Image quality of coronary CT angiography at ultra low tube voltage reconstructed with a deep-learning image reconstruction algorithm in patients of different weight
Introduction
A report from the China Health and Nutrition survey indicated that the prevalence of overweight status in adults has increased linearly over the past 20 years, and obesity is widely regarded as an important risk factor in coronary artery disease (CAD) (1). Coronary computed tomography angiography (CCTA) is the main noninvasive imaging method for detecting CAD and has high sensitivity and accuracy (2). However, compared with normal-weight patients, overweight patients have more adipose tissue, which may lead to more image noise that degrades the analysis of CCTA images. Additionally, low-dose rather than high-dose CCTA is recommended from the perspective of reducing the radiation dose to patients. However, although low-voltage scanning can effectively reduce radiation exposure to patients and improve vessel contrast in CCTA, there is a low penetration of X-rays and noisy images. Furthermore, the low photon energy spectrum peak produced by the low tube voltage may cause greater beam-hardening effects and influence image quality, especially in obese people. To satisfy the clinical demands of diagnosis, CT image reconstruction algorithms have been updated from filtered back-projection to iterative reconstruction (IR) including adaptive statistical iterative reconstruction-Veo (ASiR-V; GE Healthcare). The IR reconstruction algorithm can improve image quality by markedly reducing image noise but sometimes produces a wax-like image texture due to its nonlinearity and instability (3,4).
With the dramatic development of artificial intelligence, deep-learning image reconstruction (DLIR) has been recently introduced to reduce image noise while preserving natural image texture in clinical CT images. Specifically, compared with ASiR-V, DLIR is described as being able to significantly reduce image noise by 43% and improve image quality by 62% with equal diagnostic accuracy, while also reducing radiation exposure by 43% without degrading the image and diagnostic accuracy in CCTA (5). GE Healthcare’s next-generation CT scanner (Revolution Apex) allows low tube voltage scanning at 70 kVp with a maximal tube current of 1,300 mA and is equipped with two image noise reduction algorithms: ASiR-V and DLIR (TrueFidelity, GE Healthcare). Thus, the purpose of this study was to assess the image quality of coronary CT angiography (CCTA) at 70 kVp with the DLIR algorithm as compared to the ASiR-V algorithm in patients of different weight.
Methods
Participants
We prospectively enrolled 150 patients who underwent CCTA with a 256-detector CT scanner (Revolution Apex, GE Healthcare) between September 2021 and January 2022. All patients had experienced chest tightness and pain after activity. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Ethics Committee of the People’s Hospital of Ningxia Hui Autonomous Region. All participants provided written informed consent.
The inclusion criteria were (I) age ≥18 years, (II) no contraindications for CCTA (i.e., severe renal and/or cardiac insufficiency, severe arrhythmia, hyperthyroidism, allergic reaction to iodine contrast agent), and (III) compliance with standard CCTA indications and imaging technique based on internationally published consensuses and domestic guidelines (6-13). The exclusion criteria were (I) incomplete patient data (n=24), such as body height and weight; (II) prior history of coronary artery bypass graft surgery and/or percutaneous coronary intervention (n=21); and (III) body mass index (BMI) <18.5 kg/m2 (n=9) (Figure 1). Thus, 96 patients were finally included in this study.
Based on the “Adult Weight Determination” standard issued by the National Health and Family Planning Commission on April 18, 2013, each patient was classified as normal weight (BMI 18.5–<24 kg/m2), overweight (BMI 24–<28 kg/m2), or obese (BMI ≥28 kg/m2) (14). To simplify groups, we combined the overweight and obese patients into the overweight group. Group A is the normal-weight, and Group B is the overweight group. Each group had 48 patients, and their characteristics are listed in Table 1.
Table 1
| Characteristics | Normal-weight group | Overweight group | P value |
|---|---|---|---|
| Sex (male/female), N | 48 (23/25) | 48 (26/22) | 0.540 |
| Age (years) | 61±12 | 57±10 | 0.198 |
| BMI (kg/m2) | 21.9±1.4 | 26.4±1.5 | <0.001 |
| Heart rate (bpm) | 62.4±0.6 | 60.3±1.3 | 0.213 |
| CTDIvol (mGy) | 6.8±2.3 | 8.0±2.4 | 0.003 |
| DLP (mGy·cm) | 95.4±30.0 | 113.7±33.1 | 0.001 |
| ED (mSv) | 1.4±0.4 | 1.6±0.5 | 0.001 |
Data are presented as the mean ± standard deviation. BMI, body mass index; CTDIvol, volume CT dose index; DLP, dose-length product; ED, effective dose; CT, computed tomography.
CCTA image acquisition and reconstruction
We performed ECG-gated CCTA axial scanning using a 350 mgI/mL nonionic iodine contrast agent (iodixanol; GE Healthcare) injected into an antecubital vein at 5.0 mL/s. The volume of contrast agent was calculated at 0.8 mL/kg. Scanning began in the craniocaudal direction from 1–2 cm below the tracheal bifurcation to the level of the diaphragm with the following parameters: tube voltage, 70 kVp; tube current, noise index of 24 Hounsfield units (HU) using the automatic exposure control (minimum, 100 mA; maximum, 1,300 mA); matrix, 512×512 pixels; collimator width, 160 mm; and rotating speed, 0.28 s/rotation. The thickness of reconstruction layer was 0.625 mm. All patients were examined with a prospective axial ECG-triggering protocol (Auto Gating, GE Healthcare). The exposure windows for patients with different heart rates (HRs) were set as follows: 60% if HR <60 bpm, 65–75% if HR >61–70 bpm, and 35–45% if HR 71–90 bpm (15). The best cardiac phase was automatically recommended according to the intelligent phase technology of the CT equipment, and the coronary artery tracking freeze technology (Snapshot Freeze, GE Healthcare) (16) was used to reduce coronary vessel motion artifacts. The 2 CT image groups were reconstructed with the following algorithms: ASiR-V (40% and 80%), and DLIR (low, medium, and high). All images were transferred to a workstation (Advantage Workstation 4.7, GE Healthcare) for coronary artery image post-processing and the image quality assessments.
Objective image quality assessment
A radiologist with 5 years’ experience of diagnostic cardiovascular imaging defined a circular region of interest (ROI) within the aortic root (AO), the proximal right coronary artery (RCA), the left main artery (LMA), the proximal left anterior descending artery (LAD), the proximal left circumflex artery (LCX), and the erector spinae muscle. All ROIs were placed in the center of the vessel, with adjacent structures being avoided. Furthermore, the size of the ROI was approximately 100 mm²in the AO and the erector spinae muscle, and 2–4 mm² in each coronary artery. The attenuation values and standard deviation (SD) values of these ROIs on the axial images were measured at a fixed window width of 800 HU and a window level of 200 HU. Image noise was defined as the SD value in the erector spinae muscle. The contrast-to-noise ratio (CNR) of all the vessels was defined as follows: CNR = (attenuation value in each vessel—attenuation value in the erector spinae muscle)/image noise.
Subjective image quality assessment
Two radiologists with 5 and 10 years’ experience, respectively, in diagnostic cardiovascular imaging independently assessed the overall subjective image quality on a per-patient basis using a 5-point Likert scale (17). A score of 5 points indicated subtle image noise, no artifacts, and very clear delineation of tissue structural details with sharp edges; 4 points indicated subtle image noise, slight artifacts, and clear delineation of tissue structural details; 3 points indicated moderate image noise, some artifacts, and preserved delineation of tissue structural details; 2 points, indicated substantial image noise, significant artifacts, and limited delineation of tissue structural details; and 1 point indicated severe image noise, serious artifacts, and poor delineation of tissue structural details. Image quality was considered as diagnostically acceptable for 3–5 points, but unacceptable with scores of 1 or 2 points.
Radiation dose
For each patient, the volume CT dose index (CTDIvol) and dose-length product (DLP) automatically displayed by the scanner were recorded to calculate the effective dose (ED), which was calculated as follows: ED = DLP × K, where K is the chest conversion factor of 0.014 mSv/mGy·cm (18).
Statistical analysis
SPSS 20.0 software (IBM Corp.) was used. The measured data are presented as mean±standard deviation if they conformed to the normality test. The objective image quality and radiation dose measurements were compared among the different reconstruction algorithms using the analysis of variance (ANOVA), and the subjective image quality was compared with the Wilcoxon signed rank test. A P value <0.05 was considered statistically significant. The weighted kappa test was used to evaluate the interobserver agreement of the subjective image quality.
Results
Patients’ characteristics and radiation dose
As shown in Table 1, the mean age and sex were comparable between groups (P>0.05). The mean BMI was significantly greater in the overweight group than in the normal-weight group (P<0.001), and thus the mean radiation dose measurements (CTDIvol, DLP, and ED) were significantly greater in that group (P=0.001–0.003). The mean ED increased by 19.1% in the overweight group compared with the normal-weight group.
Objective image quality
In comparison with the normal-weight group, the CNR of the ASiR-V-reconstructed images in the overweight group was lower, but the difference between the two groups was not statistically significant (P>0.05). The CNR of the ASiR-80% images was higher than that of ASiR-40% images (P<0.05), which indicated that the noise in the ASiR-80% images was less than that in the ASiR-40% (Tables 2,3).
Table 2
| Group A | Group B | P value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ASiR-40% | ASiR-80% | ASiR-40% | ASiR-80% | ASiR-40% (Group A vs. Group B) | ASiR-80% (Group A vs. Group B) | ASiR-40% vs. ASiR-80% | |||
| CT value (Hu) | 46.26±16.29 | 46.15±16.10 | 45.20±15.88 | 45.10±15.44 | 0.921 | 0.982 | 0.896 | ||
| SD value (Hu) | 48.19±5.57 | 35.81±5.87 | 48.69±6.34 | 36.31±667 | 0.918 | 0.904 | <0.001 | ||
Data are presented as mean ± standard deviation. Group A, normal-weight group; Group B, overweight group. ASiR-V, adaptive statistical iterative reconstruction-Veo; CT, computed tomography; SD, standard deviation.
Table 3
| Group | AO | LMA | LAD | RCA | LCX |
|---|---|---|---|---|---|
| Group B | |||||
| ASiR-40% | 8.39±1.46 | 7.93±1.44 | 7.36±1.50 | 7.63±1.60 | 7.66±1.65 |
| ASiR-80% | 10.70±1.71 | 10.00±1.76 | 9.22±1.89 | 9.65±1.92 | 9.67±2.15 |
| Group A | |||||
| ASiR-40% | 8.81±1.81 | 8.55±1.64 | 7.43±1.65 | 8.06±1.61 | 8.10±1.74 |
| ASiR-80% | 11.01±2.40 | 10.70±2.34 | 9.26±2.47 | 10.04±2.40 | 10.01±2.41 |
| P value | |||||
| ASiR-40% (Group A vs. Group B) | 0.240 | 0.078 | 0.950 | 0.182 | 0.231 |
| ASiR-80% (Group A vs. Group B) | 0.238 | 0.057 | 0.817 | 0.241 | 0.343 |
| ASiR-40% vs. ASiR-80% | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
Data are presented as mean ± standard deviation. Group A, normal-weight group; Group B, overweight group. CNR, contrast-to-noise ratio; ASiR-V, adaptive statistical iterative reconstruction-Veo; AO, aortic root; LMA, left main artery; LAD, left anterior descending artery; RCA, right coronary artery; LCX, left circumflex artery.
In both groups, the noise in the DLIR reconstructed images at different levels was less than that in the ASiR-V40%-reconstructed images. Among the 5 algorithms, DLIR-H had the highest CNR while ASiR-40% had the lowest, and the difference in the CNR of the 5 methods was statistically significant (P<0.05). The noise of DLIR-M- and DLIR-H-reconstructed images in the overweight group was slightly higher than that in the normal-weight group, and the corresponding CNR was lower than that in the normal-weight group, and the difference between the two groups was statistically significant. The noise in the DLIR-H-reconstructed images was less than that in the DLIR-L reconstructed images, and the corresponding CNR was higher, with statistically significant differences (P<0.05; Tables 4-6, Figure 2).
Table 4
| Group | DLIR-L | DLIR-M | DLIR-H | ASiR-40% | ASiR-80% | P value |
|---|---|---|---|---|---|---|
| Group B | ||||||
| CT value (Hu) | 45.34±15.64 | 45.73±15.61 | 45.76±15.47 | 45.20±15.88 | 45.10±15.44 | 0.001 |
| SD value (Hu) | 44.35±7.07 | 40.41±7.16 | 32.68±7.48 | 48.69±6.34 | 36.31±667 | <0.001 |
| Group A | ||||||
| CT value (Hu) | 46.30±16.11 | 46.38±15.98 | 46.88±15.99 | 46.26±16.29 | 46.15±16.10 | 0.001 |
| SD value (Hu) | 43.63±6.04 | 39.30±6.18 | 31.36±6.16 | 48.19±5.57 | 35.81±5.87 | <0.001 |
Data are presented as mean ± standard deviation. Group A, normal-weight group; Group B, overweight group. DLIR, deep-learning image reconstruction; L, low; M, medium; H, high; CT, computed tomography; SD, standard deviation; ASiR, adaptive statistical iterative reconstruction.
Table 5
| Group | AO | LMA | LAD | RCA | LCX |
|---|---|---|---|---|---|
| Group B | |||||
| DLIR-L | 10.59±2.32 | 10.09±2.29 | 9.35±2.18 | 9.60±2.42 | 9.83±2.50 |
| DLIR-M | 12.68±2.91 | 12.04±2.93 | 11.10±2.75 | 11.54±2.95 | 11.76±3.08 |
| DLIR-H | 19.15±4.31 | 18.22±4.36 | 16.55±4.17 | 16.95±5.00 | 17.52±4.60 |
| Group A | |||||
| DLIR-L | 11.24±2.45 | 10.99±2.26 | 9.60±2.21 | 10.47±2.24 | 10.36±2.34 |
| DLIR-M | 13.74±3.23 | 13.46±3.02 | 11.74±2.94 | 12.81±3.11 | 12.61±3.15 |
| DLIR-H | 20.60±4.50 | 20.12±4.21 | 17.47±4.13 | 19.00±4.14 | 18.75±4.27 |
| P value | |||||
| DLIR-L (Group A vs. Group B) | 0.152 | 0.981 | 0.866 | 0.186 | 0.121 |
| DLIR-M (Group A vs. Group B) | 0.008 | <0.001 | 0.054 | <0.001 | 0.067 |
| DLIR-H (Group A vs. Group B) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
Data are presented as mean ± standard deviation. Group A, normal-weight group; Group B, overweight group. CNR, contrast-to-noise ratio; DLIR, deep-learning image reconstruction; AO, aortic root; LMA, left main artery; LAD, left anterior descending artery; RCA, right coronary artery; LCX, left circumflex artery; L, low; M, medium; H, high.
Table 6
| Group | AO | LMA | LAD | RCA | LCX |
|---|---|---|---|---|---|
| Group B | |||||
| DLIR-L | 10.59±2.32 | 10.09±2.29 | 9.35±2.18 | 9.60±2.42 | 9.83±2.50 |
| DLIR-M | 12.68±2.91 | 12.04±2.93 | 11.10±2.75 | 11.54±2.95 | 11.76±3.08 |
| DLIR-H | 19.15±4.31 | 18.22±4.36 | 16.55±4.17 | 16.95±5.00 | 17.52±4.60 |
| ASiR-40% | 8.39±1.46 | 7.93±1.44 | 7.36±1.50 | 7.63±1.60 | 7.66±1.65 |
| ASiR-80% | 10.70±1.71 | 10.00±1.76 | 9.22±1.89 | 9.65±1.92 | 9.67±2.15 |
| Group A | |||||
| DLIR-L | 11.24±2.45 | 10.99±2.26 | 9.60±2.21 | 10.47±2.24 | 10.36±2.34 |
| DLIR-M | 13.74±3.23 | 13.46±3.02 | 11.74±2.94 | 12.81±3.11 | 12.61±3.15 |
| DLIR-H | 20.60±4.50 | 20.12±4.21 | 17.47±4.13 | 19.00±4.14 | 18.75±4.27 |
| ASiR-40% | 8.81±1.81 | 8.55±1.64 | 7.43±1.65 | 8.06±1.61 | 8.10±1.74 |
| ASiR-80% | 11.01±2.40 | 10.70±2.34 | 9.26±2.47 | 10.04±2.40 | 10.01±2.41 |
| P value (Group B) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| P value (Group A) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
Data are presented as mean ± standard deviation. Group A, normal-weight group; Group B, overweight group. CNR, contrast-to-noise ratio; DLIR, deep-learning image reconstruction; ASiR, adaptive statistical iterative reconstruction; AO, aortic root; LMA, left main artery; LAD, left anterior descending artery; RCA, right coronary artery; LCX, left circumflex artery; L, low; M, medium; H, high.
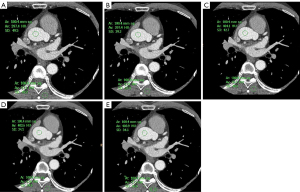
Subjective image quality
In the overweight group, radiologists A and B had the highest average quality scores for the DLIR-H-reconstructed images (close to 5 points), and the lowest scores for the ASiR-80%-reconstructed images. The overall DLIR average quality scores were significantly higher than those for the ASiR-V images (all P values <0.05). The comparison of the ASiR-V images between the overweight and normal-weight groups indicated that the subjective score of the ASiR-80% images was lower than that for ASiR-40% images (all P values <0.05); for the DLIR images, the subjective scores for DLIR-L, DLIR-M, and DLIR-H were similar (all P values >0.05) (Tables 7-9).
Table 7
| Group | DLIR-L | DLIR-M | DLIR-H | ASiR-40% | ASiR-80% | P |
|---|---|---|---|---|---|---|
| Group A | ||||||
| Doctor A | 4.00±0.41 | 4.63±0.90 | 4.90±0.31 | 3.73±0.57 | 3.65±0.48 | <0.001 |
| Doctor B | 4.00±0.36 | 4.56±0.50 | 4.85±0.30 | 3.73±0.54 | 3.67±0.48 | <0.001 |
| Group B | ||||||
| Doctor A | 4.10±0.42 | 4.75±0.44 | 4.96±0.21 | 3.85±0.50 | 3.71±0.46 | <0.001 |
| Doctor B | 4.17±0.43 | 4.73±0.45 | 4.98±014 | 3.83±0.48 | 3.73±0.45 | <0.001 |
| Kappa value (Group B) | 0.883±0.057 | 0.838±0.071 | 0.857±0.120 | 0.846±0.052 | 0.845±0.064 | – |
| Kappa value (Group A) | 0.841±0.062 | 0.871±0.072 | 0.810±0.131 | 0.958±0.041 | 0.865±0.075 | – |
Data are presented as mean ± standard deviation. Group A, normal-weight group; Group B, overweight group. DLIR, deep-learning image reconstruction; L, low; M, medium; H, high; ASiR, adaptive statistical iterative reconstruction.
Table 8
| Group | Group A | Group B | P value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ASiR-40% | ASiR-80% | ASiR-40% | ASiR-80% | ASiR-40% (Group A) vs. ASiR-40% (Group B) | ASiR-80% (Group A) vs. ASiR-80% (Group B) | ASiR-40% vs. ASiR-80% | |||
| Doctor A | 3.73±0.57 | 3.65±0.48 | 3.85±0.50 | 3.71±0.46 | 0.232 | 0.515 | <0.001 | ||
| Doctor B | 3.73±0.54 | 3.67±0.48 | 3.83±0.48 | 3.73±0.45 | 0.293 | 0.507 | <0.001 | ||
Data are presented as mean ± standard deviation. Group A, normal-weight group; Group B, overweight group. ASiR, adaptive statistical iterative reconstruction; L, low; M, medium; H, high.
Table 9
| Group | Group A | Group B | P value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DLIR-L | DLIR-M | DLIR-H | DLIR-L | DLIR-M | DLIR-H | DLIR-L (Group A vs. Group B) | DLIR-M (Group A vs. Group B) | DLIR-H (Group A vs. Group B) | |||
| Doctor A | 4.00±0.41 | 4.63±0.90 | 4.90±0.31 | 4.10±0.42 | 4.75±0.44 | 4.96±0.21 | 0.291 | 0.008 | 0.520 | ||
| Doctor B | 4.00±0.36 | 4.56±0.50 | 4.85±0.30 | 4.17±0.43 | 4.73±0.45 | 4.98±014 | 0.004 | 0.005 | 0.005 | ||
Data are presented as mean ± standard deviation. Group A, normal-weight group; Group B, overweight group. DLIR, deep-learning image reconstruction; L, low; M, medium; H, high.
Discussion
It is well known that changes in the tube voltage and tube current will affect the radiation dose of the scan. In the fixed In the fixed tube current scan mode (mA), the tube voltage and radiation dose have a quadratic relationship. In the automatic mA scan mode, mA will increase to compensate for the required radiation dose, and reducing the tube voltage can significantly improve the enhancement effect (CT value and CNR) (19). However, for overweight patients undergoing CCTA examination, low tube voltage, low penetration of X-rays, and the thicker fat layer patients increases the quantum noise of the acquired image. Thus, in overweight patients, it can be difficult to achieve an appropriate degree of enhancement of the coronary vessels, interfering with the display of the degree of coronary stenosis and the nature of atherosclerotic plaque in the image; affecting the sensitivity, accuracy, and reliability of diagnosis; and ultimately worsening the treatment and prognosis of the disease (20). Although 70-kVp low tube voltage scanning was used in this study, the automatic mA mode applied was as high as 1,300 mA, which can compensate for the insufficient X-ray electron energy caused by low tube voltage. In addition, when the 70-kVp tube voltage is used, the average electron energy of the X-ray is close to the critical value of the iodine atom attenuation in the iodine contrast medium. This critical value is called the iodine K edge. When the ray energy is equal to or slightly greater than the K electron binding energy of the iodine atom, the X-ray attenuation suddenly changes, but the attenuation coefficient of the human tissue does not have this feature. The detection efficacy of the iodine signal rises significantly, and the CT value of iodine is the largest. This causes the CT value of blood vessels to increase, with the contrast against the surrounding soft tissue structure being the strongest, which is helpful to display the diseased blood vessels (21,22).
In recent years, the application of deep learning has become widespread in the medical field (23). The DLIR represents a new generation of algorithms. Compared with ASiR-V, which can cause “blocky” artifacts or over smoothing, DLIR can maintain real image texture while significantly reducing image noise (24). With non-interventional medical image registration technology, implemented by convolutional neural networks that model the image texture of low-noise, high-resolution and high-dose filtered back-projection from millions of training parameters can achieve a favorable trade-off between noise reduction and subjective image recognition (25).
The development of contemporary CT technology focuses on high efficiency, low dose, artificial intelligence. In this study, we compared the DLIR and ASiR-V algorithms in the context of low-voltage scanning, and the results showed the possibility of performing CCTA in overweight patients. Compared with ASiR-V images, DLIR images had significantly less image noise and substantially improved image quality in overweight participants. With the graduated level of noise reduction (DLIR-L is low-level noise reduction, DLIR-H is high-level noise reduction), the CNR also increased, and the subjective image quality scores were better. A previous study comparing the image quality of ASiR-V70% standard images and high-definition kernel images reconstructed by DLIR-M and DLIR-H, reported that the latter were higher (26), which is consistent with the results of our study. Relative to that of the normal-weight group, the objective image quality score of the reconstructed images was better with the increasing noise reduction level of DILR; comparing the ASiR-V images between the overweight and normal-weight groups, we found an increase in strength (ASiR-V40% vs. 80%) and an improvement in objective image quality; however, the subjective score of images reconstructed by the high-strength ASiR-V decreased because the nonlinear and non-static characteristics of the iterative algorithm led to the image spatial resolution being dependent on contrast and radiation dose. As iterative strength increases, the mean spatial frequency left-shifting of the noise power spectrum increases, which causes changes in image texture and results in excessive smoothing of images, which is not conducive to the diagnosis of diseases (5). Consequently, in the clinical setting, in order to reduce image noise and show more detail of tissue structure, a high-level voltage is often used for image scanning overweight and obese people in order to reduce the image noise. However, the DLIR can reduce the noise of the image without changing the texture of the image. The combination of low tube voltage and DLIR can lead to better enhancement of the coronary vessels and good image quality with lower radiation dose. A study of CCTA with the ASiR-V algorithm for overweight and obese people reported an average ED of 2.21±0.05 mSv (27). In our study, although the radiation dose of the overweight patients was still higher than of the normal-weight patients, the mean ED was 1.59±0.46 mSv, and the radiation dose was still low.
The limitations of this study mainly include the following: (I) the sample size was small, and the overweight and obese patients were combined into a single group; (II) we only used 70 kVp in the overweight and obese patients, and did not compare image quality and dose with other voltage group (e.g., 80 kVp, 100 kVp, 120 kVp). It is necessary to carry out further research with more samples and with more variables.
In summary, both the ASiR-V and DLIR algorithms can improve image quality in both normal-weight and heavier-weight patients. In the normal-weight group, DLIR showed better improvement of image quality than did ASiR-V. For overweight patients undergoing CCTA examination, the combination of low tube voltage and DLIR can provide higher contrast and quality of images with a lower radiation dose. Although the objective image evaluation score improved with increasing strength of the ASiR-V algorithm, due to the change in the noise texture of the image, the subjective score decreased, which may affect the diagnosis of the disease. Therefore, compared with the ASiR-V algorithm, the DLIR algorithm can significantly reduce noise and improve image quality. With the improvement of the DLIR level, the detailed structure of coronary arteries can be displayed more clearly, which is helpful for the diagnosis and differential diagnosis of diseases.
Conclusions
As the strength of the ASiR-V reconstruction algorithm increased, the objective image quality increased accordingly, but the high-strength ASiR-V changed the noise texture of the image, resulting in a decrease in the subjective score, which affected disease diagnosis. Compared with the ASiR-V reconstruction algorithm, the DLIR reconstruction algorithm improved the image quality and diagnostic reliability for CCTA in patients with different weights, especially in heavier patients.
Acknowledgments
Funding: This work was supported by the Key R&D (General) Project of Ningxia Hui Autonomous Region (Nos. 2019BEG03046 and 2021BEG03092).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-1141/coif). XS and YS are employees of GE (China) CT Imaging Research Center. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the People’s Hospital of Ningxia Hui Autonomous Region. All participants provided written informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen W, Jiang H. Interpretation of expert consensus on medical nutrition therapy for overweight/obesity in China in 2016. Chinese Journal of Practical Internal Medicine 2017;37:430-3.
- Chen J, Gao Z, Li X, Yao B, Wang J, Ye Z. Radiation dose and image quality of low-concentration iodine contrast agent, low-dose scanning technology combined with body mass index upper abdominal CT examination. Chinese Journal of Radiology 2017;51:141-4.
- Lee S, Kwon H, Cho J. The Detection of Focal Liver Lesions Using Abdominal CT: A Comparison of Image Quality Between Adaptive Statistical Iterative Reconstruction V and Adaptive Statistical Iterative Reconstruction. Acad Radiol 2016;23:1532-8. [Crossref] [PubMed]
- Yang L, Zhu X, Ge Y, Tang LJ, Zhu YS. Effect of iterative reorganization intensity on coronary CTA images in patients with different body weights. Journal of Clinical Radiology 2018;37:2091-6.
- Benz DC, Ersözlü S, Mojon FLA, Messerli M, Mitulla AK, Ciancone D, Kenkel D, Schaab JA, Gebhard C, Pazhenkottil AP, Kaufmann PA, Buechel RR. Radiation dose reduction with deep-learning image reconstruction for coronary computed tomography angiography. Eur Radiol 2022;32:2620-8. [Crossref] [PubMed]
- Kramer CM, Budoff MJ, Fayad ZA, Ferrari VA, Goldman C, Lesser JR, et al. ACCF/AHA 2007 clinical competence statement on vascular imaging with computed tomography and magnetic resonance. A report of the American College of Cardiology Foundation/American Heart Association/American College of Physicians Task Force on Clinical Competence and Training. J Am Coll Cardiol 2007;50:1097-114. [Crossref] [PubMed]
- Thomas JD, Zoghbi WA, Beller GA, Bonow RO, Budoff MJ, Cerqueira MD, Creager MA, Douglas PS, Fuster V, Garcia MJ, Holmes DR Jr, Manning WJ, Pohost GM, Ryan TJ, Van Decker WA, Wiegers SEAmerican Heart Association (AHA). American College of Physicians (ACP) Task Force on Clinical Competence and Training. ACCF 2008 Training Statement on Multimodality Noninvasive Cardiovascular Imaging A Report of the American College of Cardiology Foundation/American Heart Association/American College of Physicians Task Force on Clinical Competence and Training Developed in Collaboration With the American Society of Echocardiography, the American Society of Nuclear Cardiology, the Society of Cardiovascular Computed Tomography, the Society for Cardiovascular Magnetic Resonance, and the Society for Vascular Medicine. J Am Coll Cardiol 2009;53:125-46. [Crossref] [PubMed]
- Jacobs JE, Boxt LM, Desjardins B, Fishman EK, Larson PA, Schoepf JAmerican College of Radiology. ACR practice guideline for the performance and interpretation of cardiac computed tomography (CT). J Am Coll Radiol 2006;3:677-85. [Crossref] [PubMed]
- Abbara S, Blanke P, Maroules CD, Cheezum M, Choi AD, Han BK, Marwan M, Naoum C, Norgaard BL, Rubinshtein R, Schoenhagen P, Villines T, Leipsic J. SCCT guidelines for the performance and acquisition of coronary computed tomographic angiography: A report of the society of Cardiovascular Computed Tomography Guidelines Committee: Endorsed by the North American Society for Cardiovascular Imaging (NASCI). J Cardiovasc Comput Tomogr 2016;10:435-49. [Crossref] [PubMed]
- Budoff MJ, Achenbach S, Berman DS, Fayad ZA, Poon M, Taylor AJ, Uretsky BF, Williams KA. Task force 13: training in advanced cardiovascular imaging (computed tomography) endorsed by the American Society of Nuclear Cardiology, Society of Atherosclerosis Imaging and Preven- tion, Society for Cardiovascular Angiography and Interventions, and Society of Cardiovascular Computed Tomography. J Am Coll Cardiol 2008;51:409-14. [Crossref] [PubMed]
- Mark DB, Berman DS, Budoff MJ, Carr JJ, Gerber TC, Hecht HS, Hlatky MA, Hodgson JM, Lauer MS, Miller JM, Morin RL, Mukherjee D, Poon M, Rubin GD, Schwartz RS. ACCF/ACR/AHA/NASCI/SAIP/SCAI/SCCT 2010 expert consensus document on coronary computed tomographic angiography: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. Circulation 2010;121:2509-43. [Crossref] [PubMed]
- Abbara S, Arbab-Zadeh A, Callister TQ, Desai MY, Mamuya W, Thomson L, Weigold WG. SCCT guidelines for performance of coronary computed tomographic angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr 2009;3:190-204. [Crossref] [PubMed]
- IEEE International Conference on Computer Vision (English). Journal of Intelligent Systems 2015;10:375.
- Yao F. Iterative algorithm to improve the image quality of dual-source CT abdominal low-dose scanning in patients with different BMI. Soochow University, 2016.
- Gao X, Li Y, Ge H, Liu G, Ma C, Fei J. The effect of personalized contrast agent scheme based on different weight on CT coronary imaging. Chinese Journal of CT and MRI 2021;19:74-6.
- Chen H, Du D, Zhao X, Gao Z, Song J, Liu B. The application value of SF technology in the evaluation of coronary artery stent implantation. Chinese Journal of CT and MRI 2021;19:97-9.
- Benz DC, Gräni C, Mikulicic F, Vontobel J, Fuchs TA, Possner M, Clerc OF, Stehli J, Gaemperli O, Pazhenkottil AP, Buechel RR, Kaufmann PA. Adaptive Statistical Iterative Reconstruction-V: Impact on Image Quality in Ultralow-Dose Coronary Computed Tomography Angiography. J Comput Assist Tomogr 2016;40:958-63. [Crossref] [PubMed]
- Park IK, Park J, Kim TH, Lee J, Han K, Oh C, Park CH. Non-inferior low-dose coronary computed tomography angiography image quality with knowledge-based iterative model reconstruction for overweight patients. PLoS One 2018;13:e0209243. [Crossref] [PubMed]
- Tan SK, Yeong CH, Raja Aman RRA, Ng KH, Abdul Aziz YF, Chee KH, Sun Z. Low tube voltage prospectively ECG-triggered coronary CT angiography: a systematic review of image quality and radiation dose. Br J Radiol 2018;91:20170874. [Crossref] [PubMed]
- Li J, Huang Y, Han D, Cai Y, Min R. The accuracy of artificial intelligence in the diagnosis of coronary heart disease in coronary CT angiography. China Medical Imaging Technology 2021;37:59-62.
- Liu P, Wang M, Wang Y, Yu M, Wang Y, Liu Z, Li Y, Jin Z. Impact of Deep Learning-based Optimization Algorithm on Image Quality of Low-dose Coronary CT Angiography with Noise Reduction: A Prospective Study. Acad Radiol 2020;27:1241-8. [Crossref] [PubMed]
- Chen Y, Liu Z, Li M, Yu Y, Jia Y, Ma G, Hu Z, Wei D, Li D, He T. Reducing both radiation and contrast doses in coronary CT angiography in lean patients on a 16-cm wide-detector CT using 70 kVp and ASiR-V algorithm, in comparison with the conventional 100-kVp protocol. Eur Radiol 2019;29:3036-43. [Crossref] [PubMed]
- Zhu B, Liu JZ, Cauley SF, Rosen BR, Rosen MS. Image reconstruction by domain-transform manifold learning. Nature 2018;555:487-92. [Crossref] [PubMed]
- Greffier J, Hamard A, Pereira F, Barrau C, Pasquier H, Beregi JP, Frandon J. Image quality and dose reduction opportunity of deep learning image reconstruction algorithm for CT: a phantom study. Eur Radiol 2020;30:3951-9. [Crossref] [PubMed]
- Hardie AD, Nelson RM, Egbert R, Rieter WJ, Tipnis SV. What is the preferred strength setting of the sinogram-affirmed iterative reconstruction algorithm in abdominal CT imaging? Radiol Phys Technol 2015;8:60-3. [Crossref] [PubMed]
- Benz DC, Benetos G, Rampidis G, von Felten E, Bakula A, Sustar A, Kudura K, Messerli M, Fuchs TA, Gebhard C, Pazhenkottil AP, Kaufmann PA, Buechel RR. Validation of deep-learning image reconstruction for coronary computed tomography angiography: Impact on noise, image quality and diagnostic accuracy. J Cardiovasc Comput Tomogr 2020;14:444-51. [Crossref] [PubMed]
- Du J, Ren A, Li Z, Guo X, Yang T. Feasibility of 80 kVp tube voltage coronary CTA combined with AsiR-V image reconstruction in overweight and class I obese patients. China Interventional Imaging and Therapeutics 2020;17:289-93.