
Revised Thyroid Imaging Reporting and Data System (TIRADS): imitating the American College of Radiology TIRADS, a single-center retrospective study
Introduction
Thyroid lumps are common, but the prevalence of palpable thyroid neoplasms varies widely, ranging from 3% to 20% (1,2). The detection rate of thyroid nodules by high-resolution ultrasound (US) reaches 68% (1). The breakneck speed of thyroid nodule detection brings challenges, and there are debates regarding the management of thyroid neoplasms (3-5). The chief objective of US imaging is to identify thyroid carcinomas from thyroid nodules, although only 7–15% of thyroid nodules are malignant (6). In a recent multi-institution investigation, Grani et al. proved that the American College of Radiology Thyroid Imaging Reporting and Data System (ACR TIRADS) achieved the largest decrease in unnecessary thyroid nodule fine needle aspiration biopsy (FNAB) (7). FNAB has been traditionally used for evaluating thyroid neoplasms (8,9). Ultrasonography was the first choice to noninvasively evaluate the malignancy risk of thyroid nodules and to decide whether nodules needed FNAB and/or excision. Consequently, risk stratification systems (RSSs) established based on US characteristics have been extensively applied to manage thyroid neoplasms and provide guidance on whether FNAB is performed (10). There are two types of US-based RSSs: pattern-based and non-pattern-based RSSs. The Korean Society of Thyroid Radiology Thyroid Imaging Reporting and Data System (K-TIRADS) is a typical pattern-based RSS that cannot classify partial lumps (11). Unlike the K-TIRADS, the ACR TIRADS uses a point-based method (12).
In a recent multi-institution investigation, the ACR TIRADS was found to compare favorably with the K-TIRADS, mainly since the ACR guidelines more efficiently diminished the number of biopsies conducted on benign neoplasms. Nevertheless, for risk stratification capabilities, the ACR TIRADS was inferior to the K-TIRADS (7). Neither of the guidelines are outstanding with respect to reducing unnecessary FNAB rates and showing good risk stratification capability. To overcome these limitations, Ruan et al. proposed a contrast-enhanced ultrasound TIRADS with thyroid neoplasm malignant peril stratification by simplifying the regression coefficients of grayscale sonography and qualitatively analyzing the characteristics of contrast-enhanced ultrasound (13). Zhang et al. also tried to distinguish between inflammatory thyroid nodules and papillary thyroid carcinoma by contrast-enhanced ultrasound (14). The contrast-enhanced ultrasound technique improves the diagnostic sensitivity and successfully reduces the rate of unnecessary biopsies. However, these methods are relatively complex, expensive, and difficult to apply widely, particularly in remote areas. We aimed to develop a practical RSS based on five US features (composition, echogenicity, shape, margin, and echogenic foci) to stratify the malignancy risk of thyroid nodules, which we designated the revised Thyroid Imaging Reporting and Data System (R-TIRADS). The R-TIRADS imitates the American College of Radiology TIRADS. We hypothesize that the R-TIRADS would improve the diagnostic sensitivity of thyroid nodules. We present the following article in accordance with the STARD reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-22-1307/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Our survey was registered on the www.chictr.org.cn platform (registration number ChiCTR2300067683). The single-center survey was approved by the institutional review board of the Seventh Affiliated Hospital, Sun Yat-sen University (No. KY-2022–066-01), and the need for informed consent was waived due to the retrospective character of the research. Informed consent for the FNAB procedures and/or resection was obtained from all patients.
Study population
We conducted a retrospective, observational study of patients with thyroid nodules referred to our center for FNAB. Between 11 May 2018 and 28 February 2022, consecutive patients were subjected to grayscale US examinations before FNAB at the Seventh Affiliated Hospital of Sun Yat-sen University in Shenzhen, China, which is a tertiary referral center. The inclusion criteria were as follows: (I) underwent FNAB and/or surgery at our institution; and (II) obtained resolute pathology outcome. The exclusion criteria were as follows: (I) nodules lacking US images and/or dynamic videos; (II) maximal diameter less than 0.5 cm; (III) thyroiditis, parathyroid nodule, and diffuse papillary carcinoma; (IV) nodules with inconclusive diagnoses based on cytologic findings, including Bethesda I, III, IV or V according to the Bethesda System for Reporting Thyroid Cytopathology (15); and (V) age younger than 18 years. The reference standard criteria were histopathological results or cytological results (Bethesda II, or VI). The time between US examinations and FNAB (or resection) was managed within 3 weeks, without any clinical intervention throughout the period.
Imaging analyses
All US images collected from the thyroid movies data bank were altered into an AVI format. US images of the thyroid glands and neck zones were obtained employing a linear-array probe (5–13 MHz Aloka ARIETTA 70, Hitachi, Tokyo, Japan; or 5–12 MHz HD15, Philips Medical Systems, Bothell, Washington). All 1,236 thyroid nodules were imaged and numbered as required by one radiologist (DNH). Image quality control was conducted for the dataset of US images and dynamic videos; all thyroid and dynamic videos were reviewed, and inferior scans, including images with serious artifacts or noticeable decreases in the resolution, were excluded. This review of the image database was completed by two radiologists (FPL and QJ) who had more than ten years of ultrasound examination experience. One senior radiologist [(FPL) with ten years of clinical practice] and a junior radiologist [(XL) with four years of clinical practice] cooperatively evaluated an extra fifty thyroid nodules to homogenize the feature task at the start of the research. The two radiologists (FPL and XL) were blinded to the cytologic and pathology outcomes of the thyroid nodules. These radiologists retrospectively analyzed the whole US images by scanning 2–5 stationary images of each neoplasm (US images with the maximal diameter and corresponding orthogonal images). The clips of the same nodule were then played back frame by frame to calculate the score based on the five US features. Each thyroid nodule was categorized, and the recommendations for FNAB were separately recorded according to the K-TIRADS and ACR TIRADS. The radiologists met and discussed disagreements to agree on a final categorization for each thyroid nodule.
US-guided FNAB
After US examination of the thyroid nodules, US-guided FNAB was performed by identical radiologists. The procedure was performed through the nonnegative pressure suction method in most cases. If sufficient samples could not be obtained, the negative pressure aspiration method was used. The target nodule was generally punctured 3 times. The three FNAB specimens were individually used for smear, liquid-based cytology, and cell blocks. The cytopathologist was on-site throughout the process. Additional special staining was performed on a case-by-case basis according to the cytopathologist’s needs. The Bethesda system was used to classify cytologic results in our institution (15). The cytopathologist and pathologist were blinded to the clinical information and TIRADS category of the thyroid nodules.
The ACR TIRADS and K-TIRADS were separately used 151 to classify each thyroid nodule. Additionally, Within each RSS, the indication for FNAB was made based on lesion size and category. According to the standard of the K-TIRADS, the size thresholds for FNAB were diameter ≥1 cm in the intermediate-risk category (K-TIRADS 4) and diameter ≥1.5 cm in the low-risk category (K-TIRADS 3). With the FNAB criteria of the ACR TIRADS, the size thresholds for FNAB were 1.5 cm and 2.5 cm for the ACR TIRADS category 4 and category 3, respectively. FNAB is recommended for nodules with high suspicion of malignancy (category: ACR TIRADS 5 and K-TIRADS 5), and the size cutoff is larger than 1 cm. According to the K-TIRADS, fine needle aspiration biopsy was selectively implemented for high-risk nodules (diameter 0.5 to 1 cm). There are no guidelines advocating FNAB for thyroid nodules whose maximum diameter is less than 0.5 cm. Therefore, those nodules were excluded.
Statistical analysis
The malignancy rate of each thyroid nodule was calculated, and the chi-square test was used to test whether the malignancy rate was different between score groups. The product-limit (Kaplan‒Meier) method was used to analyze the changes in the rate of extraglandular invasion and rate of lymph node metastasis according to nodule diameter. Patients with extraglandular invasion or lymph node metastasis were included in analyses, and patients with no extraglandular invasion or lymph node metastasis were regarded excluded. The Kaplan–Meier method (log-rank test) was used (16). According to this reclassification, an R-TIRADS classification system was developed.
For each sonographic classification system (K-TIRADS, ACR TIRADS, and R-TIRADS), we computed the quantity of neoplasms that did and did not fulfil the yardstick for FNAB (test positivity and test negativity, respectively). In this study, the biopsies ordered for test-negative nodules were considered “unnecessary”. The sonographic recommendations regarding FNAB were then compared with the reference standard in terms of obtaining a diagnosis (benign vs. malignant), and the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and area under the receiver operating characteristic (AUROC) curve [with 95% confidence intervals (CIs)] of the recommendations were estimated. The rates of biopsies that would have been deemed unnecessary by the three TIRADSs were compared by applying the McNemar test. A P value <0.05 was considered to indicate a statistically significant difference. The CIs of the proportions were reported as a two-sided exact binomial as 95% CIs. The data were analyzed with the IBM SPSS Statistics package, version 26.0 (IBM Corp., Armonk, NY) and Microsoft Office Suite.
Results
Patient characteristics
Between 11 May 2018 and 28 February 2022, 1,236 thyroid nodules in 999 consecutive patients were subjected to grayscale US examinations before FNAB at our center. Eight hundred and eleven nodules were excluded due to the following reasons: (I) without US images (n=37), only static ultrasound images and lacking dynamic video recording (n=618); (II) maximum diameter less than 0.5 cm (n=70); (III) diffuse lesions (n=4), extraglandular nodules (n=2); (IV) inconclusive cytologic results without pathologic confirmation (n=78); and (V) age <18 years (n=2) (Figure 1). The final dataset consisted of 425 nodules from 370 patients (118 men and 252 women). Among the 425 lesions, 196 nodules were <1 cm (range, 0.5–0.96 cm) in diameter, and the rest were ≥1 cm in diameter. Two hundred and twenty-eight (surgical histopathology results of 155 and cytopathology results of 73) lesions met the reference standard criteria for malignancy, and 197 were benign. Baseline features of the participants are presented in Table 1. The sizes of benign neoplasms were 17.4±11.7 mm, and those of malignancies were 10.6±7.0 mm. The average patient age of malignant nodules was younger than that of benign nodules (41.5±11.1 vs. 46.2±12.5 years). Nodule size and patient age were significantly different between the two groups (P<0.001). The difference in sex was statistically significant (P=0.005).
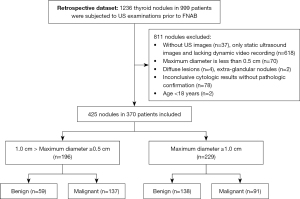
Table 1
| Variables | Malignant | Benign | P value |
|---|---|---|---|
| No. of patients | 196 | 174 | |
| Age (years), mean ± SD | 41.5±11.1 | 46.2±12.5 | <0.001* |
| Sex | 0.005* | ||
| Male | 75 | 43 | |
| Female | 121 | 131 | |
| Nodule sizes (mm), mean ± SD | 10.6±7.0 | 17.4±11.7 | <0.001* |
| 5≤ diameter <10 | 137 | 59 | |
| ≥10 | 91 | 138 |
*P<0.05 were considered as significant difference.
Construction of the R-TIRADS
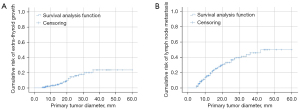
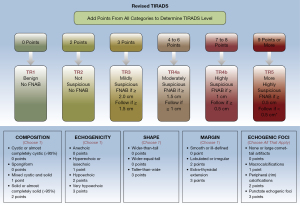
Founded on the outcomes of count data analysis, we calculated the malignancy rates of nodules per score. The total percentage of malignant nodules with 4–6 points, 7–8 points, and more than 9 points was 28.8% (32/111), 64.7% (44/68), and 84.2% (149/177), respectively (Table 2). The results of the chi-square analyses indicated that the malignancy rate was significantly different between thyroid nodules with 6 points and those with 7–8 points (P=0.006, 37.5% vs. 64.7%) and between nodules with 7–8 points and those with ≥9 points (P=0.001, 64.7% vs. 84.2%) (Table 3). Subsequently, we established the R-TIRADS. The five US features (composition, echogenicity, shape, margin, and echogenic foci) are consistent with those of the ACR TIRADS. The totality of the scores settled the nodule’s R-TIRADS sort (henceforth, TR): 1 point was parallel to TR1 (benign; fitting probability: 0), two points was parallel to TR2 (not suspicious; fitting probability: 0.04), three points was parallel to TR3 (mildly suspicious; fitting probability: 0.08), 4–6 points was parallel to TR4a (moderately suspicious; fitting probability: 0.23–0.38), 7–8 points was parallel to TR4b (moderately suspicious; fitting probability: 0.59–0.67), and more than 9 points was parallel to TR5 (highly suspicious; fitting probability: 0.80–1.0) (Table 2). We performed product-limit (Kaplan‒Meier) analyses for the cumulative incidence of extraglandular infiltration and lymph node metastasis, which demonstrated that they were positively correlated with thyroid nodule diameter, but no cutoff values were observed (Figure 2). The FNAB suggestions were based on syntheses of the greatest diameter and classification of the nodules (Figure 3).
Table 2
| Score | Percentage of benign nodules | Percentage of Malignant Nodules | ACR TIRADS (percentage of malignant nodules) | Revised TIRADS (percentage of malignant nodules) |
|---|---|---|---|---|
| 0 | 7/7 (100.00) | 0/7 (0.00) | 0/7 (0.00) | TR 1, 0/7 (0.00) |
| 2 | 25/26 (96.15) | 1/26 (3.85) | 1/26 (3.85) | TR 2, 1/26 (3.85) |
| 3 | 33/36 (91.67) | 3/36 (8.33) | 3/36 (8.33) | TR 3, 3/36 (8.33) |
| 4 | 37/48 (77.08) | 11/48 (22.92) | TR 4, 32/111 (28.83) | TR 4a, 32/111 (28.83) |
| 5 | 17/23 (73.91) | 6/23 (26.09) | ||
| 6 | 25/40 (62.50) | 15/40 (37.50) | ||
| 7 | 15/46 (32.61) | 31/46 (67.39) | TR 5, 193/245 (78.78) | TR 4b, 44/68 (64.71) |
| 8 | 9/22 (40.91) | 13/22 (59.09) | ||
| 9 | 11/66 (16.67) | 55/66 (83.33) | TR 5, 149/177 (84.18) | |
| 10 | 9/46 (19.57) | 37/46 (80.43) | ||
| 11 | 2/13 (15.38) | 11/13 (84.62) | ||
| 12 | 2/24 (8.33) | 22/24 (91.67) | ||
| 13 | 4/24 (16.67) | 20/24 (83.33) | ||
| 14 | 0/2 (0.00) | 2/2 (100.00) | ||
| 15 | 0/2 (0.00) | 2/2 (100.00) |
Data are shown as n/total (%). ACR, American College of Radiology; TIRADS(TR), Thyroid Imaging Reporting and Data System; R-TIRADS, revised Thyroid Imaging Reporting and Data System.
Table 3
| Group | ACR TIRADS (points) | Total (N) | Malignant, n (%) | χ² | P |
|---|---|---|---|---|---|
| Group 1 | 4–5 | 71 | 17 (23.9) | 2.292 | 0.13 |
| 6 | 40 | 15 (37.5) | |||
| Group 2 | 6 | 40 | 15 (37.5) | 7.521 | 0.006* |
| 7–8 | 68 | 44 (64.7) | |||
| Group 3 | 4–5 | 71 | 17 (23.9) | 24.141 | <0.001* |
| 6 | 40 | 15 (37.5) | |||
| 7–8 | 68 | 44 (64.7) | |||
| Group 4 | 7–8 | 68 | 44 (64.7) | 11.144 | 0.001* |
| ≥9 | 177 | 149 (84.2) |
P value reflects the differences of the three subcategories. *P<0.05 were considered as significant difference. ACR, American College of Radiology; TIRADS, Thyroid Imaging Reporting and Data System.
Comparison of the three RSSs
The numbers of nodules that were recommended or not recommended for FNAB by the ACR TIRADS, K-TIRADS and R-TIRADS are shown in Table 4. The three RSSs (ACR TIRADS, K-TIRADS, and R-TIRADS) were used to assess the unnecessary biopsy rates and diagnostic performances of these guidelines. The unnecessary biopsy rate of R-TIRADS was similar to that of ACR TIRADS (0.252 vs. 0.287, P>0.05) but higher than that of K-TIRADS (0.252 vs. 0.148, P<0.05). The sensitivity, specificity, positivity predictive value, and negative predictive value of the three RRSs (R-TIRADS, ACR TIRADS, and K-TIRADS) were as follows: 0.746 vs. 0.377 vs. 0.399, respectively; 0.543 vs. 0.619 vs. 0.320, respectively; 0.654 vs. 0.534 vs. 0.404, respectively; and 0.648 vs. 0.462 vs. 0.315, respectively. The R- TIRADS showed the highest AUC value {0.79 [95% confidence intervals (CI): 0.74–0.83]}, followed by the ACR TIRADS (0.69 (95% CI: 0.64–0.75), P=0.046) and K-TIRADS [0.66 (95% CI: 0.60–0.71), P=0.041] (Table 5).
Table 4
| TIRADS guidelines | Pathological diagnosis | Total | |
|---|---|---|---|
| Malignant | Benign | ||
| ACR TIRADS | |||
| Recommended FNAB | 86 | 75 | 161 |
| No-recommended FNAB | 142 | 122 | 264 |
| Total | 228 | 197 | 425 |
| K-TIRADS | |||
| Recommended FNAB | 91 | 134 | 225 |
| No-recommended FNAB | 137 | 63 | 200 |
| Total | 228 | 197 | 425 |
| R-TIRADS | |||
| Recommended FNAB | 170 | 90 | 260 |
| No-recommended FNAB | 58 | 107 | 165 |
| Total | 228 | 197 | 425 |
ACR, American College of Radiology; K, The Korean Society of Thyroid Radiology; R, revised; TIRADS, Thyroid Imaging Reporting and Data System; FNAB, fine needle aspiration biopsy.
Table 5
| RSS | Avoided unnecessary biopsies (%) | FNR % (95% CI) | Sensitivity %(95% CI) | Specificity %(95% CI) | PPV %(95% CI) | NPV %(95% CI) | AUC |
|---|---|---|---|---|---|---|---|
| ACR TIRADS | 122/425 (28.7) | 62.3 (55.9, 68.6) | 37.7 (31.4, 44.1) | 61.9 (55.1, 68.8) | 53.4 (45.6, 61.2) | 46.2 (40.2, 52.3) | 0.69 (0.64, 0.75) |
| K-TIRADS | 63/425 (14.8) | 60.1 (53.7, 66.5) | 39.9 (33.5, 46.3) | 32.0 (25.4, 38.5) | 40.4 (34.0, 46.9) | 31.5 (25.0, 38.0) | 0.66 (0.60, 0.71) |
| R-TIRADS | 107/425 (25.2) | 25.4 (19.7, 31.1) | 74.6 (68.9, 80.3) | 54.3 (47.3, 61.3) | 65.4 (59.6, 71.2) | 64.8 (57.5, 72.2) | 0.79 (0.74, 0.83) |
FNAB, fine needle aspiration biopsy; ACR, American College of Radiology; K, The Korean Society of Thyroid Radiology; R, revised; TIRADS, Thyroid Imaging Reporting and Data System; AUC, area under the receiver operating characteristic curve; CI, confidence intervals; FN, false negative; FNR, false negative rate; PPV, positive predictive value; NPV, negative predictive value; RSS, risk stratification system.
Discussion
To investigate the latent value of an RSS based on B-model US features for reducing the number of unnecessary biopsies and improving the risk stratification capability, we constructed the R-TIRADS by adding a subcategory to the point-based thyroid imaging reporting and data system. Compared with existing RSSs, the use of the R-TIRADS decreases the number of unnecessary FNABs and enhances the sensitivity and negative predictive value in the detection of thyroid cancer. Compared to the K-TIRADS, the ACR TIRADS presented superior specificity (0.619 vs. 0.32; P<0.01) in our study but a slightly lower sensitivity (0.377 vs. 0.399, P>0.05). The ACR TIRADS had a higher unnecessary biopsy rate and AUC (0.287 vs. 0.148 and 0.69 vs. 0.66, respectively). These results are consistent with those of previous studies (17-19). However, the sensitivity and specificity of the ACR TIRADS and K-TIRADS were slightly lower in our investigation than in previous papers (17-19). This is because the subcentimeter nodules in our study accounted for 196 nodules, nearly half of the total nodules. A previous study proved that the diagnostic performance of the TIRADS for subcentimeter nodules (diameter <1.0 cm) was inferior to that for large nodules (diameter >1.0 cm). Compared with subcentimeter nodules, large nodules could obtain a higher AUC [(0.87–0.92) vs. (0.68–0.70)] (20).
The R-TIRADS was developed based on ACR TIRADS (12), so the five US features (composition, echogenicity, shape, margin, and echogenic foci) of malignancy are conformal. The core distinction between the two RSSs is that a subcategory has been added in our R-TIRADS. The original ACR TR 4 category was classified as R-TIRADS 4a, and nodules with 7–8 points were classified as R-TIRADS 4b. The size cutoff for recommending FNAB is the same between ACR TIRADS 5 and R-TIRADS 4b. If nodules were category R-TIRADS 5, the threshold of biopsy was advised to be 0.5 centimeters.
The subclass classification scheme of our stratification system that moderately malignant risk nodules were divided into two subcategories (4a and 4b) resembles the K-TIRADS (subdivision: 4a, 4b, and 4c) (11). Nevertheless, there are two main variances between the two RSSs. First, our R-TIRADS was founded by a point-based approach, but the K-TIRADS was a pattern-based strategy. Second, the connotations of the two US features (composition and echogenic foci) present subtle differences. The R-TIRADS does not need to consider the fraction of a nodule that is solid, as this assessment is not objective and not more significant than the traits of solid-state constituents. Neoplasms that comprise tiny cystic parts (less than 5% of the whole nodule) should be categorized as entities. When diverse types of echogenic foci coexist in the nodule, according to our stratification system, the summation of each type should be computed. Our RSS is also feasible for managing thyroid nodules. On the one hand, our stratification system is a quantitative risk-scoring model, which is objective and repeatable; on the other hand, the counting points procedure is simple.
Many predecessors have performed pioneering work before that. Benjamin and his colleagues used an artificial intelligence method to revise the ACR TIRADS in 2019 (21). Ruan et al. abstracted contrast-enhanced US features and developed the contrast-enhanced US TIRADS to evaluate thyroid nodules (13). These previous surveys are very innovative, but they are difficult to apply to remote areas. However, our stratification system is easier to master and has better adaptability for rural regions.
There are some imperfections in our investigation that need to be noted. First, it was inaccurate to use multiple referenced criteria in our survey. For example, the Bethesda II category was thought to be adequate for diagnosing the neoplasm as benign. Nevertheless, FNAB cytological outcomes can generate missed diagnoses. Such consequences are infrequent, with an approximate prevalence rate of 3.7% in a previous study and an even lower prevalence rate (<1%) in prospective investigations of thyroid neoplasms lacking extremely dubious US characteristics (22-24). Second, we simply accepted thyroid neoplasms that had been subjected to FNAB. In our center, FNAB is generally carried out on nodules with the highest classification. If no suspicious US characteristics are noticed, this procedure will be performed on the largest neoplasm. Therefore, these choice biases can influence the risks of malignancy. In addition, we ignored the risk of malignancy in patients with a single nodule compared with those with several neoplasms. Third, however, we considered R-TIRADS categories 4a and 4b as nodules with different scores, making this scoring system more complicated than that based on ACR TIRADS. Fourth, when this system was applied, we ignored the cost-effectiveness and follow-up management of subcentimeter (0.5–1.0 cm) nodules scored as TR5, which have a relatively high malignancy rate. Papillary carcinoma accounts for most thyroid malignancies, and its fatality rate is less than that of breast cancer. Fifth, only one thyroid follicular carcinoma case was included in this study, and the application value of the R-TIRADS for follicular carcinoma is limited. When using the R-TIRADS or the ACR TIRADS, thyroid nodules receive point totals from the five US features, but the reason for the different weights of each US feature was not interpreted. This issue should be explored further in the future. Sixth, Grani et al. proved that ACR TIRADS achieved the largest decrease in unnecessary thyroid nodule FNABs and outperformed the ATA, AACE/ACE/AME, EU-TIRADS, and K-TIRADS [0.534 vs. (0. 171–0.438)], but the K-TIRADS had the highest sensitivity (91.7%) (7). Ruan and his colleagues compared the diagnostic performance of the ACR with eight other TIRADSs, and they demonstrated that the ACR2017 had the highest AUC value [0.88 vs. (0.70–0.88)] (13). Therefore, based on previous research, we selected only the ACR TIRADS and K-TIRADS to compare with the R-TIRADS. Nevertheless, our outcomes may be more persuasive if we compared the R-TIRADs with more TIRADSs. Seventh, our investigation was a retrospective single institutional study, and analysis was only performed by two radiologists. This may present an important limitation on the external validity of our research in the future.
Conclusions
In conclusion, the R-TIRADS can assist radiologists in effectively diagnosing thyroid nodules and considerably reduce the unnecessary FNAB rate in the clinical management of thyroid nodules.
Acknowledgments
We thank Xiuyuan Chen, MD, and Jing Gu, PhD (Department of Medical Statistics, School of Public Health, Sun Yat-sen University), for their help with the statistical analyses.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-22-1307/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-1307/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The single-center survey was approved by the institutional review board of the Seventh Affiliated Hospital, Sun Yat-sen University (No. KY-2022–066-01) and was granted a waiver of informed consent for the use of patient data due to the retrospective nature of the study. Informed consent for FNAB examinations and/or resection was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tunbridge WM, Evered DC, Hall R, Appleton D, Brewis M, Clark F, Evans JG, Young E, Bird T, Smith PA. The spectrum of thyroid disease in a community: the Whickham survey. Clin Endocrinol (Oxf) 1977;7:481-93. [Crossref] [PubMed]
- Nguyen XV, Job J, Fiorillo LE, Sipos J. Thyroid Incidentalomas: Practice Considerations for Radiologists in the Age of Incidental Findings. Radiol Clin North Am 2020;58:1019-31. [Crossref] [PubMed]
- Durante C, Grani G, Lamartina L, Filetti S, Mandel SJ, Cooper DS. The Diagnosis and Management of Thyroid Nodules: A Review. JAMA 2018;319:914-24. [Crossref] [PubMed]
- Fisher SB, Perrier ND. The incidental thyroid nodule. CA Cancer J Clin 2018;68:97-105. [Crossref] [PubMed]
- Alexander EK, Cibas ES. Diagnosis of thyroid nodules. Lancet Diabetes Endocrinol 2022;10:533-9. [Crossref] [PubMed]
- Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1-133. [Crossref] [PubMed]
- Grani G, Lamartina L, Ascoli V, Bosco D, Biffoni M, Giacomelli L, Maranghi M, Falcone R, Ramundo V, Cantisani V, Filetti S, Durante C. Reducing the Number of Unnecessary Thyroid Biopsies While Improving Diagnostic Accuracy: Toward the "Right" TIRADS. J Clin Endocrinol Metab 2019;104:95-102. [Crossref] [PubMed]
- Hegedüs L. Clinical practice. The thyroid nodule. N Engl J Med 2004;351:1764-71. [Crossref] [PubMed]
- Gharib H, Papini E, Garber JR, Duick DS, Harrell RM, Hegedüs L, Paschke R, Valcavi R, Vitti P. AACE/ACE/AME Task Force on Thyroid Nodules. American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi Medical Guidelines for clinical practice for the diagnosis and management of thyroid nodules--2016 update. Endocr Pract 2016;22:622-39. [PubMed]
- Kwak JY, Han KH, Yoon JH, Moon HJ, Son EJ, Park SH, Jung HK, Choi JS, Kim BM, Kim EK. Thyroid imaging reporting and data system for US features of nodules: a step in establishing better stratification of cancer risk. Radiology 2011;260:892-9. [Crossref] [PubMed]
- Shin JH, Baek JH, Chung J, Ha EJ, Kim JH, Lee YH, et al. Ultrasonography Diagnosis and Imaging-Based Management of Thyroid Nodules: Revised Korean Society of Thyroid Radiology Consensus Statement and Recommendations. Korean J Radiol 2016;17:370-95. [Crossref] [PubMed]
- Tessler FN, Middleton WD, Grant EG, Hoang JK, Berland LL, Teefey SA, Cronan JJ, Beland MD, Desser TS, Frates MC, Hammers LW, Hamper UM, Langer JE, Reading CC, Scoutt LM, Stavros AT. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): White Paper of the ACR TI-RADS Committee. J Am Coll Radiol 2017;14:587-95. [Crossref] [PubMed]
- Ruan J, Xu X, Cai Y, Zeng H, Luo M, Zhang W, Liu R, Lin P, Xu Y, Ye Q, Ou B, Luo B. A Practical CEUS Thyroid Reporting System for Thyroid Nodules. Radiology 2022;305:149-59. [Crossref] [PubMed]
- Zhang P, Liu H, Yang X, Pang L, Gu F, Yuan J, Ding L, Zhang J, Luo W. Comparison of contrast-enhanced ultrasound characteristics of inflammatory thyroid nodules and papillary thyroid carcinomas using a quantitative time-intensity curve: a propensity score matching analysis. Quant Imaging Med Surg 2022;12:5209-21. [Crossref] [PubMed]
- Cibas ES, Ali SZ. The 2017 Bethesda System for Reporting Thyroid Cytopathology. Thyroid 2017;27:1341-6. [Crossref] [PubMed]
- Kaplan EL, Meier P. Nonparametric Estimation from Incomplete Observations. Journal of the American Statistical Association 1958;53:457-81. [Crossref]
- Li W, Wang Y, Wen J, Zhang L, Sun Y. Diagnostic Performance of American College of Radiology TI-RADS: A Systematic Review and Meta-Analysis. AJR Am J Roentgenol 2021;216:38-47. [Crossref] [PubMed]
- Na DG, Paik W, Cha J, Gwon HY, Kim SY, Yoo RE. Diagnostic performance of the modified Korean Thyroid Imaging Reporting and Data System for thyroid malignancy according to nodule size: a comparison with five society guidelines. Ultrasonography 2021;40:474-85. [Crossref] [PubMed]
- Qi Q, Zhou A, Guo S, Huang X, Chen S, Li Y, Xu P. Explore the Diagnostic Efficiency of Chinese Thyroid Imaging Reporting and Data Systems by Comparing With the Other Four Systems (ACR TI-RADS, Kwak-TIRADS, KSThR-TIRADS, and EU-TIRADS): A Single-Center Study. Front Endocrinol (Lausanne) 2021;12:763897. [Crossref] [PubMed]
- Gao L, Xi X, Jiang Y, Yang X, Wang Y, Zhu S, Lai X, Zhang X, Zhao R, Zhang B. Comparison among TIRADS (ACR TI-RADS and KWAK- TI-RADS) and 2015 ATA Guidelines in the diagnostic efficiency of thyroid nodules. Endocrine 2019;64:90-6. [Crossref] [PubMed]
- Wildman-Tobriner B, Buda M, Hoang JK, Middleton WD, Thayer D, Short RG, Tessler FN, Mazurowski MA. Using Artificial Intelligence to Revise ACR TI-RADS Risk Stratification of Thyroid Nodules: Diagnostic Accuracy and Utility. Radiology 2019;292:112-9. [Crossref] [PubMed]
- Bongiovanni M, Spitale A, Faquin WC, Mazzucchelli L, Baloch ZW. The Bethesda System for Reporting Thyroid Cytopathology: a meta-analysis. Acta Cytol 2012;56:333-9. [Crossref] [PubMed]
- Durante C, Costante G, Lucisano G, Bruno R, Meringolo D, Paciaroni A, Puxeddu E, Torlontano M, Tumino S, Attard M, Lamartina L, Nicolucci A, Filetti S. The natural history of benign thyroid nodules. JAMA 2015;313:926-35. [Crossref] [PubMed]
- Ha SM, Baek JH, Choi YJ, Chung SR, Sung TY, Kim TY, Lee JH. Malignancy risk of initially benign thyroid nodules: validation with various Thyroid Imaging Reporting and Data System guidelines. Eur Radiol 2019;29:133-40. [Crossref] [PubMed]


