
Using 2D/3D ultrasound observation of endometrial thickness, endometrial volume, and blood flow changes to predict the outcome of frozen embryo transfer cycles: a prospective study
Introduction
Endometrial receptivity (ER) refers to the likelihood of embryo implantation in the endometrium during the window of implantation. ER has been the focus of research for more than 80 years since Rock and Bartlett described histological changes in the endometrium before and after implantation in 1937 (1). Advances in detection techniques have led to a better understanding of embryo-endometrial dialogue and implant-related processes (2-4). However, few advances have been made in translating this knowledge into methods to provide a clinically meaningful assessment of ER (5). Many methods for evaluating ER require endometrial biopsies, such as pinocytosis tests, endometrial receptivity array (ERA), and endometrial function tests; these tests are invasive and must be performed during the stimulation cycle, which delays the transplant cycle (6-8). In contrast, ultrasound imaging is noninvasive and reproducible. Ultrasound evaluation of ER includes measurements of endometrial thickness (EMT), endometrial patterns, endometrial volume (EV), and endometrial blood flow (9,10). However, the results of studies using a single ultrasound evaluation of ER are inconsistent (11,12). For example, EMT is widely regarded as a predictor of ER, and a thin EMT is associated with a low in vitro fertilization (IVF) success rate (13). However, some studies have shown that EMT before transplantation is not significant for predicting IVF outcomes (14-16). Therefore, we aimed to determine whether the changes in endometrial indicators are meaningful for predicting IVF outcomes in frozen embryo transfer (ET) cycles. We present the following article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-22-705/rc).
Methods
Study design and participants
This was a prospective cross-sectional study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The ethics committee of Dalian Women and Children’s Medical Group approved the research protocol (No. 2020013). All the women who agreed to participate in the study signed informed consent forms. Women who underwent IVF were enrolled between September 2020 and July 2021. The criteria for inclusion were frozen cleavage-stage ETs and transplantation of 1 or 2 high-quality embryos. The exclusion criteria were ultrasonographic findings of a poorly displayed endometrium, uterine malformation, adenomyosis, endometriosis, uterine fibroids, hydrosalpinx, or intrauterine adhesions. Patients to be enrolled underwent an ultrasound examination on the second day of menstruation for detection of items related to the exclusion criteria. For patients identified for enrollment, clinical information was recorded, including age, body mass index (BMI), time of infertility, and levels of the basic hormone indicators estradiol (E2), progesterone, and anti-Müllerian hormone (AMH).
The enrolled patients underwent ultrasound examination 3 times: the day of progesterone administration but before progesterone administration (day P), the third day after progesterone administration (day P + 2), and the day of embryo transplantation (day of ET). Ultrasound examinations were performed from 7 am to 9 am. One doctor with over 20 years of experience in conducting gynecological ultrasounds completed all the examinations. Two-dimensional ultrasound was used to record EMT, 3-dimensional (3D) ultrasound was used to record EV, and 3-dimensional power Doppler (3D-PD) ultrasound imaging was used to record the following endometrial blood flow parameters: vascular index (VI), flow index (FI), and vascular flow index (VFI). First, we validated the intraoperator 3-time measurement agreement for the 3D imaging–related measurements EV, VI, FI, and VFI. All examinations were performed using a Voluson E10 (GE Healthcare) ultrasound machine (probe model RIC5-9-D). The E2 level was tested on day P and day P + 2. For each patient, 2 consecutive changes during the 3 inspections of EMT, EV, VI, FI, and VFI and 1 change during the 2 inspections of the E2 level were categorized as “declining” or “nondeclining” (Figures 1,2). A decrease in a value was considered “declining”, and a value that did not change or that increased was considered “nondeclining”. The relationships between changes in a given indicator and the IVF outcome were analyzed.
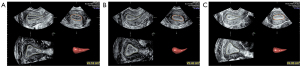
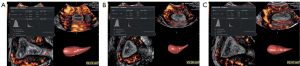
Treatment protocol
All patients received hormone replacement therapy (HRT) for endometrium preparation during frozen ET cycles. Treatment started on the second day of menstruation with oral administration of Femoston estradiol tablets (estradiol tablets/estradiol dydrogesterone tablets, 2 mg/10 mg; Abbott Healthcare Products) at a dosage of 2 mg, 2 to 3 times a day, for 10 to 21 days. The medication was adjusted according to the EMT and serum E2 level. Endometrial starting progesterone administration was performed when the EMT was ≥8 mm and the serum E2 level was ≥200 pgl/L. When Femoston estradiol tablets were stopped, 2 to 3 tablets of Femoston estradiol dydrogesterone tablets were administered per day, and progesterone was injected intramuscularly (Guangzhou Baiyun Mountain Pharmaceutical) at 40 mg/d. When the EMT was <8 mm or the serum E2 level was <200 pgl/L, the decision to cancel the transplant was based on the patient’s previous condition. E2 and progesterone levels were tested on the third day of progesterone administration, and arrangements were made for thawing and transplantation. All embryos were frozen and thawed by vitrification. One or two high-quality embryos were transferred. Femoston estradiol dydrogesterone tablets (2–3 tablets per day orally) and progesterone (40 mg intramuscularly) were applied 14 days after starting the transplantation. The blood β-human chorionic gonadotrophin (β-hCG) level was measured.
Measurement of EMT and EV
EMT was measured at the midsagittal section of the uterus. Measurements were made from the outer edge to the outer edge of the endometrial–myometrial interface in the widest part of the endometrium (17). The 3D model was started with the angle set to 120°, and the uterine volume data were collected and stored. Two sets of 3D volume data were collected. The most satisfactory 3D data were analyzed offline, and VOCAL (Virtual Organ Computer-aided Analysis) software (GE Healthcare) was used. We started from plane B, selected “manual trace”, marked every 15°, and obtained the EV after completion. The average value of 3 measurements was taken.
Measurement of VI, FI, and VFI in the 3D-PD model
The PD mode was started at the midsagittal section of the uterus, and the pulse repetition frequency was set to 0.3, and the PD gain was adjusted for a good endometrial blood flow signal. The 3D mode was started, and the angle was set to 120°. The patient was asked to breathe gently and remain as still as possible, and the observer made every effort to limit inappropriate movements of the transducer. Two sets of 3D volume data were collected. The most satisfactory 3D data were analyzed offline, and the EV was obtained. A volume histogram was used to obtain the VI, FI, and VFI. The average value of 3 measurements was taken.
Definition of clinical outcomes
A positive β-hCG blood test 14 days after ET was used to diagnose pregnancy (18). A clinical pregnancy (CP) was defined as the detection of 1 or more gestational sacs via ultrasound (19). Pregnancy over 12 weeks was defined as an ongoing pregnancy (OP) (20).
Statistical analysis
R 4.0 statistical analysis software (The R Foundation of Statistical Computing) and the Shapiro-Wilk method were used for the normality test. A 2-sample t-test was performed for comparisons between 2 groups of data that were normally distributed with a uniform variance, and a t-test was performed for data with an uneven variance. A Mann-Whitney test was used to compare 2 groups of data that did not satisfy a normal distribution. According to the distribution characteristics of the count data, a chi-squared test, adjusted chi-squared test, or Fisher exact probability test was used. In the univariate difference analysis, a P value <0.1 was included in the multivariate stepwise logistic regression based on the Akaike information criterion. The inspection level was α=0.05.
The sample size was calculated using Power Analysis and Sample Size (PASS) 15 software (NCSS LLC). According to previous reports, the EMT in IVF cycles is 10.43±1.97 mm and 11.88±2.28 mm in pregnant and nonpregnant women, respectively (21). A group sample size of 80 could achieve an 85% power to reject the null hypothesis with a significance level (α) of 0.050 according to a 2-sided 2-sample unequal-variance t-test.
Results
A total of 133 patients were initially enrolled in this study; 48 patients were excluded, and 85 patients were finally included in the statistical analysis (Figure 3). The characteristics of the study population and baseline data are shown in Table 1. The intraobserver consistency for 3D measurements of EV, VI, FI, and VFI were 0.994, 0.994, 0.969, and 0.994, respectively (Table 2). Ultrasound values measured at day P, day P + 2, and day of ET are shown in Table 3. Among the 85 patients, 61 were pregnant (71%, 61/85), 47 had a CP (55%, 47/85), and 39 had an OP (45%, 39/85). Univariate analysis was used to analyze changes in the EMT, EV, VI, FI, VFI, and E2 level. The results showed that for the outcome of pregnancy, none of the indicators analyzed were influencing factors. The changes in EMT, EV, VI, FI, and VFI, as well as P values at the 3 time points, are shown in Figure 4. Two changes in EV were indicators associated with the outcome of a CP (P=0.005 and P=0.02). For the outcome of an OP, 2 changes in EMT (P=0.04 and P=0.02) and EV were influencing factors (P=0.001 and P=0.007; Table 4). After multivariate stepwise regression analysis, the results showed that if the first change in the EV was nondeclining, then the likelihood of a CP was more unfavorable (P=0.03). In the second change, the difference between declining and nondeclining was not significant for a CP (P=0.07). Similarly, if the first change in the EV was nondeclining, then the likelihood of an OP was unfavorable (P=0.01). However, if the second change in the EV on the day of ET was nondeclining, the likelihood of an OP was more favorable (P=0.03; Table 5).
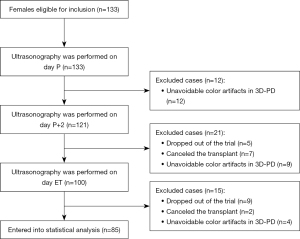
Table 1
| Variable | Pregnancy | Clinical pregnancy | Ongoing pregnancy | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No (n=24) | Yes (n=61) | P | No (n=38) | Yes (n=47) | P | No (n=46) | Yes (n=39) | P | |||
| Age (years) | 34.67±4.37 | 33.62±3.76 | 0.27 | 34.68±4.13 | 33.30±3.71 | 0.10 | 34.59±4.24 | 33.13±3.44 | 0.08 | ||
| Basal E2 (pg/mL) | 38.69 (26.85, 50.83) | 39.16 (33.44, 48.64) | 0.59 | 39.2 (30.01, 44.70) | 38.73 (33.16, 50.00) | 0.60 | 47.14 (39.02, 47.14) | 38.73 (33.71, 57.31) | 0.48 | ||
| Basal P (ng/mL) | 0.16 (0.05, 0.34) | 0.23 (0.13, 0.32) | 0.35 | 0.18 (0.08, 0.31) | 0.23 (0.13, 0.32) | 0.35 | 0.19 (0.08, 0.31) | 0.25 (0.17, 0.37) | 0.11 | ||
| AMH (ng/mL) | 2.34 (1.59, 3.83) | 3.34 (2.01, 5.28) | 0.05 | 2.33 (1.55, 3.98) | 3.50 (2.45, 6.41) | 0.002** | 2.43 (1.64, 4.03) | 3.76 (2.45, 7.00) | 0.002** | ||
| BMI (kg/m2) | 24.08±2.85 | 23.33±3.34 | 0.18 | 23.63±2.95 | 23.48±3.44 | 0.62 | 23.53±3.04 | 23.56±3.46 | 0.96 | ||
| Number of high-quality embryos | 1.00 (1.00, 2.00) | 1.00 (1.00, 2.00) | 0.50 | 1.00 (1.00, 2.00) | 1.00 (1.00, 2.00) | 0.12 | 1.00 (1.00, 2.00) | 1.00 (1.00, 2.00) | 0.36 | ||
| Duration of infertility (years) | 3.00 (2.25, 5.75) | 3.00 (2.00, 4.50) | 0.15 | 3.00 (2.00, 5.00) | 3.00 (2.00, 5.00) | 0.14 | 3.00 (2.00, 5.00) | 3.00 (2.00, 5.00) | 0.47 | ||
| E2 on day P (pg/mL) | 285.00 (230.25, 376.88) | 267.50 (214.15, 354.50) | 0.54 | 291.75 (226.50, 375.63) | 266.00 (208.50, 338.80) | 0.32 | 279.95 (225.00, 374.03) | 266.00 (208.50, 351.00) | 0.61 | ||
| P on day P + 2 (ng/mL) | 14.32 (11.70, 19.46) | 12.43 (9.11, 16.46) | 0.12 | 14.34 (11.75, 18.59) | 11.58 (9.04, 16.17) | 0.08 | 14.25 (10.86, 16.75) | 11.58 (9.04, 16.95) | 0.27 | ||
Data are presented as mean ± standard deviation or median (interquartile range). **, P<0.01. E2, estradiol; P, progesterone; AMH, anti-Müllerian hormone; BMI, body mass index.
Table 2
| Parameters | Intraclass correlation | 95% CI | P value |
|---|---|---|---|
| EV | 0.994 | 0.990–0.997 | 0.000 |
| VI | 0.994 | 0.989–0.997 | 0.000 |
| FI | 0.969 | 0.944–0.984 | 0.000 |
| VFI | 0.994 | 0.989–0.997 | 0.000 |
3D, 3-dimensional; CI, confidence interval; EV, endometrial volume; VI, vascular index; FI, flow index; VFI, vascular flow index.
Table 3
| Variable | Pregnancy | Clinical pregnancy | Ongoing pregnancy | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No (n=24) | Yes (n=61) | P | No (n=38) | Yes (n=47) | P | No (n=46) | Yes (n=39) | P | |||
| Day P | |||||||||||
| EMT, mm | 10.6±2.0 | 10.2±2.0 | 0.48 | 10.6±2.1 | 10.2±1.9 | 0.34 | 10.6±2.0 | 10.1±2.1 | 0.30 | ||
| EV, cm3 | 4.1±1.5 | 4.3±1.5 | 0.68 | 4.2±1.7 | 4.2±1.4 | 0.89 | 4.3±1.6 | 4.2±1.5 | 0.80 | ||
| VI, % | 24.9±10.0 | 26.8±12.9 | 0.52 | 25.6±10.4 | 26.8±13.4 | 0.65 | 26.8±11.9 | 25.7±12.6 | 0.67 | ||
| FI, % | 20.6±2.6 | 21.2±3.0 | 0.39 | 20.8±2.5 | 21.3±3.2 | 0.39 | 21.2±2.8 | 20.9±3.0 | 0.67 | ||
| VFI, % | 4.9 (3.1, 6.7) | 5.5 (3.2, 8.7) | 0.41 | 4.9 (3.0, 7.5) | 5.5 (3.3, 8.9) | 0.65 | 5.7 (3.0, 8.3) | 4.8 (3.3, 8.6) | 0.76 | ||
| Day P + 2 | |||||||||||
| EMT, mm | 11.1±2.9 | 10.6±2.2 | 0.05 | 11.1±2.5 | 10.5±2.3 | 0.39 | 11.0±2.3 | 10.4±2.5 | 0.55 | ||
| EV, cm3 | 4.0±1.9 | 3.8±1.4 | 0.18 | 4.1±1.7 | 3.7±1.4 | 0.14 | 4.1±1.6 | 3.7±1.4 | 0.31 | ||
| VI, % | 15.5±7.2 | 15.7±7.8 | 0.29 | 15.2±7.6 | 16.0±7.6 | 0.75 | 15.7±7.8 | 15.6±7.4 | 0.64 | ||
| FI, % | 18.6 (16.7, 20.9) | 19.3 (17.4, 20.9) | 0.47 | 18.6 (16.8, 20.9) | 19.4 (17.5, 21.0) | 0.32 | 19.3 (17.1, 21.3) | 19.2 (16.9, 20.9) | 0.71 | ||
| VFI, % | 3.0±1.7 | 3.5±2.3 | 0.09 | 3.0±1.7 | 3.7±2.5 | 0.05 | 3.2±1.8 | 3.6±2.5 | 0.18 | ||
| Day ET | |||||||||||
| EMT, mm | 11.3±2.4 | 10.9±2.4 | 0.53 | 11.1±2.5 | 10.9±2.3 | 0.47 | 11.0±2.3 | 11.0±2.5 | 0.60 | ||
| EV, cm3 | 4.1±1.8 | 4.1±1.5 | 0.43 | 4.1±1.8 | 4.0±1.4 | 0.08 | 4.2±1.7 | 4.0±1.4 | 0.20 | ||
| VI, % | 15.3±9.6 | 15.9±11.4 | 0.47 | 14.6±8.7 | 16.7±12.3 | 0.07 | 14.7±9.5 | 17.0±12.3 | 0.22 | ||
| FI, % | 17.4±4.9 | 18.4±4.0 | 0.70 | 17.7±4.6 | 18.4±4.0 | 0.97 | 18.0±4.4 | 18.2±4.1 | 0.70 | ||
| VFI, % | 2.7 (1.5, 4.4) | 2.4 (1.5, 3.6) | 0.76 | 2.7 (1.5, 3.5) | 2.4 (1.6, 4.0) | 0.89 | 2.3 (1.5, 3.5) | 2.5 (1.6, 4.0) | 0.88 | ||
Data are presented as mean ± standard deviation or median (interquartile range). EMT, endometrial thickness; EV, endometrial volume; VI, vascular index; FI, flow index; VFI, vascular flow index; day P, before progesterone administration; d P + 2, the third day after progesterone administration; day of ET, the day of embryo transplantation.

Table 4
| Variable | Pregnancy, n (%) | Clinical pregnancy, n (%) | Ongoing pregnancy, n (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No (n=24) | Yes (n=61) | P | No (n=38) | Yes (n=47) | P | No (n=46) | Yes (n=39) | P | |||
| EMT | |||||||||||
| The first change | 0.29 | 0.07 | 0.04* | ||||||||
| Declining | 8 (33.3) | 28 (45.9) | 12 (31.6) | 24 (51.1) | 15 (32.6) | 21 (53.9) | |||||
| Nondeclining | 16 (66.7) | 33 (54.1) | 26 (68.4) | 23 (48.9) | 31 (67.4) | 18 (46.1) | |||||
| The second change | 0.73 | 0.05 | 0.02* | ||||||||
| Declining | 10 (41.7) | 23 (37.7) | 19 (50.0) | 14 (29.8) | 23 (50.0) | 10 (25.6) | |||||
| Nondeclining | 14 (58.3) | 38 (62.3) | 19 (50.0) | 33 (70.2) | 23 (50.0) | 29 (74.4) | |||||
| EV | |||||||||||
| The first change | 0.17 | 0.005** | 0.001** | ||||||||
| Declining | 15 (62.5) | 47 (77.1) | 22 (57.9) | 40 (85.1) | 27 (58.7) | 35 (89.7) | |||||
| Nondeclining | 9 (37.5) | 14 (22.9) | 16 (42.1) | 7 (14.9) | 19 (41.3) | 4 (10.3) | |||||
| The second change | 0.41 | 0.02* | 0.007** | ||||||||
| Declining | 8 (33.3) | 15 (24.6) | 15 (39.5) | 8 (17.0) | 18 (39.1) | 5 (12.8) | |||||
| Nondeclining | 16 (66.7) | 46 (75.4) | 23 (60.5) | 39 (83.0) | 28 (60.9) | 34 (87.2) | |||||
| E2 | |||||||||||
| The change | 0.76 | 0.06 | 0.13 | ||||||||
| Declining | 15 (62.5) | 36 (59.0) | 27 (71.1) | 24 (51.1) | 31 (67.4) | 20 (51.3) | |||||
| Nondeclining | 9 (37.5) | 25 (41.0) | 11 (28.9) | 23 (48.9) | 15 (32.6) | 19 (48.7) | |||||
| VI | |||||||||||
| The first change | 1.00 | 0.81 | 0.75 | ||||||||
| Declining | 21 (87.5) | 52 (85.3) | 33 (86.8) | 40 (85.1) | 40 (87.0) | 33 (84.6) | |||||
| Nondeclining | 3 (12.5) | 9 (14.7) | 5 (13.2) | 7 (14.9) | 6 (13.0) | 6 (15.4) | |||||
| The second change | 0.53 | 0.70 | 0.47 | ||||||||
| Declining | 14 (58.3) | 31 (50.8) | 21 (55.3) | 24 (51.1) | 26 (56.5) | 19 (48.7) | |||||
| Nondeclining | 10 (41.7) | 30 (49.2) | 17 (44.7) | 23 (48.9) | 20 (43.5) | 20 (51.3) | |||||
| FI | |||||||||||
| The first change | 0.90 | 0.89 | 0.99 | ||||||||
| Declining | 17 (70.8) | 44 (72.1) | 27 (71.1) | 34 (72.3) | 33 (71.7) | 28 (71.8) | |||||
| Nondeclining | 7 (29.2) | 17 (27.9) | 11 (28.9) | 13 (27.7) | 13 (28.3) | 11 (28.2) | |||||
| The second change | 0.43 | 0.59 | 0.28 | ||||||||
| Declining | 16 (66.7) | 35 (57.4) | 24 (63.2) | 27 (57.5) | 30 (65.2) | 21 (53.8) | |||||
| Nondeclining | 8 (33.3) | 26 (42.6) | 14 (36.8) | 20 (42.5) | 16 (34.8) | 18 (46.2) | |||||
| No (n=24) | Yes (n=61) | P | No (n=38) | Yes (n=47) | P | No (n=46) | Yes (n=39) | P | |||
| VFI | |||||||||||
| The first change | 0.76 | 0.87 | 0.73 | ||||||||
| Declining | 21 (87.5) | 50 (82.0) | 32 (84.2) | 39 (83.0) | 39 (84.8) | 32 (82.1) | |||||
| Nondeclining | 3 (12.5) | 11 (18.0) | 6 (15.8) | 8 (17.0) | 7 (15.2) | 7 (17.9) | |||||
| The second change | 0.66 | 0.77 | 0.39 | ||||||||
| Declining | 15 (62.5) | 35 (57.4) | 23 (60.53) | 27 (57.5) | 29 (63.0) | 21 (53.9) | |||||
| Nondeclining | 9 (37.5) | 26 (42.6) | 15 (39.47) | 20 (42.5) | 17 (37.0) | 18 (46.1) | |||||
*, P<0.05; **, P<0.01. E2, estradiol; EMT, endometrial thickness; EV, endometrial volume; VI, vascular index; FI, flow index; VFI, vascular flow index.
Table 5
| Variable | Estimate | Se | Z | Wald | P | OR (95% CI) | Significant markers |
|---|---|---|---|---|---|---|---|
| Pregnancy | |||||||
| (Intercept) | 0.496 | 0.759 | 0.653 | 0.426 | 0.51 | ||
| AMH | 0.115 | 0.083 | 1.378 | 1.899 | 0.16 | 1.121 (0.953, 1.320) | |
| Clinical pregnancy | |||||||
| (Intercept) | 0.496 | 0.759 | 0.653 | 0.426 | 0.51 | ||
| The first change of EV on day P + 2 | |||||||
| Declining | ref | ||||||
| Nondeclining | −1.203 | 0.577 | −2.087 | 4.354 | 0.03 | 0.300 (0.097, 0.930) | P<0.05 |
| The change of E2 on day P + 2 | |||||||
| Declining | ref | ||||||
| Nondeclining | 0.914 | 0.515 | 1.775 | 3.152 | 0.07 | 2.494 (0.909, 6.839) | |
| P on P + 2 day | −0.07 | 0.038 | −1.844 | 3.399 | 0.06 | 0.932 (0.865, 1.004) | |
| AMH | 0.182 | 0.093 | 1.943 | 3.775 | 0.05 | 1.199 (0.998, 1.440) | |
| Ongoing pregnancy | |||||||
| (Intercept) | −1.384 | 0.63 | −2.196 | 4.823 | 0.02 | ||
| The first change of EV on day P + 2 | |||||||
| Declining | ref | ||||||
| Nondeclining | −1.549 | 0.629 | −2.463 | 6.066 | 0.01 | 0.212 (0.062, 0.729) | P<0.05 |
| The second change of EV on day of ET | |||||||
| Declining | ref | ||||||
| Nondeclining | 1.251 | 0.598 | 2.09 | 4.369 | 0.03 | 3.493 (1.081, 11.284) | P<0.05 |
| AMH | 0.156 | 0.082 | 1.889 | 3.569 | 0.05 | 1.168 (0.994, 1.373) |
AMH, anti-Müllerian hormone; E2, estradiol; P, progesterone; EV, endometrial volume; ET, embryo transfer; CI, confidence interval; OR, odds ratio.
Discussion
EMT was the earliest ultrasound indicator used to evaluate ER. In the univariate analysis, a first declining change in the EMT and a second nondeclining change in the EMT differed between the OP group and the non-OP group (P=0.04 and P=0.02). Although the changes in the EMT in the multivariate analysis was not an independent predictor, a decline in the EV after progesterone administration had predictive value for a CP and OP (P=0.03 and P=0.01). Meanwhile, a nondeclining change in EV on the day of ET had predictive value for an OP (P=0.03). Regarding the blood flow in the endometrium, some studies have shown that a good endometrial blood supply is necessary for embryo implantation (22,23). However, changes in the VI, FI, and VFI observed via 3D-PD imaging ultimately had no association with the IVF results. Regarding patient hormone levels, neither progesterone at day P + 2 nor changes in E2 at day P + 2 showed an ability to predict the pregnancy outcome. Although the AMH level was included in the multivariate analysis, the overall ability to predict pregnancy, CP and OP was not strong (P=0.16, P=0.05, P=0.05). This finding may be related to the lack of precise uniformity in the timing of hormone measurements.
With the development of ultrasound technology, the 3D imaging function of ultrasound equipment has become almost universal (24). This universality makes the measurement of EV easier to achieve in clinical practice with good repeatability (25). Some studies have used EV instead of EMT to predict ER (26). However, whether EV can be a predictor of pregnancy is controversial. Regardless of whether it is measured on the day of egg collection (21) or the day of β-hCG detection (27), EV cannot indicate pregnancy outcome. The reason for this finding may be that in addition to reflecting EMT, EV is mainly affected by the size of the uterus. Monitoring changes in the EV can eliminate the impact of uterus size and thus more accurately reflect the changes in the endometrium. In our study, as in other studies, changes in EMT did not have the ability to predict IVF outcomes (28,29). The measurement of EMT was greatly affected by endometrial peristalsis (25). In particular, endometrial peristalsis was more evident on day P, which affected the accuracy of the EMT measurement. Perhaps this explains why the thickness measurement could not accurately reflect changes in the endometrium. EV overcame this shortcoming and showed a good predictive ability, becoming an independent predictor of a CP, especially for predicting an OP. In the HRT cycle, the use of progesterone reduces the estrogen receptors in the endometrium. The receptivity of the endometrium requires a reduction in estrogen receptors (30). The effect of progesterone also decreases the thickness of the transformed endometrium (31). EV increased on the day of ET compared with day P + 2, which may be related to the rapid and progressive curve of endometrial angiogenesis and interstitial edema during the midluteal phase. These endometrial changes were thought to be conducive to embryo implantation. Therefore, a nondeclining change in the EV on the day of ET was conducive to an OP in this study.
In recent years, studies have shown that sequential changes in the endometrium during the cycle may be related to the outcome of IVF (32). This study did not use sequential ultrasound examinations during the cycle because we studied the changes in the endometrium during HRT in frozen ET cycles. In this procedure, the doctor did not need to perform daily ultrasound examinations. To avoid increasing the number of examinations for patients, we selected representative time points that were also the time at which clinical ultrasound monitoring was needed. We sought to determine the relationships between ER and the changes revealed by ultrasound at these time points. Additionally, it was hoped that the study results could be translated into clinically usable ER indicators.
In the current study, 25 patients (25/133) were excluded due to unavoidable artifacts in the 3D-PD imaging process, accounting for 18.80% of the enrolled patients, which represented the main sample size loss. The leading causes of artifacts were arterial pulsatility and peristalsis of the pelvic bowel (33). Moreover, if a patient underwent 3 consecutive ultrasound examinations in the study and color artifacts were present in any 1 examination, that patient was excluded. Our findings on 3D-PD imaging of blood flow changes to predict IVF outcomes were partially identical to those of a recent study (12). In our study, changes in endometrial blood flow were observed twice before transplantation, but neither change was predictive of the IVF outcome. This finding may be because 3D-PD imaging is not a sufficiently sensitive evaluation method, which is consistent with the findings of another study (33).
The present study differed from previous studies in that it defined a pregnancy outcome in 3 ways: a pregnancy, a CP, and an OP. Our study found that changes in the EV were a meaningful indicator, and we used the least number of inspections to predict the outcome of IVF. Additionally, compared with other 3D indicators, such as the VI, FI, and VFI, EV is easy to obtain in practice. However, we did not follow up on the birth of the fetuses. In addition, our sample size was relatively small, and we hope to do more related work in the future.
Conclusions
This study of the pattern of changes in endometrial ultrasound indices found that the pattern of changes in EV during the frozen ET cycles was a predictor of IVF outcome. We also found that a decline in EV after progesterone administration and a nondeclining EV before ET were favorable factors for pregnancy.
Acknowledgments
We sincerely thank the Reproductive Laboratory of Dalian Women and Children’s Medical Group (China) for providing information on the transplanted embryos.
Funding: This study was supported by the Dalian Science and Technology Innovation Fund (No. 2022JJ13SN087), the Guide Project for Key Research and Development Foundation of Liaoning Province (No. 2019JH8/10300008), the Guide Project for the Science Foundation of Liaoning Province (No. 2019-ZD-1019), the Scientific Research Project of the Educational Department of Liaoning Province (No. ZF2019023), and the 345 Talent Project and Liaoning BaiQianWan Talents Program.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-22-705/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-705/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), and the research protocol was approved by the ethics committee of Dalian Women and Children’s Medical Group (No. 2020013). All the women who agreed to participate in the study signed informed consent forms.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Craciunas L, Gallos I, Chu J, Bourne T, Quenby S, Brosens JJ, Coomarasamy A. Conventional and modern markers of endometrial receptivity: a systematic review and meta-analysis. Hum Reprod Update 2019;25:202-23. [Crossref] [PubMed]
- Sehring J, Beltsos A, Jeelani R. Human implantation: The complex interplay between endometrial receptivity, inflammation, and the microbiome. Placenta 2022;117:179-86. [Crossref] [PubMed]
- Paulson EE, Comizzoli P. Endometrial receptivity and embryo implantation in carnivores-commonalities and differences with other mammalian species. Biol Reprod 2021;104:771-83. [Crossref] [PubMed]
- Owusu-Akyaw A, Krishnamoorthy K, Goldsmith LT, Morelli SS. The role of mesenchymal-epithelial transition in endometrial function. Hum Reprod Update 2019;25:114-33. [Crossref] [PubMed]
- Strowitzki T, Germeyer A, Popovici R, von Wolff M. The human endometrium as a fertility-determining factor. Hum Reprod Update 2006;12:617-30. [Crossref] [PubMed]
- Casper RF. Frozen embryo transfer: evidence-based markers for successful endometrial preparation. Fertil Steril 2020;113:248-51. [Crossref] [PubMed]
- Tan J, Kan A, Hitkari J, Taylor B, Tallon N, Warraich G, Yuzpe A, Nakhuda G. The role of the endometrial receptivity array (ERA) in patients who have failed euploid embryo transfers. J Assist Reprod Genet 2018;35:683-92. [Crossref] [PubMed]
- Bassil R, Casper R, Samara N, Hsieh TB, Barzilay E, Orvieto R, Haas J. Does the endometrial receptivity array really provide personalized embryo transfer? J Assist Reprod Genet 2018;35:1301-5. [Crossref] [PubMed]
- Tong R, Zhou Y, He Q, Zhuang Y, Zhou W, Xia F. Analysis of the guidance value of 3D ultrasound in evaluating endometrial receptivity for frozen-thawed embryo transfer in patients with repeated implantation failure. Ann Transl Med 2020;8:944. [Crossref] [PubMed]
- Elsokkary M, Eldin AB, Abdelhafez M, Rateb A, Samy M, Eldorf A, Islam BA, Raafat TA, Gomaa IA, Taema M, Mohamed R, Elshourbagy M, Tawfik W, Morad A, Mostafa M, Abbas A, Assar T, Hemeda H. The reproducibility of the novel utilization of five-dimensional ultrasound and power Doppler in the prediction of endometrial receptivity in intracytoplasmic sperm-injected women: a pilot prospective clinical study. Arch Gynecol Obstet 2019;299:551-8. [Crossref] [PubMed]
- Kim A, Han JE, Yoon TK, Lyu SW, Seok HH, Won HJ. Relationship between endometrial and subendometrial blood flow measured by three-dimensional power Doppler ultrasound and pregnancy after intrauterine insemination. Fertil Steril 2010;94:747-52. [Crossref] [PubMed]
- Sadek S, Matitashvili T, Kovac A, Ramadan H, Stadtmauer L. Assessment of uterine receptivity by endometrial and sub-endometrial blood flow using SlowflowHD in hormone prepared frozen embryo transfer cycles: a pilot study. J Assist Reprod Genet 2022;39:1069-79. [Crossref] [PubMed]
- Kasius A, Smit JG, Torrance HL, Eijkemans MJ, Mol BW, Opmeer BC, Broekmans FJ. Endometrial thickness and pregnancy rates after IVF: a systematic review and meta-analysis. Hum Reprod Update 2014;20:530-41. [Crossref] [PubMed]
- Griesinger G, Trevisan S, Cometti B. Endometrial thickness on the day of embryo transfer is a poor predictor of IVF treatment outcome. Hum Reprod Open. 2018;2018:hox031. [Crossref] [PubMed]
- Stentz N, Devine K. Through thick and thin: time to stop worrying about endometrial thickness? Fertil Steril 2021;116:71-2. [Crossref] [PubMed]
- Masrour MJ, Yoonesi L, Aerabsheibani H. The effect of endometrial thickness and endometrial blood flow on pregnancy outcome in intrauterine insemination cycles. J Family Med Prim Care 2019;8:2845-9. [Crossref] [PubMed]
- Zhang M, Li J, Fu X, Zhang Y, Zhang T, Wu B, Han X, Gao S. Endometrial thickness is an independent risk factor of hypertensive disorders of pregnancy: a retrospective study of 13,458 patients in frozen-thawed embryo transfers. Reprod Biol Endocrinol 2022;20:93. [Crossref] [PubMed]
- Merviel P, Labarre M, James P, Bouée S, Chabaud JJ, Roche S, Cabry R, Scheffler F, Lourdel E, Benkhalifa M, Copin H, Drapier H, Beauvillard D. Should intrauterine inseminations still be proposed in cases of unexplained infertility? Retrospective study and literature review. Arch Gynecol Obstet 2022;305:1241-54. [Crossref] [PubMed]
- Wang M, Xi Q, Yang Q, Li Z, Yang L, Zhu L, Jin L. The relationship between a novel evaluation parameter of premature luteinization and IVF outcomes. Reprod Biomed Online 2021;42:323-31. [Crossref] [PubMed]
- Labarta E, Mariani G, Holtmann N, Celada P, Remohí J, Bosch E. Low serum progesterone on the day of embryo transfer is associated with a diminished ongoing pregnancy rate in oocyte donation cycles after artificial endometrial preparation: a prospective study. Hum Reprod 2017;32:2437-42. [Crossref] [PubMed]
- Schild RL, Knobloch C, Dorn C, Fimmers R, van der Ven H, Hansmann M. Endometrial receptivity in an in vitro fertilization program as assessed by spiral artery blood flow, endometrial thickness, endometrial volume, and uterine artery blood flow. Fertil Steril 2001;75:361-6. [Crossref] [PubMed]
- Ng EH, Chan CC, Tang OS, Yeung WS, Ho PC. Changes in endometrial and subendometrial blood flow in IVF. Reprod Biomed Online 2009;18:269-75. [Crossref] [PubMed]
- Wang J, Xia F, Zhou Y, Wei X, Zhuang Y, Huang Y. Association Between Endometrial/Subendometrial Vasculature and Embryo Transfer Outcome: A Meta-analysis and Subgroup Analysis. J Ultrasound Med 2018;37:149-63. [Crossref] [PubMed]
- Feng Y, Zhang S, Zhou Y, He G, Hong L, Shi L, Wang J, Zhang P, Zhai L. Three-dimensional measurement and analysis of morphological parameters of the uterus in infertile women. Quant Imaging Med Surg 2022;12:2224-37. [Crossref] [PubMed]
- Lam MT, Li HWR, Ng EHY. Impact of Endometrial Thickness and Volume Compaction on the Live Birth Rate Following Fresh Embryo Transfer of In Vitro Fertilization. J Ultrasound Med 2022;41:1455-63. [Crossref] [PubMed]
- Maged AM, Kamel AM, Abu-Hamila F, Elkomy RO, Ohida OA, Hassan SM, Fahmy RM, Ramadan W. The measurement of endometrial volume and sub-endometrial vascularity to replace the traditional endometrial thickness as predictors of in-vitro fertilization success. Gynecol Endocrinol 2019;35:949-54. [Crossref] [PubMed]
- Saravelos SH, Jayaprakasan K, Ojha K, Li TC. Assessment of the uterus with three-dimensional ultrasound in women undergoing ART. Hum Reprod Update 2017;23:188-210. [PubMed]
- Babayev E, Matevossian K, Hensley C, Zhang JX, Bulun SE. Baseline Endometrial Thickness or Endometrial Thickness Change in Response to Estrogen Is Not Predictive of Frozen Embryo Transfer Success in Medicated Cycles. Reprod Sci. 2020;27:2242-6. [Crossref] [PubMed]
- Jin Z, Shi H, Lu M, Bu Z, Huo M, Zhang Y. Endometrial thickness changes after progesterone administration do not affect the pregnancy outcomes of frozen-thawed euploid blastocyst transfer: a retrospective cohort study. Fertil Steril 2021;116:1502-12. [Crossref] [PubMed]
- Lessey BA, Killam AP, Metzger DA, Haney AF, Greene GL, McCarty KS Jr. Immunohistochemical analysis of human uterine estrogen and progesterone receptors throughout the menstrual cycle. J Clin Endocrinol Metab 1988;67:334-40. [Crossref] [PubMed]
- Zilberberg E, Smith R, Nayot D, Haas J, Meriano J, Barzilay E, Casper RF. Endometrial compaction before frozen euploid embryo transfer improves ongoing pregnancy rates. Fertil Steril 2020;113:990-5. [Crossref] [PubMed]
- Silva Martins R, Helio Oliani A, Vaz Oliani D, Martinez de Oliveira J. The predictive value of serial serum estradiol and serial endometrial volume on endometrial receptivity on assisted reproductive technology cycles. BMC Pregnancy Childbirth 2021;21:184. [Crossref] [PubMed]
- Nandi A, Martins WP, Jayaprakasan K, Clewes JS, Campbell BK, Raine-Fenning NJ. Assessment of endometrial and subendometrial blood flow in women undergoing frozen embryo transfer cycles. Reprod Biomed Online 2014;28:343-51. [Crossref] [PubMed]


