
Central nervous system involvement in adult-onset hemophagocytic lymphohistiocytosis secondary to lymphoma: a case presentation and literature analysis
Introduction
Hemophagocytic lymphohistiocytosis (HLH) is a hyperinflammatory syndrome characterized by activation of natural killer (NK) cells, cytotoxic T lymphocytes, and macrophages. HLH can be attributed to genetic mutations (primary HLH) or can occur secondary to an infection, malignancy, or autoimmune disorder. For adults, the most common causes are hematologic malignancies, especially T-cell/NK-cell lymphoma or leukemia (1).
Clinical and laboratory manifestations include fever, splenomegaly, cytopenia, hypertriglyceridemia, hemophagocytosis, diminished NK cell activity, hyperferritinemia, and increased sCD25 (2). In addition, central nervous system (CNS) involvement has received increasing attention. There is no precise, uniform definition of CNS-HLH, but most experts agree in suspecting CNS involvement when a patient is diagnosed with HLH with neurological symptoms or cerebrospinal fluid (CSF) abnormalities, or magnetic resonance imaging (MRI) abnormalities. MRI is an excellent tool for detecting brain lesions and is important for diagnosing and treating cranial diseases. We report a rare case of CNS involvement in adult-onset HLH secondary to diffuse large B-cell lymphoma and review the clinical and imaging features of CNS-HLH in adults.
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was given by the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
A 57-year-old male patient experienced repeated coughing and expectoration for 2 weeks and was diagnosed with pulmonary infection based on increased C-reactive protein level and chest computed tomography (CT) scan in October 2021 at a local hospital. Piperacillin–tazobactam was administered to control infection. Unexpectedly, the patient experienced nausea and vomiting 2 days later, followed shortly by consciousness disturbance and persistent fever (fluctuating between 38 °C and 40 °C). Thus, he was admitted to the intensive care unit of the local hospital’s neurology department with suspected encephalitis. Lumbar puncture and CSF testing were performed. Autoimmune encephalitis antibodies and microbial metagenomic next-generation sequencing of CSF were negative. Brain MRI showed abnormal signals in the brain stem, left temporal lobe, and subcortical projections of the parietal lobe without enhancement. Previously, in 2013, the patient had been diagnosed with viral encephalitis because of headache and unresponsiveness, and the observation of left parietal and occipitotemporal gyrus lesions according to brain MRI (Figure S1).
The patient was transferred to Xiangya Hospital owing to the failure of anti-infective therapy, persistent fever, and disturbance of consciousness.
Physical examination revealed consciousness between somnolence and sopor, disorientation of time and location, impaired memory, dyscalculia, and grade 4 muscle strength in all 4 limbs. Complete blood count (CBC) showed progressive pancytopenia (Figure 1). Laboratory tests further revealed that the patient had progressive pancytopenia together with elevated levels of serum ferritin, Ca2+, and interleukin (IL)-10. Following consultation with a hematologist, HLH was identified as a potential diagnosis in this case. Further tests showed elevated sCD25 levels and reduced NK cell activity. There was no serologic evidence of cytomegalovirus, hepatitis, tuberculosis, HIV, or Epstein–Barr virus (EBV) infection. B-ultrasound examination showed splenomegaly but without enlarged lymph nodes. Positron emission tomography–CT (PET-CT) did not disclose abnormal glucose metabolism of malignant solid tumors. CSF examination showed a high total protein content (0.73 g/L, reference = 0.15–0.45 g/L) and a slightly increased immunoglobulin G level (0.05 g/L, reference = 0–0.03 g/L). Owing to the progressive neurological symptoms, cranial MRI was ordered and demonstrated abnormal signals throughout the cortical and subcortical regions of the telencephalon and pontine (Figure 2), which had some similarities with the MRI findings in 2013.

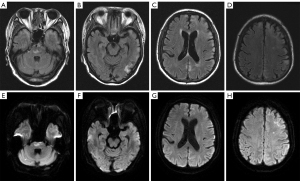
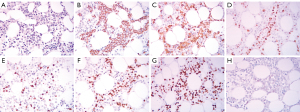
The patient fulfilled 6 of the 8 diagnostic criteria for HLH. In adult patients, the vast majority of HLH cases are secondary, so bone marrow aspiration biopsy was performed to determine the cause of HLH in this patient. Flow cytometry assessment of the bone marrow demonstrated 1.5% clonal B cells in nucleated cells, resulting in a pathological diagnosis of diffuse large B-cell lymphoma after immunohistochemistry (Figure 3). Dexamethasone was administered under the guidance of the hematologist in November 2022, and the patient’s temperature gradually decreased.
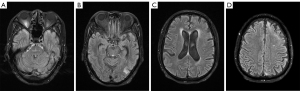
The patient was additionally treated with R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone) in the hematology department. MRI performed 3 months later showed significant regression of the intracranial lesions (Figure 4). Clinically, the patient had few neurologic symptoms, except for a slightly blunted response and impaired memory. As of this writing, he is still being followed up at our hospital.
Discussion
Despite treatment for suspected pulmonary infection, the patient still had a fever and disturbance of consciousness. MRI revealed multiple lesions, but there was no evidence of CNS infection or autoimmune diseases of the CNS. The progressive decline of the CBC, elevated serum ferritin and IL-10 levels, splenomegaly, and hyperpyrexia revealed the possibility of HLH. An elevated sCD25 level and reduced NK cell activity further supported this diagnosis. In adults, most cases of HLH are secondary to infections, tumors, or autoimmune disorders. In this case, there was no evidence of viral infection, especially EBV, or autoimmune diseases. After hematology consultation, abnormal phenotype B cells were found in the bone marrow with flow cytometry, and further bone marrow biopsy revealed diffuse large B-cell lymphoma. Thus, the HLH was confirmed as being secondary to diffuse large B-cell lymphoma.
Among children with HLH, 47–73% present with manifestations of neurological involvement (3,4), which is a marker of poor prognosis in children (5,6). On the other hand, 10% of adults with HLH have CNS involvement (7), so this patient represents a rare case of CNS-HLH. Because of the atypical initial clinical presentation of cough, expectoration, fever, and CNS symptoms, as well as abnormal cortical and subcortical signals on MRI, alternative diagnoses were considered. These included viral meningitis, autoimmune encephalitis, mitochondrial disease, and malignancy.
We searched the literature for cases of CNS-HLH with detailed MRI data that had been independently analyzed by 2 doctors. The underlying cause in 5 of 21 cases could not be found or was not mentioned. The mean age of the 21 patients was 43±17 years, and there were 11 (52%) women. The most common clinical manifestations were consciousness disturbance (15/21) and seizures (11/21) (Table 1). In CSF examination, a mildly elevated white blood cell count with lymphocyte predominance and a mildly elevated protein content were found, although some patients exhibited normal CSF. Excluding the 2 cases of diffuse cerebral hemorrhage (Table 1), Table 2 summarizes the characteristics of the remaining 19 cases. Most lesions on MRI were multiple (Table 2) and mainly located in the supratentorial area, and almost none involved the infratentorial area alone. Of the 19 cases, deep white matter (14/19) was most frequently involved, followed by cortical or subcortical white matter regions (10/19), periventricular white matter (8/19), basal ganglia (8/19), and pons (5/19). Hemorrhage, perhaps due to perivascular infiltration causing injury, was reported in some cases (5/21) (7,9,25,26). Enhancement and diffusion restriction of the lesion was also reported in some cases. A Chinese retrospective case series showed that consciousness disturbance and focal lesions in the cortical and adjacent subcortical regions were the most common signs, but the proportion of diffusion restriction and enhancement was significantly higher than that encountered in our literature review (7). According to histological examination of the brain, CNS-HLH is characterized by CD8+ T cell infiltration of the parenchymal and perivascular tissues, which activate macrophages. Most patients were treated with the guideline-directed HLH-1994 or HLH-2004 protocol, and achieved remission (12/19). Interestingly, most reported deaths were attributed to complications such as infection rather than CNS deterioration.
Table 1
| Author | Sex | Age, years | Neurological symptoms | Underlying condition | Brain pathology | CSF findings | MRI findings | Treatment | Prognosis |
|---|---|---|---|---|---|---|---|---|---|
| Ruppert et al. (8) | F | 28 | Obtunded, comatose | SLE | – | – | FLAIR: abnormal signals in bilateral thalami, adjacent portions of the posterior limb of the internal capsule bilaterally, midbrain, pons, medulla, and bilateral middle cerebellar peduncles | Cyclosporin A, dexamethasone, etoposide, IT-MTX, IVIG | Improved |
| Gratton et al. (9) | M | 38 | Disorientation, somnolence, spastic tetraparesis, seizure, cognitive slowing | Ankylosing spondylitis, SLE | – | WBC: 2 (100% L) | FLAIR: increased signal in the basal ganglia and patchy periventricular and subcortical white matter hyperintensities | Cyclosporine, dexamethasone, etoposide | Improved |
| Pro: 147 mg/dL | DWI: scattered punctate foci of restricted diffusion | ||||||||
| Contrast-enhanced T1: nodular enhancement in the basal ganglia and periventricular white matter | |||||||||
| Gratton et al. (9) | F | 62 | Encephalopathy, seizure | Rheumatoid arthritis, EBV infection | – | WBC: 11 (77% L) | FLAIR: hyperintensity in the basal ganglia, external capsule, periventricular white matter | Dexamethasone, etoposide, rituximab | Improved, but anemia and failure to thrive |
| Pro: 79 mg/dL | |||||||||
| Gratton et al. (9) | F | 21 | Seizure | Malaria | – | WBC: 43 (24% L) | FLAIR: hyperintensity in the basal ganglia, external capsule, cortex, and subcortical white matter | Dexamethasone, cladribine, IVIG | Died after prolonged hospital course because of complications |
| Pro: 1,206 mg/dL | SWI: hemorrhage in left temporal–occipital lobe | ||||||||
| Pastula et al. (10) | M | 55 | Disequilibrium, gait unsteadiness, left foot drop, right-hand paresthesia, seizure | Unknown | Brain autopsy: profound histiocytic infiltration, perivascular lymphocytosis, emperipolesis | WBC: 1 | FLAIR: hyperintensity in supra- and infratentorial areas involving the cortex and juxtacortical white matter | Solumedrol, steroid, PE | Died 1 year after diagnosis because of aspiration pneumonia |
| Pro: 69 mg/dL | Contrast-enhanced T1: cortical enhancement | ||||||||
| Gold et al. (11) | M | 63 | Seizure, left hemiparesis, deteriorating mental status | Rheumatoid arthritis | – | WBC: 4 (52% L) | FLAIR: hyperintensity in the right frontal and parietal cortical regions and throughout the subcortex | Dexamethasone, cyclosporine, etanercept, PE | Improved |
| Pro: 82 mg/dL | |||||||||
| Anderson et al. (12) | F | 56 | Encephalopathy | Unknown | – | Pro: 40 mg/dL | FLAIR: hyperintensity in bihemispheric white matter and basal ganglia | Steroid, chemotherapy | Died 6 years after diagnosis because of polymicrobial bacteremia |
| DWI: diffusion restriction | |||||||||
| Contrast-enhanced T1: enhancement | |||||||||
| Magaki et al. (13) | M | 41 | Altered mental status, headache, apraxia, coma | EBV infection | Brain autopsy: lymphocytic inflammation, necrosis, gliosis, focal hemorrhage | WBC: L↑ | FLAIR: hyperintensity in multifocal, gyriform cortical, diffusion restriction | Dexamethasone, etoposide, acyclovir, methotrexate, ganciclovir | Death from CNS relapsing |
| Pro: >620 mg/dL | Contrast-enhanced T1: hyperintensity in multifocal, gyriform cortical, subtle enhancement | ||||||||
| Barmettler et al. (14) | F | 24 | Unresponsive, seizure | Familial HLH | – | – | FLAIR: hyperintensity in periventricular and subcortical white matter | Antibiotics, IVIG, prednisone, anakinra, cyclosporin A, dexamethasone, etoposide | Died after 44 days in hospital because of intracranial hemorrhage with herniation |
| Shah et al. (15)‡ | M | 25 | Confusion, seizure | Cutaneous T-cell lymphoma | Brian biopsy: encephalitis | Histiocytes and hemophagocytosis | FLAIR: diffuse white matter signal in the right temporal, occipital and parietal lobes, edema | Alemtuzumab, etoposide, dexamethasone, IT-MTX | Improved |
| SWI: hemorrhage in left anterior capsular/caudate | |||||||||
| Algahtani et al. (16) | M | 20 | Headache, decrease in vision, seizure | Familial HLH | – | Pleocytosis | FLAIR: multiple confluent white matter demyelinating lesions in both cerebral hemispheres (mostly involving the parietal and occipital lobes), corpus callosum, cerebellar hemispheres, dorsal pons, brain volume loss | Steroid, PE, cyclosporin A, dexamethasone, etoposide, allogeneic stem cell transplantation | – |
| Pro: 0.76 mg/dL | Contrast-enhanced T1: nodular and linear enhancement | ||||||||
| Han et al. (17) | F | 52 | Alert, abnormal behavior, seizure | Infection | – | IgG: 43.6 mg/dL | FLAIR: hyperintensity in bilateral deep white matter | Doxorubicin, etoposide, methylprednisolone | >6 months no seizures or limb convulsions |
| Verma et al. (18)‡ | F | 68 | Confused, forgetful, seizure | Intravascular large B-cell lymphoma | – | Macrophages: ↑ | FLAIR: hyperintensity in the right hemisphere, medial left occipital lobe, and pons | Dexamethasone, etoposide, IT-MTX, hydrocortisone, rituximab, cyclophosphamide, doxorubicin, prednisone | Improved |
| Radmanesh et al. (19)† | F | 44 | Altered consciousness | Unknown | – | – | SWI: innumerable microscopic and small hemorrhages | Dexamethasone, etoposide | Improved |
| Oppegard et al. (20)‡ | F | 70 | Encephalopathy, visual hallucinations, diplopia, gaze | Intravascular large B-cell lymphoma | – | WBC: 5 | T2: hyperintensity in the pons and supratentorial lesions | Antimicrobials, etoposide, IT-MTX, R-CHOP | – |
| Pro: 89 mg/dL | |||||||||
| Hiraldo et al. (21) | M | 43 | Disturbance of consciousness, cognitive and language impairment, left limbs hemiparesis, seizure | Unknown | Brain biopsy: lymphohistiocytic parenchymal and perivascular inflammatory infiltrates and foci of necrosis with macrophages | WBC: 2 (100% L) | T2: heterogeneous mass located in the right hemisphere parenchyma with associated vasogenic edema | Antibiotherapy, acyclovir, corticosteroids, anakinra | Improved |
| Pro: 90 mg/dL | Contrast-enhanced T1: leptomeningeal enhancement and nodular enhancement in right basal ganglia and subcortical white matter | ||||||||
| Fohle et al. (22) | M | 51 | Lightheadedness, confusion | Unknown | Brain biopsy: no evidence of malignancy or infection | – | FLAIR: hyperintensity in the right frontal lobe with a small hemorrhagic focus | Dexamethasone, etoposide, prophylactic Bactrim DS, acyclovir | Recovered |
| Lehrer et al. (23)‡ | F | 32 | Headache, irritability, right leg weakness, urinary hesitancy | Anaplastic large cell lymphoma | – | WBC: 11 (48% L) | FLAIR: hyperintensity in the basal ganglia, lateral ventricles, and corona radiata | IT-MTX, anakinra, dexamethasone, CHOP-E | Sustained remission |
| Pro: 56.4 mg/dL | Contrast-enhanced T1: enhancement in the gangliocapsular region, temporal lobe, and corona radiata | ||||||||
| Southam et al. (24) | F | 22 | Diplopia, hemianesthesia, hemiparesis, incoordination, headache, tremor | Familial HLH | – | WBC: ↑ | T2: hyperintensity in the cerebellar hemispheres and white matter with leptomeningeal involvement | Dexamethasone, etoposide, IT-MTX | Died 2.5 years after diagnosis because of infectious complications |
| Pro: ↑ | |||||||||
| Southam et al. (24)† | M | 30 | Dysarthria, hemiplegia, cognitive decline | Familial HLH | – | – | SWI: hemorrhage involving unilateral thalamus and midbrain and innumerable microhemorrhages | Cyclosporin A, dexamethasone, etoposide, IT-MTX, rituximab | Died several years after diagnosis |
| Present case‡ | M | 57 | Disturbance of consciousness, anomalous behavior | Diffuse large B-cell lymphoma | – | Pro: 73 mg/dL | FLAIR: hyperintensity in the pons and cortical and subcortical of the telencephalon, cerebral atrophy Contrast-enhanced T1: no enhancement |
Dexamethasone, PE, R-CHOP | Improved |
| DWI: no diffusion restriction |
†, MRI indicated diffuse cerebral hemorrhage; ‡, CNS involvement in HLH secondary to lymphoma. HLH, hemophagocytic lymphohistiocytosis; CNS, central nervous system; CSF, cerebrospinal fluid; MRI, magnetic resonance imaging; SLE, systemic lupus erythematosus; EBV, Epstein–Barr virus; WBC, white blood cell; L, lymphocytes; Pro, protein; FLAIR, fluid-attenuated inversion recovery; DWI, diffusion-weighted imaging; SWI, susceptibility-weighted imaging; IT-MTX, intrathecal methotrexate; IVIG, intravenous immunoglobulin; PE, plasma exchange; R-CHOP, cyclophosphamide, doxorubicin, vincristine, prednisone, and rituximab; CHOP-E, cyclophosphamide, doxorubicin, vincristine, prednisone, and etoposide.
Table 2
| MRI findings | No. of cases |
|---|---|
| Lesion localization | |
| Supratentorial | 12/19 |
| Infratentorial | 0/19 |
| Both | 7/19 |
| Quantity of lesions | |
| Solitary | 0/19 |
| Multiple | 17/19 |
| Diffuse infiltrative | 2/19 |
| Lesion enhancement‡ | 7/19 |
| Diffusion restriction§ | 2/19 |
| Lesion localization details | |
| Periventricular white matter | 8/19 |
| Deep white matter | 14/19 |
| Cortical and/or subcortical regions | 10/19 |
| Thalamus | 1/19 |
| Basal ganglia | 8/19 |
| Pons | 5/19 |
| Cerebellar | 3/19 |
| Meningeal | 2/19 |
| Hemorrhage | 5/19 |
†, statistics based on MRI provided in the article. ‡, presence of enhancement was assessed in only 9 patients, and there was no enhancement in 2 patients. Enhancement-weighted imaging was not mentioned for the others. §, presence of diffusion restriction was assessed in only 2 patients; diffusion restriction was reported in 3 patients but without diffusion-weighted imaging in the study. Diffusion-weighted imaging was not mentioned in the others. HLH, hemophagocytic lymphohistiocytosis; CNS, central nervous system; MRI, magnetic resonance imaging.
Secondary CNS lymphoma mostly involves deep gray or white matter, and lesions show enhancement on enhanced T1-weighted image and diffusion restriction (27,28). The patient described in this report had no limited space-occupying lesions and no enhancement, which is inconsistent with lymphoma. Despite the lack of a brain biopsy, based on the clinical presentation and imaging findings, we deemed that the lesions observed by MRI were CNS-HLH rather than lymphoma. Few studies have reported CNS involvement in HLH secondary to lymphoma. We searched the literature for studies on CNS involvement in HLH secondary to lymphoma that provided detailed MRI data (15,18,20,23) (Table 1). The mean age of the 5 patients (including the present patient) was 50±21 years, and 3 were female (60%). Neurological symptoms and CSF changes were similar to other cases of secondary HLH, and CSF flow cytometry showed lymphoma infiltration in 1 case. Imaging showed that white matter lesions (5/5) and pons (3/5) were more common, with punctate enhancement reported in 1 case and diffusion restriction in 1 case. Notably, HLH secondary to intravascular large B-cell lymphoma (IVLBCL) was the most common. It has been reported that neurological symptoms are present in 35% of patients with IVLBCL, lesions mostly involve the pons, and the number of patients with a hyperintense lesion in the pons with HLH is higher than that without HLH (29). The clinical features of IVLBCL include a hemophagocytic syndrome-associated variant (30). Therefore, it is difficult to definitively determine whether CNS symptoms are caused by HLH or IVLBCL, but it is clear that HLH can aggravate intracranial lesions. The underlying disease increases the complexity of the MRI findings. Thus, the CNS symptoms and MRI findings should be considered in conjunction with other tests in the early stage, and a brain biopsy should be considered if diagnosis is difficult.
This patient had neurological symptoms in 2013, and MRI showed similar cortical and subcortical involvement to the episode described here. Thus, we should consider the possibility that the “encephalitis” developed 8 years ago was associated with HLH, but this is hard to confirm. Similar observations have been reported in the literature: a patient with CNS-HLH of unknown cause had a similar MRI lesion 10 years earlier, which was also deemed to be an acute-on-chronic manifestation of CNS-HLH (10). Therefore, we believe that MRI can provide more information than expected, and the diagnosis of HLH can be considered in the absence of systemic features.
Conclusions
If prominent fever and neurological symptoms occur in combination with the observation of multiple lesions involving the cortex and subcortex, periventricular white matter, and basal ganglia on MRI, then encephalitis, autoimmunity encephalitis, infection, and diagnosis of HLH syndrome should be considered. We report a rare case of hemophagocytic syndrome secondary to B-cell lymphoma involving the CNS and have summarized the features of MRI in adult-onset HLH. The most common clinical manifestations of CNS-HLH in adults are impaired consciousness and seizures. Most of the brain lesions are located in the white matter and basal ganglia. Importantly, the underlying disease increases the complexity of MRI findings. We believe future larger-sample studies with an analysis of MRI features grouped according to the underlying disease would yield insightful findings.
Acknowledgments
Funding: This work was supported by grants from the National Science Foundation of China (Grant No. 81801203) and Hunan Academician Expert Workstation (Shi Xuemin) Open-end Fund Project (No. 2019YSZJJ12).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-1151/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ricard JA, Charles R, Tommee CG, Yohe S, Bell WR, Flanagan ME. Epstein Virus Barr-Positive Diffuse Large B-Cell Lymphoma Associated with Hemophagocytic Lymphohistiocytosis. J Neuropathol Exp Neurol 2020;79:915-20. [Crossref] [PubMed]
- Al-Samkari H, Berliner N. Hemophagocytic Lymphohistiocytosis. Annu Rev Pathol 2018;13:27-49. [Crossref] [PubMed]
- Yang S, Zhang L, Jia C, Ma H, Henter JI, Shen K. Frequency and development of CNS involvement in Chinese children with hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer 2010;54:408-15. [Crossref] [PubMed]
- Haddad E, Sulis ML, Jabado N, Blanche S, Fischer A, Tardieu M. Frequency and severity of central nervous system lesions in hemophagocytic lymphohistiocytosis. Blood 1997;89:794-800. [Crossref] [PubMed]
- Goo HW, Weon YC. A spectrum of neuroradiological findings in children with haemophagocytic lymphohistiocytosis. Pediatr Radiol 2007;37:1110-7. [Crossref] [PubMed]
- Zhao YZ, Zhang Q, Li ZG, Zhang L, Lian HY, Ma HH, Wang D, Zhao XX, Wang TY, Zhang R. Central Nervous System Involvement in 179 Chinese Children with Hemophagocytic Lymphohistiocytosis. Chin Med J (Engl) 2018;131:1786-92. [Crossref] [PubMed]
- Cai G, Wang Y, Liu X, Han Y, Wang Z. Central nervous system involvement in adults with haemophagocytic lymphohistiocytosis: a single-center study. Ann Hematol 2017;96:1279-85. [Crossref] [PubMed]
- Ruppert P, Edmonds EC, Brook M, Musil S, Han SD. Neuropsychological assessment in a case of adult-onset hemophagocytic lymphohistiocytosis (HLH). Clin Neuropsychol 2012;26:1038-52. [Crossref] [PubMed]
- Gratton SM, Powell TR, Theeler BJ, Hawley JS, Amjad FS, Tornatore C. Neurological involvement and characterization in acquired hemophagocytic lymphohistiocytosis in adulthood. J Neurol Sci 2015;357:136-42. [Crossref] [PubMed]
- Pastula DM, Burish M, Reis GF, Bollen A, Cha S, Ralph J, Douglas VC. Adult-onset central nervous system hemophagocytic lymphohistiocytosis: a case report. BMC Neurol 2015;15:203. [Crossref] [PubMed]
- Gold CA, Sheth SJ, Agarwal S, Claassen J, Foreman B. New-onset seizures in two adults with hemophagocytic lymphohistiocytosis. J Neurol 2015;262:1063-5. [Crossref] [PubMed]
- Anderson TL, Carr CM, Kaufmann TJ. Central nervous system imaging findings of hemophagocytic syndrome. Clin Imaging 2015;39:1090-4. [Crossref] [PubMed]
- Magaki S, Ostrzega N, Ho E, Yim C, Wu P, Vinters HV. Hemophagocytic lymphohistiocytosis associated with Epstein-Barr virus in the central nervous system. Hum Pathol 2017;59:108-12. [Crossref] [PubMed]
- Barmettler S, Nowak RJ, Parker T, Price C. Previously undiagnosed fatal familial haemophagocytic lymphohistiocytosis in a 24-year-old woman. BMJ Case Rep 2016; [Crossref] [PubMed]
- Shah AR, Muzzafar T, Assi R, Schellingerhout D, Estrov Z, Tamamyan G, Kantarjian H, Daver N. Hemophagocytic lymphohistiocytosis in adults: An under recognized entity. BBA Clin 2016;7:36-40. [Crossref] [PubMed]
- Algahtani H, Absi A, Bassuni W, Shirah B. Adult-onset hemophagocytic lymphohistiocytosis type 2 presenting as a demyelinating disease. Mult Scler Relat Disord 2018;25:77-82. [Crossref] [PubMed]
- Han C, Chen K, Gao D, Tuo H. New-onset non-convulsive status epilepticus in an adult with hemophagocytic lymphohistiocytosis: a case report. Quant Imaging Med Surg 2020;10:1559-65. [Crossref] [PubMed]
- Verma A, Sharma A, Robetorye R, Porter A, Hilal T. Intravascular Large B-cell Lymphoma Associated with Systemic and Central Nervous System Hemophagocytic Lymphohistiocytosis: A Case Report. Perm J 2020; [Crossref] [PubMed]
- Radmanesh F, Rodriguez-Pla A, Pincus MD, Burns JD. Severe cerebral involvement in adult-onset hemophagocytic lymphohistiocytosis. J Clin Neurosci 2020;76:236-7. [Crossref] [PubMed]
- Oppegard L, O'Donnell M, Piro K, Shatzel J, Christian R, Raess PW, Desai S. Going Skin Deep: Excavating a Diagnosis of Intravascular Large B Cell Lymphoma. J Gen Intern Med 2020;35:3368-71. [Crossref] [PubMed]
- Hiraldo JDG, Domínguez-Mayoral A, García-Gómez FJ, Fouz-Rosón N, Rivas-Infante E, Cano MAM, Fernández AR, Morillo SG, Fernández NA, de León JAP, Mascarell GN, Lebrón CV. Central nervous system involvement in adult-onset relapsing hemophagocytic lymphohistiocytosis responsive to maintenance treatment with anakinra. J Neuroimmunol 2021;355:577552. [Crossref] [PubMed]
- Fohle E, Afriyie F, Dekowski SS. Central Nervous System Involvement in Adult-Onset Hemophagocytic Lymphohistiocytosis. Cureus 2021;13:e14792. [Crossref] [PubMed]
- Lehrer H, Scigliano E, Chan A. Central Nervous System Hemophagocytic Lymphohistiocytosis (CNS-HLH) from Leptomeningeal Anaplastic Large Cell Lymphoma: Mild Clinical Neurologic Syndrome with Extensive Multifocal White Matter Disease. Clin Neuroradiol 2021;31:881-3. [Crossref] [PubMed]
- Southam C, Grossman J, Hahn C. Primary Adult-Onset Hemophagocytic Lymphohistiocytosis with Neurologic Presentation. Can J Neurol Sci 2022;49:441-4. [Crossref] [PubMed]
- Song Y, Pei RJ, Wang YN, Zhang J, Wang Z. Central Nervous System Involvement in Hemophagocytic Lymphohistiocytosis in Adults: A Retrospective Analysis of 96 Patients in a Single Center. Chin Med J (Engl) 2018;131:776-83. [Crossref] [PubMed]
- Foley JM, Borders H, Kurt BA. A Diagnostic Dilemma: Similarity of Neuroradiological Findings in Central Nervous System Hemophagocytic Lymphohistiocytosis and Aspergillosis. Pediatr Blood Cancer 2016;63:1296-9. [Crossref] [PubMed]
- Wu Y, Wang Y, Sun X, Bai X, Qian J, Zhu H, Cui Q, Xing R, Chen Y, Liu Q, Guo J, Ji N, Sun S, Liu Y. Parenchymal central nervous system involvement in aggressive B-cell lymphoma: retrospective analysis of clinical and MRI features in a Chinese population. BMC Neurol 2019;19:268. [Crossref] [PubMed]
- Malikova H, Burghardtova M, Koubska E, Mandys V, Kozak T, Weichet J. Secondary central nervous system lymphoma: spectrum of morphological MRI appearances. Neuropsychiatr Dis Treat 2018;14:733-40. [Crossref] [PubMed]
- Abe Y, Narita K, Kobayashi H, Kitadate A, Takeuchi M, Kikuchi Y, Ouchi T, Takeuchi K, Matsue K. Clinical value of abnormal findings on brain magnetic resonance imaging in patients with intravascular large B-cell lymphoma. Ann Hematol 2018;97:2345-52. [Crossref] [PubMed]
- Ponzoni M, Campo E, Nakamura S. Intravascular large B-cell lymphoma: a chameleon with multiple faces and many masks. Blood 2018;132:1561-7. [Crossref] [PubMed]


