
The effect of cerebral blood perfusion on the correlation between cerebral stroke onset time and synthetic T2 mapping: a pilot study
Introduction
The latest treatment guidelines for acute ischemic stroke extend the time window for endovascular treatment to a maximum of 24 h for some large-vessel lesions and 4.5 h for small-vessel lesions. Studies have confirmed the clinical benefit of endovascular treatment within this time window (1-6). However, in clinical practice, the exact time of stroke onset is difficult to determine in some acute stroke survivors with wake-up stroke or unconscious acute stroke, making clinical management difficult (7-10). Therefore, accurate stroke time determination is crucial for clinical management. Recent multicenter studies have shown that determination of the stroke onset time with diffusion-weighted imaging–T2-weighted fluid-attenuated inversion recovery (DWI–T2-FLAIR) mismatch has good efficacy in guiding intravenous thrombolytic therapy in patients with uncertain stroke timing, such as a wake-up stroke. However, the T2-FLAIR signal is subject to subjective judgment and the interference of scanning parameters. As seen from previous studies, the correlation between DWI–T2-FLAIR mismatch and onset time deviates significantly (11,12).
The T2 relaxation time (qT2) arises from molecular motion and proton–proton interactions, and it is directly influenced by the local biophysical structure and biochemical environment. Quantitative qT2 is a structural magnetic resonance imaging (MRI) technique that potentially offers a more detailed characterization of tissue compared with conventional qualitative or weighted imaging approaches. Recent preclinical (13-15) and clinical (16,17) studies showed that qT2 could be used to detect brain ischemia and estimate the onset time more accurately than can MRI parameters obtained from weighted images. The qT2 and qT2 ratios in DWI-restricted areas also correlate with stroke onset time, and this correlation is more significant compared to that of T2-FLAIR (18-20).
However, the T2-FLAIR and the qT2 values reflect the local cerebral blood supply deficit that leads to cerebral edema and increased fluid content, and this change will be affected by cerebral blood flow (CBF) (21,22). Previous animal studies have reported that the qT2 and its correlation with onset time were affected by CBF status (23,24). The CBF difference between patients may be one reason for the weak correlation with the time of stroke onset for qT2 and T2-FLAIR changes. Synthetic MRI is a quantitative MRI technology that uses multiecho and multidelay acquisition methods to obtain multiple groups of contrast images and quantitative values of brain relaxometry. Duchaussoy et al. (25) confirmed that the magnetic resonance image compilation (MAGiC) conventional sequence and quantitative imaging could be used as a tool to estimate stroke onset time. The aim of this study was thus to examine the changes of qT2 of the ischemia area and stroke onset time under different CBF conditions and further evaluate the effect of different CBF statuses on the correlations between qT2, T2-FLAIR ratio, and stroke time. T2-weighted image acquisition has a long scanning time due to multiple echo acquisitions; therefore, the qT2 of the lesion was quantified with the MAGiC technique (26,27), and the CBF values of the lesion area were quantified with the 3-dimensional (3D) pseudo-continuous arterial spin labeling perfusion (pcASL) technique (28-32). We present the following article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-22-991/rc).
Methods
Patient selection
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Ethics Review Board of the Liaoning Thrombus Treatment Center of Integrated Chinese and Western Medicine. Patients’ informed consent for this retrospective analysis was waived. From December 2020 to December 2021, data from 112 patients with acute ischemic stroke (symptom onset time ≤24 h) admitted to the Liaoning Thrombus Treatment Center of Integrated Chinese and Western Medicine, Liaoning, China, were collected in this cross-sectional retrospective study. All patients underwent MRI examinations within 24 h of stroke onset and before treatment. An acute ischemic lesion was defined as a parenchymal hyperintensity on DWI, explaining the clinical deficit [hypointensity on the apparent diffusion coefficient (ADC) map]. Time from symptom onset, clinical National Institutes of Health Stroke Scale (NIHSS) score, blood pressure, glucose level, and other clinical information were recorded.
The exclusion criteria were as follows: patients with a stroke onset time longer than 24 h or with uncertain onset times, patients without obviously restricted lesions on their ADC map, patients with both periventricular and parenchymal white matter hyperintensity on T2-FLAIR images, patients with claustrophobia and other contraindications for MRI, patients with poor image quality (e.g., movement artifacts), and patients for whom the acquisition of the full MRI protocol was incomplete.
All patients were divided into 2 groups according to cerebral perfusion results assessed using CBF maps: the good CBF group (CBF >25 mL/100 g/min) and the poor CBF group (CBF ≤25 mL/100 g/min). In addition, the total case group (including all enrolled cases) was primarily used to compare the accuracy of MR quantitative measures in determining stroke onset times of less than 6 h and less than 24 h with those of the 2 previously mentioned subgroups.
Examination methods
All MRI examinations were performed on a 3T GE Pioneer scanner (GE Healthcare) with a 21-channel phased-array head coil. The MRI protocol included DWI, T2-FLAIR, 3D pcASL, and MAGiC sequences. These main technical parameters are detailed in Table 1.
Table 1
| Sequence | Slice thickness (mm) | Matrix | TR (ms) | TE (ms) | Acquisition time (min: s) | Other parameters |
|---|---|---|---|---|---|---|
| T2-FLAIR | 5 | 256×256 | 9,000 | 137 | 1:57 | TI =2,470 ms |
| DWI | 5 | 130×160 | 4,221 | 80.2 | 0:34 | B value =1,000 s/mm2 |
| 3D pcASL | 4 | 512×6 | 4,649 | 10.9 | 3:18 | PLD =2,500 ms |
| MAGiC | 5 | 320×256 | 4,000 | 23/92 | 4:00 |
TE, time of echo; TR, time of repetition; TI, time of inversion; T2-FLAIR, T2-weighted fluid-attenuated inversion recovery; DWI, diffusion-weighted imaging; PLD, postlabel delay time; pcASL, pseudo-continuous arterial spin labeling; MAGiC, magnetic resonance imaging compilation; 3D, 3-dimensional; MRI, magnetic resonance imaging.
Image processing and analysis
The T2 and CBF maps were processed with the MAGiC postprocessing software (MAGIC software version 100.1.1, GE Healthcare) and 3D pcASL, respectively, on an Advantage Workstation (AW version 4.7, GE Healthcare). For each patient, DWI, ADC, CBF, T2-FLAIR, and T2 map images were registered to the AW 4.7 workstation.
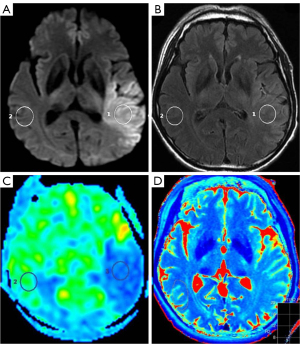
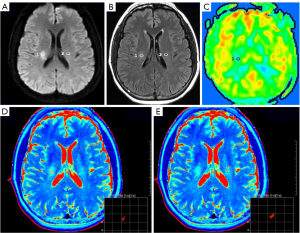
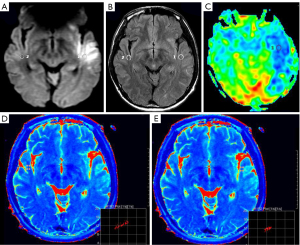
First, the central slice with a maximum area covering the core of the ischemic lesion was selected on DWI with parenchymal hyperintensity. Then, two 1-cm2 2-dimensional regions of interest (ROIs) were placed in the same location on both the individual T2 map and the FLAIR imaging. One ROI was placed at the core of the acute ischemic lesion in the same location as the area of the highest signal intensity on DWI, and the other ROI was placed in the normal contralateral parenchyma (Figures 1-3). The qT2, T2-FLAIR signal intensity, and CBF values were measured on the T2 map, T2-FLAIR image, and CBF map, respectively. The ratio of qT2 (the qT2 ratio) between ischemic and nonischemic contralateral parenchyma, as well as the T2-FLAIR signal intensity ratio (T2-FLAIR ratio), were then calculated. One radiologist who was trained beforehand assessed the whole data set during the same session. The formulae for calculating the qT2 ratio and T2-FLAIR ratio are as follows:
Statistical analysis
All statistical analyses were performed using SPSS 25.0 (IBM Corp.). Qualitative variables are described with numbers and frequencies, and quantitative variables are described with the mean ± standard deviation (SD). The Kolmogorov-Smirnov test was used to check whether MR quantitative data followed a normal distribution. The Pearson (for normally distributed data) or Spearman (for nonnormally distributed data) correlation coefficient (r) was used to evaluate the correlation between stroke onset time and the qT2, qT2 ratio, and T2-FLAIR ratio between DWI-restricted areas and contralateral normal areas for each group. In addition, the sensitivity and specificity of the qT2, qT2 ratio, and T2-FLAIR ratio in predicting the time from symptom onset of less than 6 h was assessed. The significance level was set at a P value <0.05.
Results
Patient population and clinical characteristics
A total of 112 patients were considered for the study, 9 of whom were accepted, with the other patients being excluded for the following reasons: the time of stroke could not be determined or exceeded 24 h (9 cases), no obvious lesion was seen on the DWI map (5 cases), and the scan sequence was incomplete or had poor image quality or obvious artifacts (4 cases; see flow diagram, Figure 4). These 94 patients were divided into 2 groups: the good CBF group (CBF >25 mL/100 g/min; 36 cases, 23 males and 13 female) and the poor CBF group (CBF ≤25 mL/100 g/min; 58 cases, 36 male and 22 females). The patient population and clinical information are shown in Table 2.
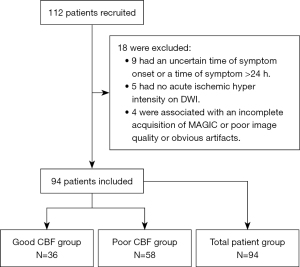
Table 2
| Variables | Good CBF group (n=36) | Poor CBF group (n=58) | t | P value |
|---|---|---|---|---|
| Age (years) | 61.44±9.90 | 61.83±10.03 | −0.181 | 0.857 |
| Female | 13 (36.1) | 22 (37.9) | −0.503 | 0.625 |
| Time from symptom onset (h) | 6.67±4.86 | 8.10±7.17 | −1.060 | 0.292 |
| CBF (mL/100 g/min) | 27.36±9.35 | 18.60±7.22 | 5.096 | 0.000 |
Data are shown as mean ± standard deviation or number (percentage). CBF, cerebral blood flow; poor CBF group, CBF ≤20 mL/100 g/min; good CBF group, CBF >20 mL/100 g/min.
Quantitative measurement and statistical analysis
The qT2 and T2-FLAIR signal intensity of the ischemic area and the contralateral normal area were measured, and then the qT2 ratio and T2-FLAIR ratio were calculated (Table 3). Statistical analysis showed no significant difference in any parameters between the 2 groups.
Table 3
| Parameters | Good CBF group (n=36) | Poor CBF group (n=58) | t | P value |
|---|---|---|---|---|
| qT2 (ms) | 108.28±15.19 | 106.53±15.33 | 0.538 | 0.592 |
| qT2 ratio | 1.30±0.23 | 1.32±0.22 | −0.409 | 0.683 |
| T2-FLAIR ratio | 1.27±0.22 | 1.30±0.23 | −0.577 | 0.565 |
Data are shown as mean ± standard deviation. qT2, T2 relaxation time; T2-FLAIR, T2-weighted fluid-attenuated inversion recovery; CBF, cerebral blood flow.
The effect of CBF status on the correlations between MR quantitative indicators and stroke onset time
The data of all MR quantitative parameters for the 3 groups were normally distributed according to the Kolmogorov-Smirnov test. The correlations between quantitative MR indicators and the time from stroke onset were assessed using Pearson correlation analysis.
In the total patient group, the stroke onset time moderately correlated with the qT2 ratio (r=0.438; P<0.001) but weakly correlated with qT2 (r=0.314; P=0.002) and T2-FLAIR ratio of the lesion area (r=0.352; P=0.001; Figure 5).
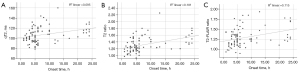
In the good CBF group, the time of stroke onset was not correlated with the qT2 in the ischemic lesion area (r=0.109; P=0.52) and was not correlated with the qT2 (r=0.285; P=0.09) or T2-FLAIR ratio (r=0.226; P=0.18; Figure 6).
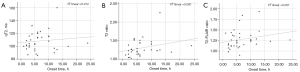
In the poor CBF group, there was a correlation between the time of stroke onset and all MR quantitative indicators (qT2: r=0.409, P=0.001; qT2 ratio: r=0.493, P<0.001; T2-FLAIR ratio: r=0.385, P=0.003; Figure 7). In comparison, the qT2 ratio had a higher correlation with stroke onset time.
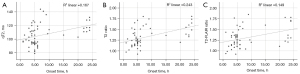
The receiver operating characteristic (ROC) curves of the qT2, qT2 ratio, and T2-FLAIR ratio in the ischemic lesion area in distinguishing whether the stroke onset time was less than 6 h are shown in Figures 8-10. The area under the ROC curve (AUC) and P values are displayed for each MR quantitative indicator in Table 4. The results showed that the AUCs of all parameters in the poor CBF group were higher than those in the good CBF group and the total patient group. The AUC of the qT2 ratio in the total patient group was the closest to that of the poor CBF group.
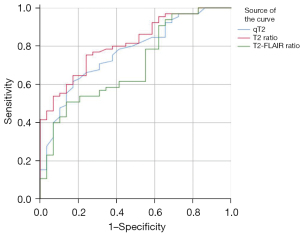
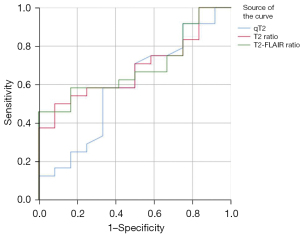
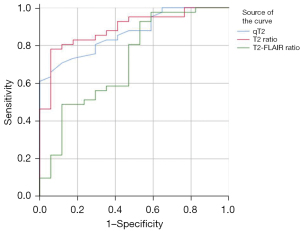
Table 4
| Parameters | Total patient group (n=94) | Good CBF group (n=36) | Poor CBF group (n=58) |
|---|---|---|---|
| qT2 (ms) | 0.767 (0.000) | 0.592 (0.374) | 0.867 (0.000) |
| qT2 ratio | 0.815 (0.000) | 0.684 (0.075) | 0.890 (0.000) |
| T2-FLAIR ratio | 0.700 (0.002) | 0.691 (0.065) | 0.716 (0.010) |
P values are shown in parentheses. qT2, T2 relaxation time; T2-FLAIR, T2-weighted fluid-attenuated inversion recovery; CBF, cerebral blood flow; AUC, area under the curve.
Discussion
This study demonstrated that the correlation between synthetic T2 mapping and stroke onset time could be influenced by the cerebral perfusion status of patients. In patients in the poor CBF group, the change of qT2 in the DWI-restricted lesions significantly correlated with stroke onset time, but there was no obvious correlation between qT2 and stroke onset time in patients in the good CBF group. In comparison, the qT2 ratio had a higher correlation with stroke onset time than did the qT2 and T2-FLAIR ratios. Our results generally support the previous conclusions drawn from animal and patient studies indicating that the qT2 can be used to detect brain ischemia and estimate the onset time of stroke more accurately than can MRI parameters obtained from weighted images (13-17). However, the focal qT2 also varies with stroke duration. Both qT2 and T2-FLAIR signal intensity values reflect the increased fluid content of brain tissue due to postischemic edema. However, T2-FLAIR is a weighted image that is affected by MR scan parameters, whereas qT2 is a quantitative imaging method. Quantitative T2 mapping seeks to parameterize the qT2 in an image. The change in qT2 underpins the FLAIR signal intensity changes. Animal models using rats, cats, and primates, as well as theoretical models, have demonstrated that the qT2 increases in ischemic tissue roughly linearly for the first few hours after onset consistently across species. In particular, local cerebral perfusion has been shown to significantly influence the progression of the infarct lesion; for example, the correlation between the T2-FLAIR ratio and stroke time is significantly higher in patients with poor collateral circulation, whereas it is not significantly correlated in patients with good collateral circulation (22). This result is consistent with our conclusion concerning the significant correlation between the qT2 and T2-FLAIR ratio with stroke onset time in the poor CBF group.
Synthetic MRI, a new MR imaging technique, has a scan time of within 3 to 5 min. It can reduce the scan time by acquiring multiple contrast images such as T2-weighted imaging (T2WI), T1WI, and phase-sensitive inversion recovery (PSIR; vessel images) at the same time and also acquire qT2, qT1, and quantitative proton density value (qPD) simultaneously. Previous research has validated the use of the MAGiC conventional sequence and quantitative imaging in patients with stroke. Its difference and ratio of the measured qT2 correlated significantly with a time to stroke onset of within 4.5 h (25). However, in this study, the most significant correlation between the time to stroke and qT2 ratio was seen in the total patient group, with the second-strongest correlation being with T2-FLAIR values and the weakest being with qT2. This finding differs from the results of previous studies (17,18,25). This difference may be due to the inconsistent onset times of the included patients. It could also be due to inconsistent data, as the effect of CBF was not considered in previous studies. In our study, the time from stroke onset did not correlate with the qT2, qT2 ratio, or T2-FLAIR ratio in the good CBF group. In contrast, the time from stroke onset was significantly more correlated with the qT2, qT2 ratio, and T2-FLAIR ratio in the poor CBF group than in the total patient group. The AUC was higher in the poor CBF group than in the good CBF group and in the total patient group; meanwhile, the AUC of the qT2 ratio was higher than that of the T2-FLAIR ratio, suggesting that changes in CBF values may affect the accurate assessment of stroke onset time by MRI.
This study has some limitations. First, the sample size was small, and a follow-up study with a larger sample is needed. Second, this was a pilot study; therefore, the sizes and sites of strokes were not distinguished, and some studies have shown the correlation between stroke time and qT2 to be not significant in white and gray matter (16). Third, some patients with recurrence never underwent intravenous thrombolytic therapy, but some studies have shown that intravenous thrombolytic therapy does not affect the correlation between the qT2 and time from stroke onset (22).
Our study shows that cerebral perfusion conditions have an impact on the correlations of the qT2, qT2 ratio, and T2-FLAIR ratio with the stroke onset time. This may affect the accuracy of predicting stroke onset time when the qT2, qT2 ratio, and T2-FLAIR ratio are used in patients who have experienced a wake-up stroke. The application of this method for the determination of clinical treatment may include an additional proportion of patients with normal cerebral perfusion.
Conclusions
Our preliminary results showed that the correlation between synthetic T2 mapping and stroke onset time could be influenced by the cerebral perfusion status of patients. The change of the qT2 in DWI-restricted lesions significantly correlated with the stroke onset time in the poor CBF group, but there was no obvious correlation between the qT2 and stroke onset time in the good CBF group. This could influence the accuracy of synthetic T2 mapping in predicting the stroke onset time in patients who experience wake-up stroke. Further study is required to expand the sample size and clarify the correlation between the qT2 and a stroke onset time of within 6 h in patients with poor CBF status.
Acknowledgments
Funding: This study was supported by the Special Clinical Research Program of Wu Jieping Medical Foundation (No. 320.6750.2020-11-22).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-22-991/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-991/coif). FB is an employee of Philips (China) Investment Co., Ltd. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Review Board of the Liaoning Thrombus Treatment Center of Integrated Chinese and Western Medicine, and the patient’s informed consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, et al. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N Engl J Med 2018;378:708-18. [Crossref] [PubMed]
- Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N Engl J Med 2018;378:11-21. [Crossref] [PubMed]
- Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 2015;372:2285-95. [Crossref] [PubMed]
- Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015;372:2296-306. [Crossref] [PubMed]
- Martins SO, Mont'Alverne F, Rebello LC, Abud DG, Silva GS, Lima FO, et al. Thrombectomy for Stroke in the Public Health Care System of Brazil. N Engl J Med 2020;382:2316-26. [Crossref] [PubMed]
- Román LS, Menon BK, Blasco J, Hernández-Pérez M, Dávalos A, Majoie CBLM, et al. Imaging features and safety and efficacy of endovascular stroke treatment: a meta-analysis of individual patient-level data. Lancet Neurol 2018;17:895-904. [Crossref] [PubMed]
- Zhang YL, Zhang JF, Wang XX, Wang Y, Anderson CS, Wu YC. Wake-up stroke: imaging-based diagnosis and recanalization therapy. J Neurol 2021;268:4002-12. [Crossref] [PubMed]
- Silva GS, Lima FO, Camargo EC, Smith WS, Singhal AB, Greer DM, Ay H, Lev MH, Harris GJ, Halpern EF, Sonni S, Koroshetz W, Furie KL. Wake-up stroke: clinical and neuroimaging characteristics. Cerebrovasc Dis 2010;29:336-42. [Crossref] [PubMed]
- Mackey J, Kleindorfer D, Sucharew H, Moomaw CJ, Kissela BM, Alwell K, Flaherty ML, Woo D, Khatri P, Adeoye O, Ferioli S, Khoury JC, Hornung R, Broderick JP. Population-based study of wake-up strokes. Neurology 2011;76:1662-7. [Crossref] [PubMed]
- Thomalla G, Boutitie F, Fiebach JB, Simonsen CZ, Nighoghossian N, Pedraza S, Lemmens R, Roy P, Muir KW, Ebinger M, Ford I, Cheng B, Galinovic I, Cho TH, Puig J, Thijs V, Endres M, Fiehler J, Gerloff C. WAKE-UP Investigators. Stroke With Unknown Time of Symptom Onset: Baseline Clinical and Magnetic Resonance Imaging Data of the First Thousand Patients in WAKE-UP (Efficacy and Safety of MRI-Based Thrombolysis in Wake-Up Stroke: A Randomized, Doubleblind, Placebo-Controlled Trial). Stroke 2017;48:770-3. [Crossref] [PubMed]
- Petkova M, Rodrigo S, Lamy C, Oppenheim G, Touzé E, Mas JL, Méder JF, Oppenheim C. MR imaging helps predict time from symptom onset in patients with acute stroke: implications for patients with unknown onset time. Radiology 2010;257:782-92. [Crossref] [PubMed]
- Thomalla G, Simonsen CZ, Boutitie F, Andersen G, Berthezene Y, Cheng B, et al. MRI-Guided Thrombolysis for Stroke with Unknown Time of Onset. N Engl J Med 2018;379:611-22. [Crossref] [PubMed]
- McGarry BL, Rogers HJ, Knight MJ, Jokivarsi KT, Sierra A, Gröhn OH, Kauppinen RA. Stroke onset time estimation from multispectral quantitative magnetic resonance imaging in a rat model of focal permanent cerebral ischemia. Int J Stroke 2016;11:677-82. [Crossref] [PubMed]
- McGarry BL, Rogers HJ, Knight MJ, Jokivarsi KT, Gröhn OHJ, Kauppinen RA. Determining stroke onset time using quantitative MRI: high accuracy, sensitivity and specificity obtained from magnetic resonance relaxation times. Cerebrovasc Dis Extra 2016;6:60-5. [Crossref]
- Rogers HJ, McGarry BL, Knight MJ, Jokivarsi KT, Gröhn OH, Kauppinen RA. Timing the ischaemic stroke by 1H-MRI: improved accuracy using absolute relaxation times over signal intensities. Neuroreport 2014;25:1180-5. [Crossref] [PubMed]
- Damion RA, Knight MJ, McGarry BL, Bosnell R, Jezzard P, Harston GW, Carone D, Kennedy J, El-Tawil S, Elliot J, Muir KW, Clatworthy P, Kauppinen RA. Quantifying T (2) relaxation time changes within lesions defined by apparent diffusion coefficient in grey and white matter in acute stroke patients. Phys Med Biol 2019;64:095016. [Crossref] [PubMed]
- Siemonsen S, Mouridsen K, Holst B, Ries T, Finsterbusch J, Thomalla G, Ostergaard L, Fiehler J. Quantitative t2 values predict time from symptom onset in acute stroke patients. Stroke 2009;40:1612-6. [Crossref] [PubMed]
- Cheng B, Brinkmann M, Forkert ND, Treszl A, Ebinger M, Köhrmann M, Wu O, Kang DW, Liebeskind DS, Tourdias T, Singer OC, Christensen S, Luby M, Warach S, Fiehler J, Fiebach JB, Gerloff C, Thomalla GSTIR and VISTA Imaging Investigators. Quantitative measurements of relative fluid-attenuated inversion recovery (FLAIR) signal intensities in acute stroke for the prediction of time from symptom onset. J Cereb Blood Flow Metab 2013;33:76-84. [Crossref] [PubMed]
- Lin SP, Schmidt RE, McKinstry RC, Ackerman JJ, Neil JJ. Investigation of mechanisms underlying transient T2 normalization in longitudinal studies of ischemic stroke. J Magn Reson Imaging 2002;15:130-6. [Crossref] [PubMed]
- Campbell BC, Christensen S, Tress BM, Churilov L, Desmond PM, Parsons MW, Barber PA, Levi CR, Bladin C, Donnan GA, Davis SM. EPITHET Investigators. Failure of collateral blood flow is associated with infarct growth in ischemic stroke. J Cereb Blood Flow Metab 2013;33:1168-72. [Crossref] [PubMed]
- Wouters A, Dupont P, Christensen S, Norrving B, Laage R, Thomalla G, Albers G, Thijs V, Lemmens R. Association Between Time From Stroke Onset and Fluid-Attenuated Inversion Recovery Lesion Intensity Is Modified by Status of Collateral Circulation. Stroke 2016;47:1018-22. [Crossref] [PubMed]
- Leng X, Lan L, Liu L, Leung TW, Wong KS. Good collateral circulation predicts favorable outcomes in intravenous thrombolysis: a systematic review and meta-analysis. Eur J Neurol 2016;23:1738-49. [Crossref] [PubMed]
- Kauppinen RA. Multiparametric magnetic resonance imaging of acute experimental brain ischaemia. Prog Nucl Magn Reson Spectrosc 2014;80:12-25. [Crossref] [PubMed]
- Jokivarsi KT, Hiltunen Y, Tuunanen PI, Kauppinen RA, Gröhn OH. Correlating tissue outcome with quantitative multiparametric MRI of acute cerebral ischemia in rats. J Cereb Blood Flow Metab 2010;30:415-27. [Crossref] [PubMed]
- Duchaussoy T, Budzik JF, Norberciak L, Colas L, Pasquini M, Verclytte S. Synthetic T2 mapping is correlated with time from stroke onset: a future tool in wake-up stroke management? Eur Radiol 2019;29:7019-26. [Crossref] [PubMed]
- Wang Q, Wang G, Sun Q, Sun DH. Application of MAGnetic resonance imaging compilation in acute ischemic stroke. World J Clin Cases 2021;9:10828-37. [Crossref] [PubMed]
- Zaharchuk G. Arterial spin-labeled perfusion imaging in acute ischemic stroke. Stroke 2014;45:1202-7. [Crossref] [PubMed]
- de Havenon A, Haynor DR, Tirschwell DL, Majersik JJ, Smith G, Cohen W, Andre JB. Association of Collateral Blood Vessels Detected by Arterial Spin Labeling Magnetic Resonance Imaging With Neurological Outcome After Ischemic Stroke. JAMA Neurol 2017;74:453-8. [Crossref] [PubMed]
- van Osch MJ, Teeuwisse WM, Chen Z, Suzuki Y, Helle M, Schmid S. Advances in arterial spin labelling MRI methods for measuring perfusion and collateral flow. J Cereb Blood Flow Metab 2018;38:1461-80. [Crossref] [PubMed]
- Wang X, Dou W, Dong D, Wang X, Chen X, Chen K, Mao H, Guo Y, Zhang C. Can 3D Pseudo-Continuous Territorial Arterial Spin Labeling Effectively Diagnose Patients With Recanalization of Unilateral Middle Cerebral Artery Stenosis? J Magn Reson Imaging 2021;54:175-83. [Crossref] [PubMed]
- Aracki-Trenkic A, Law-Ye B, Radovanovic Z, Stojanov D, Dormont D, Pyatigorskaya N. ASL perfusion in acute ischemic stroke: The value of CBF in outcome prediction. Clin Neurol Neurosurg 2020;194:105908. [Crossref] [PubMed]
- Chen CY, Li CW, Mak HKF, Lin MF, Chan WP. Combined native magnetic resonance angiography, flow-quantifying, and perfusion-imaging for impending second-stroke assessment. Quant Imaging Med Surg 2019;9:521-9. [Crossref] [PubMed]


