
Accuracy of aortic annulus measurements by three-dimensional transesophageal echocardiography and predictive value for thoracic aorta aneurysm in patients with aortic regurgitation
Introduction
Aortic regurgitation (AR) is a common form of valvular disease. The prevalence of AR has also gradually increased as the average human lifespan has lengthened (1). Consequently, more patients undergo aortic valve replacement/valvuloplasty due to severe AR. Meanwhile, for high-risk or inoperable patients, transcatheter aortic valve implantation may be considered for selected patients with AR in experienced centers (2,3). An accurate preoperative evaluation of the aortic annulus (AA) is of critical importance to reduce the occurrence of complications, regardless of the type of surgery. Currently, multi-slice computed tomography (MSCT) is a highly valuable tool for the workup of patients with aortic valve disease and can accurately evaluate AA dimensions (4). Three-dimensional transesophageal echocardiography (3D-TEE) has also been widely applied as a routine preoperative and intraoperative evaluation for aortic valve surgery. However, relevant studies primarily focused on patients with aortic stenosis. Thus, the first purpose of the present study is to explore whether 3D-TEE can accurately measure AA dimensions in patients with AR, with MSCT as a standard approach.
Meanwhile, the etiology of AR is complex and multifactorial, and most patients have concomitant different degrees of aortic dilatation. Preoperative imaging evaluation of severe AR should focus not only on the accurate measurement of AA but also on the detection of high-risk thoracic aorta aneurysms, which is important for the selection of surgical protocols and postoperative follow-up observation. At the anatomical level, the thoracic aorta and the aortic root, including the sinotubular junction, sinus of Valsalva, and AA, are continuous integral structures. A previous study pointed out that aortic annuloplasty can efficiently reduce the annulus and the rate of reoperation in patients with ascending aorta aneurysms, which may be associated with preventing further dilation of the aorta (5). In contrast, postoperative dilation of the AA may lead to the early or late recurrence of AR and aortic aneurysms (6). Thus, we hypothesized that AA dimensions may have predictive value in the existence of thoracic aorta aneurysms. The second purpose of the present study is to explore whether AA dimensions can predict the occurrence of thoracic aorta aneurysm detected by MSCT scan, based on the foundation of accurate measurement of AA dimensions by 3D-TEE. We present the following article in accordance with the GRRAS reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-22-341/rc).
Methods
Study population
From August 2018 to December 2020, 160 patients indicated for aortic valve replacement/valvuloplasty due to pure moderate-to-severe AR undergoing intraoperative 3D-TEE and preprocedural MSCT examination at our institution were consecutively enrolled in this cross-sectional study. The two examinations were performed no more than one week apart. The exclusion criteria were evidence of aortic valve stenosis (n=18), aortic acute or chronic dissection (n=10), aortic coarctation and/or other forms of congenital heart disease (n=7), Marfan syndrome or a family history of Marfan syndrome (n=3), and previous surgery or intervention (n=3). In addition, patients with poor imaging quality were also excluded from the study (n=8). Eventually, 111 patients were included in this study. Clinical characteristics were prospectively collected from the hospital information system and retrospectively analyzed. This study was conducted following the Declaration of Helsinki (as revised in 2013). The protocol was approved by the Institutional Ethics Committee of Fuwai Hospital (No. 2019-1201), and informed consent was obtained from all individual participants.
MSCT acquisition protocol and AA measurements
MSCT data sets were obtained using a second-generation dual source CT system (SOMATOM Definition Flash, Siemens Healthcare, Germany). The data acquisition protocol was described previously in the published article of our research group (7). The imaging volume extended from the aortic arch to the level of the diaphragm in a craniocaudal direction. For assessment of AA dimensions, MSCT images were transferred to an offline post-processing workstation (Leonardo Workstation, Siemens Healthcare, Forchheim, Germany). MSCT measurements were performed by one experienced radiologist. The concept of a basal annular plane comprises a virtual plane connecting the three lowest insertion points of aortic valve cusps in each of the three sinuses. Multiplanar reconstructions were manually oriented to display AA (8). After creating an oblique multiplanar reconstruction exactly aligned with the AA plane, the following dimensions of AA were measured: minimum diameter, maximum diameter, area, and perimeter (Figure 1). All examinations were analyzed between 35–45% R-R intervals, when the annulus was in the systolic period, and the clearest image was selected. In addition, the measurement of thoracic aortic dimensions at standard anatomic landmarks and the definition of aortic dilatation were performed according to the guidelines of the American College of Radiology (9).
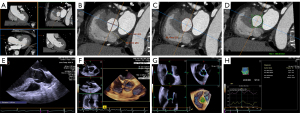
Transthoracic and transesophageal echocardiography measurements
Two-dimensional transthoracic echocardiography (2D-TTE) was performed as a part of preoperative evaluation within 1 week before surgery using a Philips EPIQ 7C ultrasound device (Philips Medical Systems, Andover, MA, USA). The left ventricular end-diastolic diameter and end-systolic diameter were obtained from the left parasternal long-axis view. The left ventricular ejection fraction was assessed by the biplane Simpson method. The vena contracta width of the AR jet was also measured according to the American Society of Echocardiography guidelines (10).
TEE was performed using a Siemens ACUSON SC2000 ultrasound system (Siemens Medical Systems, Mountainview, CA) equipped with the transesophageal Z6Ms True Volume transducer. For 2D-TEE evaluation of AA, measurements were taken at the point of the insertion of the valve cusps in the mid-esophageal long axis view with scanning plans from 115–160°. 3D-TEE images for subsequent reconstruction were acquired in the same position using ZOOM mode of the aortic valve apparatus after adjustment of lateral and elevation width to optimize the frame rate (range, 16–25 fps). Three beat, ECG-gated, and full-volume images were used for data analysis.
Online semiautomated 3D measurements were conducted using eSie Valve analytical software (Siemens Medical Systems, Mountainview, CA) that could model the annulus automatically. Observers could verify the landmarks of the automated model and manually correct them from a long axis view of the 3D image shown in different long planes and a short axis view at different levels. Finally, the cyclic changes in the parameters were displayed as a graph (Figure 1). The available parameters also included the AA minimum diameter (AAmin), maximum diameter (AAmax), area (AAarea), and perimeter (AAperi). The mid-systolic frame was selected for this analysis. The circularity of AA was indicated by the eccentricity index (maximum diameter/minimum diameter). All measurements (MSCT and TEE) were measured three times by the same operator based on the same image, and the results shown were averaged. The results were blinded to the different methods of measurement (MSCT and 3D-TEE).
Statistical analysis
Normality of distributions for continuous variables was tested using the Kolmogorov-Smirnov test. Quantitative values are expressed as the mean ± SD, and categorical variables are represented as absolute numbers or proportions. An independent t-test was used to compare continuous values between two groups, and categorical variables were compared using the χ2 test or Fisher’s exact test. Pearson correlation coefficients were used to assess the correlation between MSCT and 3D-TEE measurements. Agreement between techniques was plotted using the Bland-Altman method. Predictors of thoracic aorta aneurysms were identified through univariate and multivariate logistic regression models. The cutoff value was determined from the area under the receiver operating characteristic (ROC) curve (AUC) to provide the best sensitivity and specificity for each parameter for prediction. Comparisons of AUCs were performed using the method described by DeLong et al. (11). Differences were considered significant at P<0.05. All analyses were performed using SPSS 24.0 (IBM Corp., Armonk, NJ, USA), MedCalc 18.2.1 (MedCalc Software, Ltd., Ostend, Belgium), and GraphPad Prism 8.0.2 (GraphPad Software, San Diego, CA, USA).
Results
Study population
The population consisted of 15 women and 96 men with a mean age of 54.5±12.1 years. Forty-seven patients were judged as having severe AR and 64 had moderate AR. According to the surgical results, all patients in the AR group had tricuspid aortic valves. The etiology of AR was determined via de novo review of the intraoperative TEE, surgical direct observation, and pathology reports. The AR mechanisms were categorized as cusp prolapse (n=34, 30.6%), aortic root dilatation (n=35, 31.5%), and cusp restriction/retraction (n=42, 37.9%). In the present study, all patients received aortic valve surgery (aortic valve replacement or valvuloplasty). Concomitant surgical procedures included coronary artery bypass grafting surgery and/or mitral valve annuloplasty. The demographic features and preoperative clinical characteristics of the patients are presented in Table 1.
Table 1
| Variables | All patients (n=111) | Group I (n=71) | Group II (n=40) | P value (Group I vs. Group II) |
|---|---|---|---|---|
| Demographic features | ||||
| Age (years) | 54.5±12.1 | 55.4±11.1 | 52.9±13.8 | 0.31 |
| Sex (M/F) | 96/15 | 63/8 | 33/7 | 0.19 |
| Weight (kg) | 73.6±17.0 | 73.9±19.7 | 72.9±10.8 | 0.76 |
| Height (cm) | 168.9±15.2 | 167.0±17.7 | 172.4±7.7 | 0.07 |
| BSA (m2) | 1.8±0.2 | 1.8±0.2 | 1.8±0.2 | 0.59 |
| Clinical characteristics, n (%) | ||||
| NYHA functional class III–IV | 36 (32.43) | 24 (33.80) | 12 (30.00) | 0.18 |
| Smoking | 53 (47.75) | 35 (49.30) | 18 (45.00) | 0.81 |
| Hypertension | 51 (45.95) | 44 (61.97) | 17 (42.50) | 0.08 |
| Peripheral vascular disease | 3 (2.70) | 1 (1.41) | 2 (5.00) | 0.28 |
| Previous cardiac surgery | 7 (6.31) | 3 (4.23) | 4 (10.00) | 0.21 |
| 2D Transthoracic echocardiography findings | ||||
| Mitral regurgitation, n (%) | 69 (62.16) | 50 (70.42) | 19 (47.50) | 0.03* |
| AR vena contracta width (mm) | 6.5±2.0 | 6.6±1.7 | 6.3±2.5 | 0.46 |
| LV end-diastolic diameter (mm) | 62.7±9.7 | 63.4±8.5 | 61.5±11.6 | 0.35 |
| LV end-systolic diameter (mm) | 41.3±10.1 | 42.2±8.8 | 39.6±12.1 | 0.24 |
| LV ejection fraction (%) | 59.7±6.8 | 59.39±6.8 | 60.3±6.9 | 0.50 |
| Transesophageal echocardiography parameters | ||||
| 2D-Aortic annulus diameter (mm) | 25.95±2.72 | 25.51±2.79 | 26.78±2.41 | 0.02* |
| 3D-Aortic annulus min diameter (mm) | 26.07±3.57 | 25.27±3.53 | 27.53±3.19 | <0.01* |
| 3D-Aortic annulus max diameter (mm) | 32.30±2.68 | 31.65±3.66 | 33.50±3.44 | 0.01* |
| 3D-Aortic annulus area (mm2) | 669.76±155.19 | 643.73±161.39 | 717.83±131.89 | 0.02* |
| 3D-Aortic annulus perimeter (mm) | 93.52±10.42 | 91.40±10.56 | 97.43±9.02 | <0.01* |
| Ellipticity index | 1.25±0.12 | 1.26±0.12 | 1.22±0.11 | 0.11 |
Data are presented as mean ± standard deviation or number (frequency). *, P<0.05 for independent-sample t tests. BSA, body surface area; NYHA, New York Heart Association; AR, aortic regurgitation; LV, left ventricle.
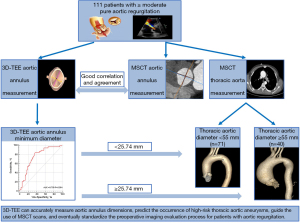
All patients underwent a standard MSCT examination containing the whole thoracic aorta. The diameters of the aorta were measured at eight anatomical landmarks. High-risk thoracic aortic aneurysms requiring surgical intervention were defined as aortic diameters larger than 55 mm at a certain position as measured by MSCT reconstruction. Eventually, the AR group was further divided into Group I (aortic diameter <55 mm, n=71) and Group II (aortic diameter ≥55 mm, n=40). No significant differences were found between Group I and Group II in age, sex, body surface area, medical history, or primary 2D-TTE findings. Compared to Group I, Group II had a significantly larger AA diameter, area, and perimeter (Table 1).
Accuracy and reproducibility of AA measurements by 3D-TEE
As reported in Table 2 and Figure 2, AA parameters derived from 3D-TEE had moderate to high correlation with the MSCT reference values (AAmin: r=0.63; AAmax: r=0.64; AAarea: r=0.74; AAperi: r=0.69, P<0.05 for all). Good agreement between 3D-TEE and MSCT measurements was also observed. Bland-Altman comparison demonstrated a mean difference of 0.19 mm between 3D-TEE and MSCT for AAmin with a 95% CI of −0.40 to 0.78 for this bias, 0.51 mm for AAmax with a 95% CI of −0.10 to 1.13, 9.71 mm2 for AAarea with a 95% CI of −12.22 to 31.64, and 1.25 mm for AAperi with a 95% CI of −0.40 to 2.90, respectively. No differences were found in average bias between methods (P>0.05). On average, the 3D-TEE method overestimated all AA parameters compared to MSCT.
Table 2
| 3D-TEE measurement | MSCT measurement | r | Mean bias (95% CI) | 95% LOA | |
|---|---|---|---|---|---|
| Aortic annulus min diameter (mm) | 26.07±3.57 | 25.88±3.68 | 0.63* | 0.19 (−0.40 to 0.78) | −5.95 to 6.32 |
| Aortic annulus max diameter (mm) | 32.30±2.68 | 31.78±4.06 | 0.64* | 0.51 (−0.10 to 1.13) | −5.91 to 6.94 |
| Aortic annulus area (mm2) | 669.76±155.19 | 660.05±168.28 | 0.74* | 9.71 (−12.22 to 31.64) | −218.78 to 238.20 |
| Aortic annulus perimeter (mm) | 93.52±10.42 | 92.26±11.71 | 0.69* | 1.25 (−0.40 to 2.90) | −15.96 to 18.47 |
Data are presented as mean ± standard. *, P<0.05 for Pearson correlation analysis. 3D-TEE, three-dimensional transesophageal echocardiography; MSCT, multi-slice computed tomography; CI, confidence interval; LOA, limits of agreement.

Meanwhile, to assess the reproducibility of 3D-TEE measurements, 50 subjects were randomly selected. All echocardiography findings were evaluated independently by two experienced echocardiographers. For intraobserver variability, the same operator performed a second measurement after the initial analysis. For interobserver variability, measurements were taken by a second operator. The second investigator was blinded to the initial results. Through Bland-Altman analysis, the intraclass correlation coefficient for intraobserver variability ranged from 0.77 to 0.90, and interobserver variability ranged from 0.75 to 0.88 (Table 3). The results showed that AA measurements performed by 3D-TEE had good reproducibility.
Table 3
| Intraobserver | Interobserver | ||||
|---|---|---|---|---|---|
| Intraclass correlation coefficient | 95% CI | Intraclass correlation coefficient | 95% CI | ||
| Aortic annulus min diameter (mm) | 0.83* | 0.71–0.90 | 0.75* | 0.56–0.86 | |
| Aortic annulus max diameter (mm) | 0.77* | 0.63–0.87 | 0.76* | 0.61–0.86 | |
| Aortic annulus area (mm2) | 0.90* | 0.83–0.94 | 0.88* | 0.79–0.93 | |
| Aortic annulus perimeter (mm) | 0.86* | 0.77–0.92 | 0.80* | 0.68–0.88 | |
*, P<0.05 for Bland–Altman analysis. CI, confidence interval.
Predictors of high-risk thoracic aorta aneurysm and ROC curve analysis
In the univariate logistic regression analysis, 2D-TEE AA diameter, 3D-TEE AAmin, 3D-TEE AAmax, 3D-TEE AAarea, and 3D-TEE AAperi correlated with high-risk thoracic aorta aneurysm. Due to significant correlations between these parameters, multivariate logistic regression analysis was performed separately to determine independent variables of AA parameters associated with thoracic aorta aneurysm in patients with AR. After multivariate adjustment for traditional risk factors, including age, sex and BSA (12), all of these parameters remained independent (Table 4).
Table 4
| Variables | Logistic regression analysis | β | 95% CI | P value |
|---|---|---|---|---|
| 2D-TEE Aortic annulus diameter (mm) | Univariate analysis | 1.19 | 1.03–1.39 | 0.02* |
| Adjusted for age, gender, and BSA | 1.21 | 1.02–1.44 | 0.03* | |
| 3D-TEE Aortic annulus min diameter (mm) | Univariate analysis | 1.21 | 1.07–1.37 | <0.01* |
| Adjusted for age, gender, and BSA | 1.23 | 1.07–1.41 | <0.01* | |
| 3D-TEE Aortic annulus max diameter (mm) | Univariate analysis | 1.16 | 1.03–1.30 | 0.01* |
| Adjusted for age, gender, and BSA | 1.15 | 1.01–1.31 | 0.04* | |
| 3D-TEE Aortic annulus area (mm2) | Univariate analysis | 1.00 | 1.00–1.01 | 0.02* |
| Adjusted for age, gender, and BSA | 1.00 | 1.00–1.01 | 0.04* | |
| 3D-TEE Aortic annulus perimeter (mm) | Univariate analysis | 1.06 | 1.02–1.11 | <0.01* |
| Adjusted for age, gender, and BSA | 1.07 | 1.02–1.12 | 0.01* | |
| Ellipticity index | Univariate analysis | 0.05 | 0.01–1.96 | 0.11 |
| Adjusted for age, gender, and BSA | 0.02 | 0.01–0.77 | 0.06 |
*, P<0.05 for logistic regression analysis. 2D-TEE, two-dimensional transesophageal echocardiography; 3D-TEE, three-dimensional transesophageal echocardiography; BSA, body surface area; CI, confidence interval.
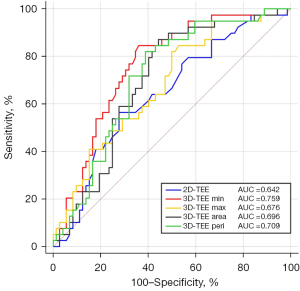
ROC curves were generated to determine the accuracy of the AA parameters most associated with high-risk thoracic aorta aneurysm. As presented in Figure 3, AAmin measured by 3D-TEE had the largest AUC (AUC: 0.759, 95% CI: 0.668–0.850, P<0.05), and it was used to calculate optimal cutoff values for prediction. A cutoff value of 25.74 mm for 3D-TEE AAmin provided a sensitivity of 84.6% and a specificity of 63.9%. Comparisons of AUCs showed that the AUC of 3D-TEE AAmin was superior to the AA diameter measured by 2D-TEE and AAmax (0.759 vs. 0.642, and 0.759 vs. 0.676; P<0.05). The AUC of 3D-TEE AAmin was similar to the aortic annular parameters obtained by 3D-TEE (AAarea, 0.696, and AAperi, 0.709; all P>0.05).
Discussion
The main findings of the present study are as follows: For patients with pure moderate-to-severe AR, (I) 3D-TEE measurement of AA is feasible, reliable with a good correlation between 3D-TEE and MSCT measurements, and reproducible with a low intraobserver and interobserver variability. (II) The AA minimum diameter obtained by 3D-TEE was a predictive parameter to indicate the presence of high-risk thoracic aorta aneurysm detected by MSCT examination.
Accuracy of AA measurements by 3D-TEE in patients with AR
Accurate AA measurements are essential for the selection of surgical strategies and the sizing of valve prostheses. Previous studies have reported that for patients with severe aortic stenosis, MSCT can provide accurate AA measurements (13-17). However, unlike aortic stenosis, the etiology of AR is complex and multifactorial. Calcification of the aortic valve leaflets and annulus is much less common in AR. Thus, the advantages of CT imaging for identifying calcifications cannot be entirely highlighted. Moreover, in real clinical practice, not all patients with AR undergo preoperative MSCT examination for annulus evaluation. The primary aim of MSCT examination for AR patients is to investigate concomitant diseases, such as coronary arteriosclerotic disease or congenital coronary artery anomalies, aortic diseases and other anatomical variations. In addition, MSCT scans are not available for patients with severe renal dysfunction and young patients. Thus, for patients with AR, the MSCT examination may not play as large a role in aortic annular quantification as previously reported. The present study only enrolled patients with pure AR and clarified whether 3D-TEE can serve as an alternative to MSCT scans in such patients to perform AA measurements. To our knowledge, this study is the first to address this issue.
The results of this study showed that the AA measurements obtained by 3D-TEE were accurate and highly correlated with the MSCT method with acceptable interobserver and intraobserver variability. However, with regard to the differences between the two measurements, some degree of overestimation and greater variability of AA dimensions obtained by 3D-TEE was observed, which was somewhat different from the results of previous studies (13-17). The different results can be explained based on several factors. First, the present study population was different compared with other studies. All the above studies included some patients with severe aortic stenosis, which may have interfered with the results. The results of our study also provide indirect evidence that the AA measurement obtained by 3D-TEE in AR patients is quite different from that in patients with aortic stenosis. Meanwhile, AR patients differ significantly regarding their AA dimensions compared to aortic stenosis patients (18). Greater variability in the measurements obtained by 3D-TEE is partially due to the larger AA dimensions in patients with AR. Second, 3D-TEE analytical software was different in the present study, which can perform automated reconstruction and analysis of the aortic root using a predefined algorithm. However, this imaging reconstruction algorithm is created based on a normal AA structure. There will be some deviation in the extraction of corresponding data from the abnormal AA. Moreover, because the analysis was performed in semiautomatic mode during the reconstruction process, this result may be explained by a “correction bias” either in the software’s algorithm or introduced by the operator during the editing of reference points to correct the expected underestimation previously reported with 3D-TEE. A previous study using the same image processing software also reported overestimation of the AA diameter (19). Although this overestimation was not significant in statistical analyses, the results of the present study still need to be interpreted with caution and validated in additional studies.
Predictive value of AA dimensions for high-risk thoracic aorta aneurysm
According to the guidelines for the diagnosis and management of thoracic aortic disease, excessive thoracic aortic dilatation (maximum diameter ≥55 mm) poses a significant risk for the occurrence of acute aortic events, and patients with aneurysms beyond this size should be referred for preventive surgical intervention (20). We therefore selected this value as a grouping criterion for patients. Currently, MSCT is the preferred technique for accurately measuring aortic dimensions from different directions by the 3D reconstruction method (21). The present study indicated that clinicians should be vigilant about the presence of high-risk thoracic aortic aneurysms when 3D-TEE AAmin is greater than 25.74 mm, and MSCT scans are recommended for accurate assessments of the thoracic aorta condition in these patients. It is generally accepted that the AA is not entirely a circular structure. The annular eccentricity originates predominantly from the difference in AAmin. Dilatation of the aortic root or its distal artery generally results in AA morphological abnormalities. Jilaihawi and colleagues suggested that patients with larger ascending aorta dimensions had a slightly less elliptical annulus, as reflected by a larger eccentricity ratio, which was driven by a larger AAmin (22). Petersen and coworkers also found that the increase in AAmin gradually became obvious as the dimensions of the annulus extended (23). All of these studies illustrated that the AAmin was relatively sensitive to aortic morphological changes. Owing to the interrelations, the finding that thoracic aorta aneurysm could be predicted by AAmin can be partially explained.
Meanwhile, we noted that the AA diameter measured by 2D-TEE also had a certain predictive value by logistic regression analyses. From the perspective of viewing angle, the mid-esophageal long-axis view on TEE is equivalent to the single oblique sagittal view on MSCT scans (24). Thus, the AA diameter measured by 2D-TEE actually also reflects the situation of AAmin. In real clinical settings, the AA diameter measured by 2D-TEE can be conveniently obtained. However, the AUC value of this parameter was relatively smaller than that of 3D-TEE AAmin through statistical comparison. Hence, methods of selecting suitable aortic annular parameters applied to clinical decision-making remain worthy of exploration. Next, our research group will perform more comparative studies to clarify the superiority and inferiority of various indicators.
Clinical implications
Currently, surgical procedures have increasingly higher requirements for preoperative imaging assessment. However, there are no certain cardiovascular imaging examinations to achieve a complete preoperative diagnosis and evaluation of patients with AR alone. Different approaches have advantages and disadvantages (4,21). Previous studies showed that TEE had unique advantages in differentiating the etiology and confirming the degree of AR (25,26). The current study confirmed that the 3D-TEE method may also be used to accurately measure primary AA dimensions, which increased the clinical application value of 3D-TEE for patients with AR. Meanwhile, MSCT has unique advantages in the evaluation of thoracic aorta, especially in the detection of high-risk thoracic aorta aneurysms. The results of the current study demonstrated that AAmin obtained by 3D-TEE showed a suggestive effect on the presence of high-risk thoracic aorta aneurysms. At our institute, almost all the patients with severe AR received MSCT reconstruction of the whole aorta before surgery. It seems unreasonable for some of the patients. We expect that our results will help reduce (at least to some extent) unnecessary examinations in these patients and standardize the preoperative imaging evaluation process, especially in the era of rapid development of transcatheter repair or replacement techniques. However, the preoperative evaluation process includes more complicated situations and factors and cannot be determined by a single study. The present study only provides references from one aspect, and additional research is needed to explore this further.
Limitations
The study was a single-center observational study, without long-term follow-up of the patients to assess clinical outcomes. The conclusion of this study requires verification in large and prospective studies. The AA dimensions were only analyzed when MSCT imaging was in the systolic period. Many studies have confirmed that AA dimensions measured during systole and diastole are different (27,28). However, MSCT scans were performed by prospectively ECG-triggered acquisition mode, and therefore, only images at 25–75% of the R-R interval were available in the present study, which was based on the principle to maximally limit the amount of patient radiation exposure and satisfied the ethical considerations. Meanwhile, it is widely recognized by clinicians that AA measurements should ideally be performed in the systolic period to achieve the greatest annular stretch (29). Thus, the measurement schemes met the practical clinical requirements. Furthermore, the consistency between the 3D-TEE measurements and prosthetic valve size was not examined in this study primarily because the prosthetic aortic valves were produced from different manufacturers. We also did not measure the diameter of the prosthetic aortic valve size using a standardized ruler. Relevant data require further collection and exploration. Finally, the high reproducibility of these measurements is likely dependent on training and experience. Thus, these results cannot necessarily be generalized to less-experienced physicians. The present study did not make distinctions between the etiologies of AR. The AA morphometric characteristics may vary among patients with different etiologies. These results should be interpreted with caution, and more nuanced stratified analyses and subgroup comparisons are still needed.
Conclusions
The main conclusion of the present study is summarized in Figure 4. 3D-TEE allows accurate assessment of the AA dimensions in patients with pure AR and shows good reproducibility. The AA minimum diameter measured by 3D-TEE could emerge as the most valuable predictive factor for high-risk thoracic aorta aneurysm detected by MSCT examination. 3D-TEE and MSCT need to be combined to complement each other to improve the diagnostic value and minimize the damage to patients.
Acknowledgments
We are grateful to the staff of the Department of Anesthesiology at our institute for their invaluable assistance in this study. We thank American Journal Experts (www.aje.cn) for English language editing.
Funding: This work was supported by the Beijing Science and Technology Program of China (No. Z161100000516097).
Footnote
Reporting Checklist: The authors have completed the GRRAS reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-22-341/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-341/coif). All authors report that this work was supported by the Beijing Science and Technology Program of China (No. Z161100000516097). DW is an employee of Siemens Healthineers Ltd. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted following the Declaration of Helsinki (as revised in 2013). The protocol was approved by the Institutional Ethics Committee of Fuwai Hospital (No. 2019-1201), and informed consent was obtained from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Singh JP, Evans JC, Levy D, Larson MG, Freed LA, Fuller DL, Lehman B, Benjamin EJ. Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham Heart Study) Am J Cardiol 1999;83:897-902. [Crossref] [PubMed]
- Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2022;43:561-632. Erratum in: Eur Heart J 2022;43:561-632. [Crossref] [PubMed]
- Sawaya FJ, Deutsch MA, Seiffert M, Yoon SH, Codner P, Wickramarachchi U, et al. Safety and Efficacy of Transcatheter Aortic Valve Replacement in the Treatment of Pure Aortic Regurgitation in Native Valves and Failing Surgical Bioprostheses: Results From an International Registry Study. JACC Cardiovasc Interv 2017;10:1048-56. [Crossref] [PubMed]
- Hakim D, Ghimire G, Alli OO, Singh S, Sasse MF, Booker OJ, Arora G, Leesar T, Jernigan L, Melby SJ, Davies JE, Leesar MA. Large-field intravascular ultrasound for annular sizing and predicting paravalvular regurgitation during TAVR: comparisons with multidetector computed tomography and transoesophageal echocardiography. Eur Heart J Cardiovasc Imaging 2017;18:1404-13. [Crossref] [PubMed]
- Lansac E, Di Centa I, Sleilaty G, Lejeune S, Khelil N, Berrebi A, Diakov C, Mankoubi L, Malergue MC, Noghin M, Zannis K, Salvi S, Dervanian P, Debauchez M. Long-term results of external aortic ring annuloplasty for aortic valve repair. Eur J Cardiothorac Surg 2016;50:350-60. [Crossref] [PubMed]
- Schäfers H, Fries R, Langer F, Nikoloudakis N, Graeter T, Grundmann U. Valve-preserving replacement of the ascending aorta: remodeling versus reimplantation. J Thorac Cardiovasc Surg 1998;116:990-6. [Crossref] [PubMed]
- Zhang M, Wan L, Liu K, Wu W, Li H, Wang Y, Lu B, Wang H. Aortic roots assessment by an automated three-dimensional transesophageal echocardiography: an intra-individual comparison. Int J Cardiovasc Imaging 2019;35:2029-36. [Crossref] [PubMed]
- Blanke P, Weir-McCall JR, Achenbach S, Delgado V, Hausleiter J, Jilaihawi H, Marwan M, Norgaard BL, Piazza N, Schoenhagen P, Leipsic JA. Computed tomography imaging in the context of transcatheter aortic valve implantation (TAVI) / transcatheter aortic valve replacement (TAVR): An expert consensus document of the Society of Cardiovascular Computed Tomography. J Cardiovasc Comput Tomogr 2019;13:1-20. [Crossref] [PubMed]
- Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE Jr, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the diagnosis and management of patients with thoracic aortic disease. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology,American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons,and Society for Vascular Medicine. J Am Coll Cardiol 2010;55:e27-e129. [Crossref] [PubMed]
- Zoghbi WA, Adams D, Bonow RO, Enriquez-Sarano M, Foster E, Grayburn PA, Hahn RT, Han Y, Hung J, Lang RM, Little SH, Shah DJ, Shernan S, Thavendiranathan P, Thomas JD, Weissman NJ. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr 2017;30:303-71. [Crossref] [PubMed]
- DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837-45. [Crossref] [PubMed]
- Biaggi P, Matthews F, Braun J, Rousson V, Kaufmann PA, Jenni R. Gender, age, and body surface area are the major determinants of ascending aorta dimensions in subjects with apparently normal echocardiograms. J Am Soc Echocardiogr 2009;22:720-5. [Crossref] [PubMed]
- Stella S, Italia L, Geremia G, Rosa I, Ancona F, Marini C, Capogrosso C, Giglio M, Montorfano M, Latib A, Margonato A, Colombo A, Agricola E. Accuracy and reproducibility of aortic annular measurements obtained from echocardiographic 3D manual and semi-automated software analyses in patients referred for transcatheter aortic valve implantation: implication for prosthesis size selection. Eur Heart J Cardiovasc Imaging 2019;20:45-55. [Crossref] [PubMed]
- Guez D, Boroumand G, Ruggiero NJ, Mehrotra P, Halpern EJ. Automated and Manual Measurements of the Aortic Annulus with ECG-Gated Cardiac CT Angiography Prior to Transcatheter Aortic Valve Replacement: Comparison with 3D-Transesophageal Echocardiography. Acad Radiol 2017;24:587-93. [Crossref] [PubMed]
- Sherif MA, Ince H, Maniuc O, Reiter T, Voelker W, Ertl G, Öner A. Two-dimensional transesophageal echocardiography for aortic annular sizing in patients undergoing transcatheter aortic valve implantation. BMC Cardiovasc Disord 2015;15:181. [Crossref] [PubMed]
- Khalique OK, Hamid NB, White JM, Bae DJ, Kodali SK, Nazif TM, Vahl TP, Paradis JM, George I, Leon MB, Hahn RT. Impact of Methodologic Differences in Three-Dimensional Echocardiographic Measurements of the Aortic Annulus Compared with Computed Tomographic Angiography Before Transcatheter Aortic Valve Replacement. J Am Soc Echocardiogr 2017;30:414-21. [Crossref] [PubMed]
- Prihadi EA, van Rosendael PJ, Vollema EM, Bax JJ, Delgado V, Ajmone Marsan N. Feasibility, Accuracy, and Reproducibility of Aortic Annular and Root Sizing for Transcatheter Aortic Valve Replacement Using Novel Automated Three-Dimensional Echocardiographic Software: Comparison with Multi-Detector Row Computed Tomography. J Am Soc Echocardiogr 2018;31:505-514.e3. [Crossref] [PubMed]
- Neumann N, Petersen J, Sinning C, Sequeira-Gross T, Schofer N, Reichenspurner H, Girdauskas E. Focus on the annuloplasty in aortic valve repair: implications from a quantitative multislice computed tomography analysis. Quant Imaging Med Surg 2020;10:853-61. [Crossref] [PubMed]
- Maia J, Ladeiras-Lopes R, Guerreiro C, Carvalho M, Fontes-Carvalho R, Braga P, Sampaio F. Accuracy of three-dimensional echocardiography in candidates for transcatheter aortic valve replacement. Int J Cardiovasc Imaging 2020;36:291-8. [Crossref] [PubMed]
- Erbel R, Aboyans V, Boileau C, Bossone E, Bartolomeo RD, Eggebrecht H, et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2873-926. [Crossref] [PubMed]
- Quint LE, Liu PS, Booher AM, Watcharotone K, Myles JD. Proximal thoracic aortic diameter measurements at CT: repeatability and reproducibility according to measurement method. Int J Cardiovasc Imaging 2013;29:479-88. [Crossref] [PubMed]
- Jilaihawi H, Wu Y, Yang Y, Xu L, Chen M, Wang J, Kong X, Zhang R, Wang M, Lv B, Wang W, Xu B, Makkar RR, Sievert H, Gao R. Morphological characteristics of severe aortic stenosis in China: imaging corelab observations from the first Chinese transcatheter aortic valve trial. Catheter Cardiovasc Interv 2015;85:752-61. [Crossref] [PubMed]
- Petersen J, Voigtländer L, Schofer N, Neumann N, von Kodolitsch Y, Reichenspurner H, Girdauskas E. Geometric changes in the aortic valve annulus during the cardiac cycle: impact on aortic valve repair. Eur J Cardiothorac Surg 2018;54:441-5. [Crossref] [PubMed]
- Achenbach S, Delgado V, Hausleiter J, Schoenhagen P, Min JK, Leipsic JA. SCCT expert consensus document on computed tomography imaging before transcatheter aortic valve implantation (TAVI)/transcatheter aortic valve replacement (TAVR). J Cardiovasc Comput Tomogr 2012;6:366-80. [Crossref] [PubMed]
- le Polain de Waroux JB, Pouleur AC, Goffinet C, Vancraeynest D, Van Dyck M, Robert A, Gerber BL, Pasquet A, El Khoury G, Vanoverschelde JL. Functional anatomy of aortic regurgitation: accuracy, prediction of surgical repairability, and outcome implications of transesophageal echocardiography. Circulation 2007;116:I264-9. [Crossref] [PubMed]
- Van Dyck MJ, Watremez C, Boodhwani M, Vanoverschelde JL, El Khoury G. Transesophageal echocardiographic evaluation during aortic valve repair surgery. Anesth Analg 2010;111:59-70. [Crossref] [PubMed]
- Suchá D, Tuncay V, Prakken NH, Leiner T, van Ooijen PM, Oudkerk M, Budde RP. Does the aortic annulus undergo conformational change throughout the cardiac cycle? A systematic review. Eur Heart J Cardiovasc Imaging 2015;16:1307-17. [Crossref] [PubMed]
- Queirós S, Morais P, Fehske W, Papachristidis A, Voigt JU, Fonseca JC, D'hooge J, Vilaça JL. Assessment of aortic valve tract dynamics using automatic tracking of 3D transesophageal echocardiographic images. Int J Cardiovasc Imaging 2019;35:881-95. [Crossref] [PubMed]
- Jurencak T, Turek J, Kietselaer BL, Mihl C, Kok M, van Ommen VG, van Garsse LA, Nijssen EC, Wildberger JE, Das M. MDCT evaluation of aortic root and aortic valve prior to TAVI. What is the optimal imaging time point in the cardiac cycle? Eur Radiol 2015;25:1975-83. [Crossref] [PubMed]


