
Patterns and implications of artery remodeling based on high-resolution vessel wall imaging in symptomatic severe basilar artery stenosis
Introduction
Symptomatic severe basilar artery (BA) stenosis is a major cause of ischemic stroke, accounting for 15% of all strokes and 10.7% of ischemic strokes annually, despite the existence of aggressive medical therapies (1). Endovascular treatment, such as angioplasty and stenting, are available for stroke prevention in symptomatic patients with severe (≥70%) BA stenosis. Periprocedural stroke or death is significantly higher in the posterior circulation than the anterior circulation, with a reported risk of 21.6% in the BA (2). Perforator infarction is the main cause of periprocedural stroke or death in patients with severe BA stenosis after endovascular treatment (2,3).
Arterial remodeling, comprising positive, intermediate, and negative remodeling (NR), reflects incremental changes of an arterial wall in response to stress-related atherosclerotic processes and may affect the outcomes of endovascular treatment for ischemic lesions (4). Positive remodeling (PR) is regarded as the compensatory outward expansion of the arterial wall to maintain lumen patency despite plaque development. Luminal compromise occurs principally due to plaque expansion exceeding the limit of compensatory remodeling (5,6). In contrast, NR is defined as the adaptive constriction of the vessel, which may exacerbate luminal stenosis. Intermediate remodeling is a state between PR and NR, in which marginal changes of the outer wall are exhibited despite luminal stenosis (7,8). A previous study reported that NR was associated with an increased risk of perforator stroke in patients with basilar stenosis; however, this study was limited by its small sample size (9). Several studies have shown that PR may increase the risk of major adverse cardiac events in patients undergoing coronary intervention, whereas NR may increase the risk of perioperative complications, such as vascular injury, during treatment (6,8). It is still unclear whether remodeling patterns are related to the occurrence of perioperative complications after endovascular treatment in symptomatic patients with severe BA stenosis.
High-resolution MRI (HR-MRI) is a reliable imaging modality for the qualitative and quantitative assessment of arterial remodeling. Several studies to evaluate HR-MRI have suggested a relationship between remodeling patterns and plaque characteristics in extracranial and intracranial arteries (10-12). Nevertheless, studies focusing on the plaque characteristics and different remodeling patterns in patients with BA stenosis are scarce. In the current study, we sought to determine the relationship between plaque characteristics and perioperative complications in patients with severe BA stenosis, with a focus on remodeling patterns. We present the following article in accordance with the Strengthening the Reporting of Cohort Studies in Surgery (STROCSS) reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-22-771/rc) (13).
Methods
Study design
The study cohort was derived from the Clinical Registration Trial of Intracranial Stenting for Patients with Symptomatic Intracranial Artery Stenosis (CRTICAS) database (ClinicalTrials.gov Identifier: NCT01994161) (14). The protocol, which adhered to the ethical principles of the Declaration of Helsinki (as revised in 2013) (15) and the Guideline for Good Clinical Practice, was approved by the Ethics Committee of Xuanwu Hospital, Capital Medical University (No. [2013] 004). Informed consent was obtained from all patients.
From December 2013 to December 2015, patients with BA stenosis at several high-volume tertiary centers in China were enrolled for analysis according to the following criteria: (I) had BA stenosis of more than 70% confirmed by digital subtraction angiography (DSA); (II) had ischemic symptoms caused by BA stenosis including dizziness, vertigo, headache, double vision, double vision, slurred speech, numbness, or weakness of limbs; (III) treated with endovascular therapy; (IV) underwent HR-MRI before intervention; and (V) underwent MRI within the 72 hours before and the 72 hours after intervention. The following patients were excluded: (I) patients with a history of acute stroke caused by BA occlusion; (II) patients who had received simultaneous endovascular therapy of another intracranial or extracranial vessel; (III) patients with BA stenosis in combination with moderate-to-severe vertebral artery stenosis; and (IV) patients whose BA stenosis had a non-atherosclerotic cause (e.g., Moyamoya disease, vasculitis, or dissection).
The baseline clinical information of all eligible patients was collected for analysis.
Imaging protocols
A 3.0-T MRI scanner (Magnetom Spectra; Siemens Healthineers, Erlangen, Germany) with a standard 8-channel head coil was used to image all eligible patients with BA stenosis. The multi-sequence protocol is described in Table S1. Imaging acquisition was performed in the sagittal plane covering the BA vessel, and multiplanar reconstructions were obtained for image analysis.
Imaging measurement and analysis
All images were independently reviewed by radiologists at the IsCore Image Corelab (http://imagecorelabcn.com/en/). The radiologists did not participate in the statistical analyses and were blinded to the clinical data of the patients. Five percent of the data in the cohort were used to train raters before a formal assessment of the imaging data was performed. The formal assessment was conducted when the agreement achieved between the two raters (reliability >0.75) was graded ‘excellent’.
The characteristics of BA lesions, such as diameter, area, and signal intensity, were measured. The lumen diameter, vessel area (VA), and lumen area (LA) at the site of maximal luminal narrowing (MLN) and the reference site were manually traced for measurement. According to the Warfarin versus Aspirin for Symptomatic Intracranial Disease (WASID) criteria, the reference site was defined as the normal segment proximal to the stenosis and the distal vessel was used when the proximal segment was diseased (16).
The parameters based on HR-MRI and DSA were defined as follows: wall area (WA), VA – LA; plaque burden, [(WAMLN − WAreference)/VAMLN] ×100%; and remodeling index, [VAMLN/VAreference]. The classification of vessel remodeling was divided according to the remodeling index into the following three types: PR (≥1.05), NR (≤0.95), and intermediate remodeling (0.95–1.05; Figure 1A). For simplicity, PR and intermediate remodeling were classified as non-NR.
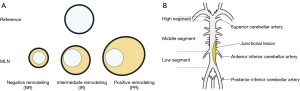
The cross-sectional culprit lesion was divided by two perpendicular lines into the following quadrants, as described in a previous study: ventral, dorsal, left, and right (17). For simplicity, the left and right sites were classified as lateral sites. If a plaque was large and the thickest part spanned two quadrants, it was defined as being distributed across more than two quadrants.
In DSA imaging, the BA was divided by branches of the anterior inferior cerebellar artery and superior cerebellar artery into three segments: low, middle, and high (18). Due to its rarity (1.2% of the incidence reported), the single high segment identified was sorted into the middle segment (18). Classification as a junctional segment lesion was considered when lesions crossed the anterior inferior cerebellar artery (Figure 1B).
The stenotic diameter based on DSA was calculated as [1 − (luminal diameter at MLN)/(luminal diameter at reference site)] ×100%. The degree of stenosis by area based on HR-MRI was calculated as [1 − LA at MLN/LA at reference site] ×100%.
Interventional procedure and outcomes assessment
All patients were treated by neurointerventionalists with at least 15 years of experience in endovascular treatment. Therapeutic strategies, including primary angioplasty, balloon-mounted stent, and self-expanding stent, were determined by experienced operators and informed by lesion characteristics (19). In the days leading up to the procedure, each patient was prescribed aspirin (100 mg daily) and clopidogrel (75 mg daily). A 2/3 mg/kg dose of systemic heparin was injected intravenously during the procedure. An additional half dose of heparin was injected after 1 hour (20). After intervention, dual antiplatelet therapy was maintained for 3 months with aspirin (100 mg daily) and clopidogrel (75 mg daily).
Perioperative cerebrovascular events, including stroke, transient ischemic attack (TIA), and new ischemic cerebral lesions (NICLs), were assessed by neurosurgeons and neuroradiologists based on patients’ clinical information and imaging. Stroke, including ischemic and hemorrhage stroke, was defined as a neurological deficit lasting for more than 24 hours. A TIA was defined as a neurological deficit lasting for less than 24 hours (21). An NICL of the BA territory was identified as a new high signal on diffusion-weighted imaging and a new low signal on apparent diffusion coefficient imaging within 72 hours after the operation (22). The mechanism of stroke or NICLs in the distribution of the BA was divided into artery-to-artery (A-A) embolism, local perforator infarction, and mixed mechanism (19). For simplicity, mixed mechanisms involving local perforator infarction were also classified as perforator infarction in this study.
Statistical analysis
Analyses were conducted with Statistical Analysis System (SAS) software, v. 9.4 (SAS Institute Inc., Cary, NC, USA). Quantitative variables were presented as mean ± standard deviation or median with interquartile range, and qualitative variables were presented as number and percentage. We performed descriptive analyses of all study participants, dividing them into NR and non-NR groups. Comparisons of categorical variables were performed using the χ2 test or Fisher’s exact test, as appropriate. The Student’s t-test or Wilcoxon tests were used to compare quantitative variables. Scatter diagrams and fitted curves of the remodeling index, plaque burden, and degree of stenosis by area were produced using the ggplot2 package in R v. 4.0.1 (R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org/) software. The evaluation of correlations between these variables was performed by a Spearman rank correlation analysis with two-tailed significance. The value of P<0.05 was considered statistically significant.
Logistic regression was performed to assess whether remodeling patterns were associated with the perioperative composite outcome of TIA, stroke, or both. Age and sex were treated as prognostic factors when adjusting the model. In cases where the comparison of the two groups revealed an imbalance between them (P=0.2), the imbalance factors were also introduced as adjustment variables. The same strategy was used for analysis of NICLs on diffusion-weighted imaging.
Results
Baseline characteristics
There were 281 consecutive patients with symptomatic severe BA stenosis treated with endovascular therapy. Following screening, 140 patients were eligible for inclusion in the study (Figure S1). The average age of these patients was 61.9±7.7 years. According to the remodeling pattern, 91 (65.0%) patients were classified as NR and 49 (35.0%) patients as non-NR. There was no significant difference between the two groups in terms of baseline characteristics, such as age, sex, and preoperative modified Rankin Scale score (Table 1).
Table 1
| Items | All patients (n=140) | NR (n=91) | Non-NR (n=49) | P value |
|---|---|---|---|---|
| Age, year, mean ± SD | 61.9±7.7 | 61.5±7.9 | 62.6±7.5 | 0.442 |
| Sex, n (%) | 0.386 | |||
| Male | 106 (75.7) | 71 (78.0) | 35 (71.4) | |
| Female | 34 (24.3) | 20 (22.0) | 14 (28.6) | |
| BMI, kg/m2, mean ± SD | 26.2±3.0 | 26.4±2.9 | 26.0±3.4 | 0.540 |
| Hypertension, n (%) | 117 (83.6) | 77 (84.6) | 40 (81.6) | 0.650 |
| DM, n (%) | 58 (41.4) | 36 (39.6) | 22 (44.9) | 0.541 |
| Hyperlipidemia, n (%) | 36 (25.7) | 23 (25.3) | 13 (26.5) | 0.871 |
| CAD, n (%) | 14 (10.0) | 10 (11.0) | 4 (8.2) | 0.813 |
| Smoking, n (%) | 58 (41.4) | 36 (39.6) | 22 (44.9) | 0.541 |
| Drinking, n (%) | 38 (27.1) | 23 (25.3) | 15 (30.6) | 0.498 |
| OTA, day, median (IQR) | 44.7 (40.9) | 42.6 (40.0) | 50.0 (40.5) | 0.420 |
| Qualifying event, n (%) | 0.650 | |||
| TIA | 23 (16.4) | 14 (15.4) | 9 (18.4) | |
| Stroke | 117 (83.6) | 77 (84.6) | 40 (81.6) | |
| Preoperative mRS, n (%) | 0.115 | |||
| <2 | 125 (89.3) | 84 (92.3) | 41 (83.7) | |
| ≥2 | 15 (10.7) | 7 (7.7) | 8 (16.3) |
NR, negative remodeling; SD, standard deviation; BMI, body mass index; DM, diabetes mellitus; CAD, coronary artery disease; OTA, onset to admission; IQR, interquartile range; TIA, transient ischemic attack; mRS, modified Rankin Scale score.
Characteristics based on HR-MRI, DSA, and treatment
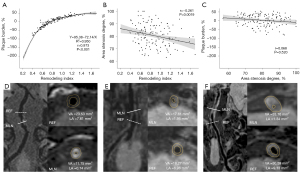
The NR group had a higher degree of stenosis by area than did the non-NR group (81.8%±9.1% vs. 78.1%±8.3%, respectively; P=0.019) but lower proportions of low and junctional segment stenosis (28.6% vs. 46.9%, 2.2% vs. 8.2%, respectively; P=0.010). The plaque burden (−8.6%±40.4 % vs. 33.6%±12.2%, respectively; P<0.001) and the remodeling index (0.7±0.2 vs. 1.2±0.2, respectively; P<0.001) were lower in the NR group than in the non-NR group (Tables 2,3). There was no difference in the treatment type, diameter ratio of balloon/stent to stenosis, or reference site between the two groups (Table 3). Regarding the relationships between the remodeling index, plaque burden, and degree of stenosis by area, the remodeling index was strongly associated with plaque burden, fitting an inverse equation between them (r=0.973, P<0.001; Figure 2A) and marginally associated with the degree of stenosis by area (r=−0.261, P=0.0019; Figure 2B). The degree of stenosis by area was not related to plaque burden (r=0.068, P=0.520; Figure 2C).
Table 2
| Items | All patients (n=140) | NR (n=91) | Non-NR (n=49) | P value |
|---|---|---|---|---|
| OTE, day, median (IQR) | 37.5 (44.8) | 32.0 (42.0) | 43.0 (43.5) | 0.512 |
| Lesion location (axial view), n (%) | 0.865 | |||
| Ventral | 18 (12.9) | 13 (14.3) | 5 (10.2) | |
| Lateral | 31 (22.1) | 21 (23.1) | 10 (20.4) | |
| Dorsal | 48 (34.3) | 30 (32.9) | 18 (36.7) | |
| ≥2 quadrants | 43 (30.7) | 27 (29.7) | 16 (32.7) | |
| Stenosis (area), %, mean ± SD | 80.5±9.0 | 81.8±9.1 | 78.1±8.3 | 0.019* |
| Plaque burden, %, median (IQR) | 9.3 (43.9) | −8.6 (40.4) | 33.6 (12.2) | <0.001* |
| Remodeling index, median (IQR) | 0.8 (0.4) | 0.7 (0.2) | 1.2 (0.2) | <0.001* |
*, P<0.05. NR, negative remodeling; OTE, onset to examination of HR-MRI; IQR, interquartile range; SD, standard deviation; HR-MRI, high-resolution MRI.
Table 3
| Items | All patients (n=140) | NR (n=91) | Non-NR (n=49) | P value |
|---|---|---|---|---|
| Lesion location (coronal view), n (%) | 0.010* | |||
| Low segment | 49 (35.0) | 26 (28.6) | 23 (46.9) | |
| Middle segment | 85 (60.7) | 63 (69.2) | 22 (44.9) | |
| Junctional segment | 6 (4.3) | 2 (2.2) | 4 (8.2) | |
| Diameter at MLN, mm, mean ± SD | 0.6±0.2 | 0.6±0.2 | 0.6±0.2 | 0.876 |
| Diameter at reference, mm, mean ± SD | 2.7±0.5 | 2.8±0.6 | 2.6±0.5 | 0.141 |
| Plaque length, mm, median (IQR) | 5.9 (3.8) | 5.6 (3.6) | 6.2 (3.3) | 0.139 |
| Stenosis (diameter), %, median (IQR) | 77.7 (9.3) | 77.8 (8.9) | 77.2 (9.7) | 0.287 |
| Treatment type, n (%) | 0.106 | |||
| PA | 26 (18.6) | 14 (15.4) | 12 (24.5) | |
| BMS | 30 (21.4) | 24 (26.4) | 6 (12.2) | |
| SES | 84 (60.0) | 53 (58.2) | 31 (63.3) | |
| Diameter ratio of stenosis#, median (IQR) | 5.2 (3.0) | 5.2 (3.1) | 5.4 (2.8) | 0.756 |
| Diameter ratio of reference#, median (IQR) | 1.2 (0.3) | 1.1 (0.3) | 1.2 (0.3) | 0.428 |
*, P<0.05; #, the diameter ratio was defined as the maximal diameter of the implant divided by the vessel diameter at the MLN reference site. NR, negative remodeling; MLN, maximal luminal narrowing; SD, standard deviation; IQR, interquartile range; PA, primary angioplasty; BMS, balloon-mounted stent; SES, self-expansion stent.
Periprocedural outcomes
Regarding the composite outcome of TIA/stroke, there were 11 (12.1%) events in the NR group (3 TIAs, 5 A-A strokes, and 3 perforator strokes) and 5 (10.2%) events in the non-NR group (3 A-A strokes and 2 perforator strokes). Based on univariable analysis, none of the subcategories of TIA/stroke (TIA, A-A stroke, perforator stroke) showed significant differences (Table 4). After multivariable adjustment for age, sex, preoperative modified Rankin Scale score, stenosis site, plaque length, stenosis by area, plaque burden, and remodeling pattern, the NR group showed no significant difference in perioperative outcome compared to the non-NR group for these composite events.
Table 4
| Outcomes | NR group (n=91) | Non-NR group (n=49) | Unadjusted P | Adjusted P* | Adjusted OR (95% CI) |
|---|---|---|---|---|---|
| TIA/stroke, n (%) | 11 (12.1) | 5 (10.2) | 0.739 | 0.226 | 2.86 (0.52–14.29) |
| TIA | 3 (3.3) | 0 (0) | 0.552 | NA | NA |
| A-A stroke | 5 (5.5) | 3 (6.1) | 1.000 | 0.345 | 2.86 (0.32–25.00) |
| Perforator stroke | 3 (3.3) | 2 (4.1) | 1.000 | 0.601 | 0.44 (0.02–9.09) |
| NICLs on DWI, n (%) | 47 (51.6) | 29 (59.2) | 0.394 | 0.663 | 0.82 (0.34–2.00) |
| A-A NICLs | 38 (41.7) | 19 (38.8) | 0.732 | 0.945 | 0.97 (0.40–2.38) |
| Perforator NICLs | 9 (9.9) | 10 (20.4) | 0.089 | 0.718 | 0.77 (0.19–3.13) |
*, outcomes adjusted by age, sex, preoperative modified Rankin Scale score, lesion location (coronal view), plaque length (mm), degree of stenosis (area), and plaque burden. NR, negative remodeling; OR, odds ratio; CI, confidence interval; TIA, transient ischemic attack; A-A, artery-to-artery; NICLs, new ischemic cerebral lesions; DWI, diffusion-weighted imaging.
In addition, postoperative NICLs on diffusion-weighted imaging were not rare in either group, with 47 (51.6%) cases in the NR group (38 A-A NICLs and 9 perforator NICLs) and 29 (59.2%) cases in the non-NR group (19 A-A NICLs and 10 perforator NICLs). Univariable analysis showed no significant difference between the NR and non-NR groups in total postoperative NICLs or all NICLs, and the difference remained insignificant after multivariable adjustment for confounders (Table 4).
Discussion
Arterial remodeling is an adaptive process vessels undergo in response to mechanical or chemical factors, which arise principally from blood flow and inflammation, respectively. Remodeling patterns may indicate differences in pathophysiology during the atherosclerotic process. It is likely that PR is a mechanism of compensation for plaque accumulation to prevent luminal stenosis. However, a larger burden ultimately increases the risk of plaque rupture; thus, there is an apparent limit to this compensatory process. In contrast, NR appears to accelerate luminal narrowing but the risk of plaque rupture is smaller when the plaque itself is diminutive (23). In the current study, we found that NR is dominant in symptomatic patients with severe BA stenosis and is associated with a lower plaque burden. Moreover, the relationship between the mathematical formulas associated with the remodeling index and plaque burden has a high degree of fit to an inverse equation.
The prevalence of arterial remodeling patterns varies among arteries and clarification of this variation may provide deeper insight into atherosclerotic progress and the occurrence of clinical events (24). For example, PR is prevalent in the renal artery and common carotid artery, whereas NR is prevalent in the femoral artery (23). Compared to the anterior circulation, the posterior circulation appears to be more capable of PR as it has a lower blood flow and less sympathetic innervation (25-28). However, we observed NR to be more common in patients with severe BA stenosis. Studies on coronary atherosclerosis have shown NR to occur more frequently than PR in lesions imparting symptomatic severe stenosis, which is in keeping with our findings for the BA (29). We speculate that the reasons for the high prevalence of NR in this study may include the following. Firstly, plaques with NR are relatively stable, and symptomatic presentations are mainly caused by a luminal stenosis obstructing the blood flow. In contrast, symptoms are more likely to occur in patients with PR following plaque rupture due to a large plaque size. Therefore, symptomatic patients with PR may not have severe stenosis and, thus, were not enrolled for endovascular treatment, in keeping with global practice patterns for intracranial atherosclerosis (30). Secondly, histopathological studies suggest that PR may transform into NR due to intimal healing and proliferation in response to repetitive micro-ruptures of a high-risk plaque (31,32). Furthermore, the risk of endovascular treatment is higher for the posterior circulation than it is for the anterior circulation (2). Therefore, we focused our research on severe BA stenosis. The present study, to our knowledge, is the first to analyze distribution differences in remodeling patterns in patients with severe BA stenosis. NR is more common in the middle segment of the BA than in the low or junctional segment. This difference in remodeling patterns in the BA may be associated with anatomical and hemodynamic factors (33), and understanding it would in turn provide researchers with a unique basis for understanding BA atherosclerosis.
Importantly, we have provided a new perspective on the relationships between remodeling patterns and plaque characteristics. The remodeling index was strongly associated with plaque burden using an inverse equation, which may provide a simple and objective radiographic approach to quantify the plaque burden for BA stenosis by overcoming the complexity of plaque burden measurement (Figure 2D-2F). On the other hand, this study observed that the plaque burden did not infinitely increase but reached a plateau along with arterial PR. Additionally, the remodeling index was marginally associated with the degree of stenosis by area. Previous studies have suggested that NR accelerates luminal narrowing, leading to a higher degree of stenosis by area (34,35). The association between the remodeling index, plaque burden, and degree of stenosis by area may simplify the assessment of plaque characteristics in future clinical practice.
In terms of perioperative complications, no association was found between BA remodeling patterns and the risk of an adverse perioperative outcome, which differs from the findings of related studies in coronary artery research. A previous coronary investigation reported that selecting balloon or stent size based solely on the reference luminal diameter but ignoring constriction of the outer wall at the lesion site may increase the risk of vessel dissection and rupture (5). Several subsequent studies on intracranial atherosclerotic stenosis reported that submaximal balloon inflation may lower the risk of perioperative complications compared with maximal balloon inflation (36). Endovascular treatment for intracranial stenosis has been progressively more judicious in the post-Stenting and Aggressive Medical Management for Preventing Recurrent stroke in Intracranial Stenosis (SAMMPRIS) trial era (37). Strict selection of patients, submaximal balloon inflation and stenting, and operator experience may be factors that improve the outlook of endovascular therapy (19).
Study limitations
In this study, there were some limitations. Firstly, despite of the small sample size, it is the largest study to date to analyze HR-MRI-based plaque characteristics and clinical outcomes in patients with symptomatic BA stenosis. Secondly, only half of the patients with symptomatic BA stenosis in CRTICAS were enrolled into the study because HR-MRI was not available at several centers in the early stage of the study, which may have resulted in a slight selection bias. In addition, the measurement of several variables, including the remodeling index, plaque burden, and degree of stenosis, was dependent on the reference site adjacent to the lesion. There is evidence that the reference site may have also undergone remodeling, resulting in the underestimation or overestimation of these variables (5). Furthermore, the current study included only patients with symptomatic severe BA stenosis. Consequently, the findings in the current study regarding remodeling patterns and implications may not be applicable to patients with mild to moderate symptomatic BA stenosis, or to patients with asymptomatic BA stenosis. Finally, arterial remodeling, as a dynamic process, along with plaque development, requires further research to examine the impact it has on patients’ long-term outcomes.
Conclusions
Under the current treatment paradigms for BA stenosis, remodeling patterns may not be associated with perioperative outcomes of ischemic events or NICLs but with plaque characteristics, especially plaque burden. A fitted curve and equation between the remodeling index and plaque burden may provide a straightforward and objective method for quantifying the plaque burden in severe BA stenosis. However, further studies on the relationship among plaque characteristics in BA stenosis are warranted.
Acknowledgments
This study’s abstract has been submitted to the 7th European Stroke Organisation Conference (ESOC 2021) with the following Receipt Number: R-ESOC21-1240.
Funding: This study was funded by the National Key Research and Development Program of China (No. 2016YFC1301703) and Beijing Science and Technology Planning Project (No. Z201100005520019).
Footnote
Reporting Checklist: The authors have completed the STROCSS reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-22-771/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-771/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The protocol adheres to the ethical principles of the Declaration of Helsinki (as revised in 2013) and Guideline for Good Clinical Practice, and was approved by the Ethics Committee of Xuanwu Hospital, Capital Medical University (No. [2013] 004). Informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Prognosis of patients with symptomatic vertebral or basilar artery stenosis. The Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) Study Group. Stroke 1998;29:1389-92. [Crossref] [PubMed]
- Gröschel K, Schnaudigel S, Pilgram SM, Wasser K, Kastrup A. A systematic review on outcome after stenting for intracranial atherosclerosis. Stroke 2009;40:e340-7. [Crossref] [PubMed]
- Fiorella D, Derdeyn CP, Lynn MJ, Barnwell SL, Hoh BL, Levy EI, et al. Detailed analysis of periprocedural strokes in patients undergoing intracranial stenting in Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS). Stroke 2012;43:2682-8. [Crossref] [PubMed]
- Gutierrez J, Goldman J, Honig LS, Elkind MS, Morgello S, Marshall RS. Determinants of cerebrovascular remodeling: do large brain arteries accommodate stenosis? Atherosclerosis 2014;235:371-9. [Crossref] [PubMed]
- Zhu XJ, Du B, Lou X, Hui FK, Ma L, Zheng BW, Jin M, Wang CX, Jiang WJ. Morphologic characteristics of atherosclerotic middle cerebral arteries on 3T high-resolution MRI. AJNR Am J Neuroradiol 2013;34:1717-22. [Crossref] [PubMed]
- Wexberg P, Gyöngyösi M, Sperker W, Kiss K, Yang P, Hassan A, Pasterkamp G, Glogar D. Pre-existing arterial remodeling is associated with in-hospital and late adverse cardiac events after coronary interventions in patients with stable angina pectoris. J Am Coll Cardiol 2000;36:1860-9. [Crossref] [PubMed]
- Birnbaum Y, Fishbein MC, Luo H, Nishioka T, Siegel RJ. Regional remodeling of atherosclerotic arteries: a major determinant of clinical manifestations of disease. J Am Coll Cardiol 1997;30:1149-64. [Crossref] [PubMed]
- Yamagishi M, Terashima M, Awano K, Kijima M, Nakatani S, Daikoku S, Ito K, Yasumura Y, Miyatake K. Morphology of vulnerable coronary plaque: insights from follow-up of patients examined by intravascular ultrasound before an acute coronary syndrome. J Am Coll Cardiol 2000;35:106-11. [Crossref] [PubMed]
- Ma N, Xu Z, Lyu J, Li M, Hou Z, Liu Y, Yang M, Mo D, Gao F, Song L, Sun X, Liu L, Liu X, Sui B, Shen M, Ma L, Wang Y, Wang Y, Miao Z, Lou X. Association of Perforator Stroke After Basilar Artery Stenting With Negative Remodeling. Stroke 2019;50:745-9. [Crossref] [PubMed]
- Qiao Y, Anwar Z, Intrapiromkul J, Liu L, Zeiler SR, Leigh R, Zhang Y, Guallar E, Wasserman BA. Patterns and Implications of Intracranial Arterial Remodeling in Stroke Patients. Stroke 2016;47:434-40. [Crossref] [PubMed]
- Astor BC, Sharrett AR, Coresh J, Chambless LE, Wasserman BA. Remodeling of carotid arteries detected with MR imaging: atherosclerosis risk in communities carotid MRI study. Radiology 2010;256:879-86. [Crossref] [PubMed]
- Zhu T, Ren L, Zhang L, Shao Y, Wan L, Li Y, Liang D, Zheng H, Liu X, Zhang N. Comparison of plaque characteristics of small and large subcortical infarctions in the middle cerebral artery territory using high-resolution magnetic resonance vessel wall imaging. Quant Imaging Med Surg 2021;11:57-66. [Crossref] [PubMed]
- Agha R, Abdall-Razak A, Crossley E, Dowlut N, Iosifidis C, Mathew G. STROCSS 2019 Guideline: Strengthening the reporting of cohort studies in surgery. Int J Surg 2019;72:156-65. [Crossref] [PubMed]
- Wang Y, Wang T, Dmytriw AA, Yang K, Jiao L, Shi H, et al. Safety of endovascular therapy for symptomatic intracranial artery stenosis: a national prospective registry. Stroke Vasc Neurol 2022;7:166-71. [Crossref] [PubMed]
- World Medical Association Declaration of Helsinki. ethical principles for medical research involving human subjects. JAMA 2013;310:2191-4. [Crossref] [PubMed]
- Samuels OB, Joseph GJ, Lynn MJ, Smith HA, Chimowitz MI. A standardized method for measuring intracranial arterial stenosis. AJNR Am J Neuroradiol 2000;21:643-6. [PubMed]
- Yu J, Li ML, Xu YY, Wu SW, Lou M, Mu XT, Feng F, Gao S, Xu WH. Plaque distribution of low-grade basilar artery atherosclerosis and its clinical relevance. BMC Neurol 2017;17:8. [Crossref] [PubMed]
- Jia B, Liebeskind DS, Ma N, Gao F, Mo D, Luo G, Li X, Sui X, Peng G, Miao Z. Factors associated with perforator stroke after selective basilar artery angioplasty or stenting. J Neurointerv Surg 2017;9:738-42. [Crossref] [PubMed]
- Luo J, Wang T, Gao P, Krings T, Jiao L. Endovascular Treatment of Intracranial Atherosclerotic Stenosis: Current Debates and Future Prospects. Front Neurol 2018;9:666. [Crossref] [PubMed]
- Gao P, Zhao Z, Wang D, Wu J, Cai Y, Li T, Wu W, Shi H, He W, Zhu F, Jiao L, Ling F. China Angioplasty and Stenting for Symptomatic Intracranial Severe Stenosis (CASSISS): A new, prospective, multicenter, randomized controlled trial in China. Interv Neuroradiol 2015;21:196-204. [Crossref] [PubMed]
- Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44:2064-89. [Crossref] [PubMed]
- Jiang L, Ai Z, Geng W, Chen H, Zhao B, Su H, Yin X, Chen YC. Predictive value of perfusion weighted imaging for early new lesions after stroke patients receive endovascular treatment. Quant Imaging Med Surg 2021;11:3643-54. [Crossref] [PubMed]
- Pasterkamp G, Galis ZS, de Kleijn DP. Expansive arterial remodeling: location, location, location. Arterioscler Thromb Vasc Biol 2004;24:650-7. [Crossref] [PubMed]
- Gibbons GH, Dzau VJ. The emerging concept of vascular remodeling. N Engl J Med 1994;330:1431-8. [Crossref] [PubMed]
- Buijs PC, Krabbe-Hartkamp MJ, Bakker CJ, de Lange EE, Ramos LM, Breteler MM, Mali WP. Effect of age on cerebral blood flow: measurement with ungated two-dimensional phase-contrast MR angiography in 250 adults. Radiology 1998;209:667-74. [Crossref] [PubMed]
- Beausang-Linder M, Bill A. Cerebral circulation in acute arterial hypertension--protective effects of sympathetic nervous activity. Acta Physiol Scand 1981;111:193-9. [Crossref] [PubMed]
- Roth W, Morgello S, Goldman J, Mohr JP, Elkind MS, Marshall RS, Gutierrez J. Histopathological Differences Between the Anterior and Posterior Brain Arteries as a Function of Aging. Stroke 2017;48:638-44. [Crossref] [PubMed]
- Ma N, Jiang WJ, Lou X, Ma L, Du B, Cai JF, Zhao TQ. Arterial remodeling of advanced basilar atherosclerosis: a 3-tesla MRI study. Neurology 2010;75:253-8. [Crossref] [PubMed]
- Nishioka T, Luo H, Eigler NL, Berglund H, Kim CJ, Siegel RJ. Contribution of inadequate compensatory enlargement to development of human coronary artery stenosis: an in vivo intravascular ultrasound study. J Am Coll Cardiol 1996;27:1571-6. [Crossref] [PubMed]
- Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, Fang MC, Fisher M, Furie KL, Heck DV, Johnston SC, Kasner SE, Kittner SJ, Mitchell PH, Rich MW, Richardson D, Schwamm LH, Wilson JA. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45:2160-236. [Crossref] [PubMed]
- Burke AP, Kolodgie FD, Farb A, Weber DK, Malcom GT, Smialek J, Virmani R. Healed plaque ruptures and sudden coronary death: evidence that subclinical rupture has a role in plaque progression. Circulation 2001;103:934-40. [Crossref] [PubMed]
- Ward MR, Pasterkamp G, Yeung AC, Borst C. Arterial remodeling. Mechanisms and clinical implications. Circulation 2000;102:1186-91. [Crossref] [PubMed]
- Bkaily G, Abou Abdallah N, Simon Y, Jazzar A, Jacques D. Vascular smooth muscle remodeling in health and disease. Can J Physiol Pharmacol 2021;99:171-8. [Crossref] [PubMed]
- Shi MC, Wang SC, Zhou HW, Xing YQ, Cheng YH, Feng JC, Wu J. Compensatory remodeling in symptomatic middle cerebral artery atherosclerotic stenosis: a high-resolution MRI and microemboli monitoring study. Neurol Res 2012;34:153-8. [Crossref] [PubMed]
- Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med 1987;316:1371-5. [Crossref] [PubMed]
- Dumont TM, Kan P, Snyder KV, Hopkins LN, Siddiqui AH, Levy EI. Revisiting angioplasty without stenting for symptomatic intracranial atherosclerotic stenosis after the stenting and aggressive medical management for preventing recurrent stroke in intracranial stenosis (SAMMPRIS) study. Neurosurgery 2012;71:1103-10. [Crossref] [PubMed]
- Chimowitz MI, Lynn MJ, Derdeyn CP, Turan TN, Fiorella D, Lane BF, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med 2011;365:993-1003. [Crossref] [PubMed]


