed non-invasive volumetric and functional analysis possible at this moment. Elevated LV pumping function (increased LV-EF) was associated with increased global LV-CS (and LV-AS) with primarily midventricular regional strain abnormalities. The use of MCS had no effect on global LV strains, moreover, tendentious lowering of LV-CS could be detected. Only midventricular LV-LS and LV-3DS proved to be elevated.
LV contractility has a special 3D pattern in systole, the efficacy of LV contractility is facilitated by LV rotational mechanics. In this special LV movement, walls of the LV not only contract, but the basal LV segments rotate in clockwise direction, while the apical LV segments rotate in counterclockwise direction leading to a towel-wringing-like motion called LV twist (15). The results of this study should be considered in the context of LV rotational parameters using the same protocol as published in a recent study (8). At rest, significant abnormalities were seen in one-fifth of the patients without reduction/elevation of LV rotational parameters in the remaining cases. Following the use of MCS, reduction of basal LV rotation with preservation of LV twisting mechanism could be detected (8). According to these findings, changes in LV strains could be considered as a compensation mechanism designed to maintain LV pumping function at a certain level.
Although lipedema and LE are considered to be clinically similar obesity-masquerading disorders, causes and origins of these disorders are different (16). Although many similarities in LV rotational mechanics were noticeable in these two diseases, some significant abnormalities highlighted the opportunity to differentiate them in cases that are difficult to distinguish clinically (16). In lipedema, approximately 30% of the cases had significant LV rotational abnormalities with tendentiously deteriorated apical LV rotation and twist in the remaining cases, which showed special LV rotational pattern after the use of MCS: basal LV rotation decreased, while apical LV rotation increased with preserved LV twist (17). LV deformation showed a compensatory increase in global LV-CS and LV-AS. The use of MCS did not change these alterations, although some regional parameters improved (18).
These results could highlight our attention to several facts. Firstly, LE-related edema has effects not only on LV rotational mechanics, but compensatory LV strain abnormalities are also seen. MCS causes more fluid to enter the blood circulation, and hence the fluid balance changes from hypovolemic towards normovolemic, leading to altered LV deformation and rotational mechanics. A compensatory response means that changes in LV rotational mechanics can have an effect on the LV strain response (and vice versa) finding the optimal myocardial mechanics for the actual clinical situation. Secondly, the MCS use have effects on these abnormalities, but primarily on regional LV function without significant effects on global LV function. However, with using MCS, LV deformation parameters changed towards the normal range. Thirdly, although lipedema and LE clinically seems to be similar, their effects on myocardial mechanics are different with more pronounced abnormalities in lipedema making an opportunity for clinical differentiation. However, further studies are warranted to confirm our findings.
Limitation section
- Known technical limitations of 3DSTE are reduced spatial resolution and lower frame rate as compared to 2DE (4-7,19,20).
- The present study did not aim to validate 3DSTE-derived LV deformation parameters due to their validated nature (10-12).
- The study involved only a limited number of LE patients who were willing to participate in this study. Moreover, a large number of parameters were investigated. Hence, the risk of type I and type II errors are significant.
The statistical power of this study would have been better if more patients had participated. - Similar functional analysis could have been performed for the left and right atria, which can be a topic of future investigations as performed for lipedema patients (21).
Conclusions
Increased global LV-CS is seen LE. With using MCS, LV deformation parameters change towards the normal range emphasizing their importance on cardiac function.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form. (available at https://qims.amegroups.com/article/view/10.21037/qims-23-243/coif). Attila Nemes serves as an unpaid editorial board member of Quantitative Imaging in Medicine and Surgery. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Institutional and Regional Human Biomedical Research Committee of University of Szeged (Hungary) approved the study on the following registration number: 71/2011 (and updated versions). Informed consent was taken from all the subjects.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Szolnoky G, Dobozy A, Kemény L. Towards an effective management of chronic lymphedema. Clin Dermatol 2014;32:685-91. [Crossref] [PubMed]
- International Society of Lymphology. The diagnosis and treatment of peripheral lymphedema: 2013 Consensus Document of the International Society of Lymphology. Lymphology 2013;46:1-11.
- Yoshida S, Koshima I, Imai H, Uchiki T, Sasaki A, Fujioka Y, Nagamatsu S, Yokota K, Yamashita S. Modified intraoperative distal compression method for lymphaticovenous anastomosis with high success and a low venous reflux rates. J Plast Reconstr Aesthet Surg 2021;74:2050-8. [Crossref] [PubMed]
- Urbano-Moral JA, Patel AR, Maron MS, Arias-Godinez JA, Pandian NG. Three-dimensional speckle-tracking echocardiography: methodological aspects and clinical potential. Echocardiography 2012;29:997-1010. [Crossref] [PubMed]
- Ammar KA, Paterick TE, Khandheria BK, Jan MF, Kramer C, Umland MM, Tercius AJ, Baratta L, Tajik AJ. Myocardial mechanics: understanding and applying three-dimensional speckle tracking echocardiography in clinical practice. Echocardiography 2012;29:861-72. [Crossref] [PubMed]
- Nemes A, Kalapos A, Domsik P, Forster T. Three-dimensional speckle-tracking echocardiography -- a further step in non-invasive three-dimensional cardiac imaging. Orv Hetil 2012;153:1570-7. [Crossref] [PubMed]
- Muraru D, Niero A, Rodriguez-Zanella H, Cherata D, Badano L. Three-dimensional speckle-tracking echocardiography: benefits and limitations of integrating myocardial mechanics with three-dimensional imaging. Cardiovasc Diagn Ther 2018;8:101-17. [Crossref] [PubMed]
- Nemes A, Kormányos Á, Domsik P, Kalapos A, Kemény L, Szolnoky G. The effects of lower body compression on left ventricular rotational mechanics in lymphoedema (from the MAGYAR-Path Study). ESC Heart Fail 2021;8:4328-33. [Crossref] [PubMed]
- Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233-70. [Crossref] [PubMed]
- Nesser HJ, Mor-Avi V, Gorissen W, Weinert L, Steringer-Mascherbauer R, Niel J, Sugeng L, Lang RM. Quantification of left ventricular volumes using three-dimensional echocardiographic speckle tracking: comparison with MRI. Eur Heart J 2009;30:1565-73. [Crossref] [PubMed]
- Kleijn SA, Brouwer WP, Aly MF, Rüssel IK, de Roest GJ, Beek AM, van Rossum AC, Kamp O. Comparison between three-dimensional speckle-tracking echocardiography and cardiac magnetic resonance imaging for quantification of left ventricular volumes and function. Eur Heart J Cardiovasc Imaging 2012;13:834-9. [Crossref] [PubMed]
- Kleijn SA, Aly MFA, Terwee CB, van Rossum AC, Kamp O. Reliability of left ventricular volumes and function measurements using three-dimensional speckle tracking echocardiography. Eur Heart J Cardiovasc Imaging 2012;13:159-68. [Crossref] [PubMed]
- Partsch H, Mosti G. Comparison of three portable instruments to measure compression pressure. Int Angiol 2010;29:426-30.
- Chassagne F, Helouin-Desenne C, Molimard J, Convert R, Badel P, Giraux P. Superimposition of elastic and nonelastic compression bandages. J Vasc Surg Venous Lymphat Disord 2017;5:851-8. [Crossref] [PubMed]
- Nakatani S. Left ventricular rotation and twist: why should we learn? J Cardiovasc Ultrasound 2011;19:1-6.
- Nemes A, Kormanyos A, Domsik P, Kalapos A, Kemeny L, Forster T, Szolnoky G. Left ventricular rotational mechanics differ between lipedema and lymphedema: Insights from the three-dimensional speckle tracking echocardiographic MAGYAR-Path Study. Lymphology 2018;51:102-8.
- Nemes A, Kormányos Á, Domsik P, Kalapos A, Kemény L, Szolnoky G. The impact of lower body compression garment on left ventricular rotational mechanics in patients with lipedema-Insights from the three-dimensional speckle tracking echocardiographic MAGYAR-Path Study. Clin Obes 2020;10:e12380. [Crossref] [PubMed]
- Nemes A, Kormányos Á, Domsik P, Kalapos A, Gyenes N, Kemény L, Szolnoky G. Are increased left ventricular strains compensatory effects in lipedema? Detailed analysis from the three-dimensional speckle-tracking echocardiographic MAGYAR-Path Study. J Clin Ultrasound 2020;48:470-5. [Crossref] [PubMed]
- Nemes A, Kormányos Á, Domsik P, Kalapos A, Gyenes N, Lengyel C, Valkusz Z. Diabetes mellitus deteriorates left ventricular deformation in acromegaly-analysis from the three-dimensional speckle-tracking echocardiographic MAGYAR-Path Study. Quant Imaging Med Surg 2021;11:410-4. [Crossref] [PubMed]
- Nemes A, Kormányos Á, Gyenes N, Ambrus N, Horváth Á, Lengyel C, Valkusz Z. Is treated hypopituitarism associated with increased left ventricular strains?-detailed analysis from the three-dimensional speckle-tracking echocardiographic MAGYAR-Path Study. Quant Imaging Med Surg 2022;12:838-45. [Crossref] [PubMed]
- Nemes A, Kormányos Á, Domsik P, Kalapos A, Gyenes N, Kemény L, Szolnoky G. Lipoedema: detailed left atrial volumetric and strain analysis by three-dimensional speckle-tracking echocardiography from the MAGYAR-Path Study. Postepy Dermatol Alergol 2022;39:580-6. [Crossref] [PubMed]


