
Developmental retardation of femoral head size and femoral head ossification in mild and severe developmental dysplasia of the hip in infants: a preliminary cross-sectional study based on ultrasound images
Introduction
Developmental dysplasia of the hip (DDH) is a common developmental disorder in infants, with an incidence of 1.5 to 20 per 1,000 births (1,2). DDH refers to a broad spectrum of abnormalities affecting the hip joint, ranging from dysplasia to subluxation to dislocation. Unrecognized and untreated DDH can lead to premature osteoarthritis and is responsible for up to 15–20% of hip replacements in adults under the age of 50 years (3).
For infants up to 6 months, ultrasound (US) screening of DDH using the Graf method is recommended by Chinese, European, and American imaging guidelines (4-6). There is no doubt that dislocation or decentered hips (Graf D, III or IV) should be treated immediately once diagnosed (7,8). However, it is controversial how and when to treat well-centered stable infant hips with acetabular dysplasia (Graf II) (9-11). From 6 weeks of age, if the US examination does not show that the hip has improved to Graf IIa (+) or Graf I, Graf II hips should be treated according to international DDH guidelines (5).
Regarding the influence of DDH on the development of the femoral head, Wanner et al. (12) found that the femoral head diameter (FHD) in the severe DDH (Graf D, III, or IV) group was significantly smaller than that in the normal hip group. The severe DDH femoral head growth rate was significantly slower than the normal femoral head growth rate. The ossification center of the femoral head typically appears between 4 months and 6 months of age, which is often delayed in DDH-affected hips (13). However, there have been few reports of the impact of mild DDH (Graf II) on the development of the femoral head and ossification.
According to our experience, we speculated that both mild and severe DDH would affect the development of the femoral head and the occurrence of femoral head ossification in infants, which requires appropriate treatment. Thus, we conducted this retrospective study to investigate the developmental data of femoral head and femoral head ossification in mature hips and the impact of mild and severe DDH on femoral head development. We present the following article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-22-513/rc).
Methods
Participant selection
This retrospective study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the institutional review board of West China Hospital, Sichuan University (No. 2022-1372). As it was a retrospective study, the requirement for informed consent was waived. We reviewed all hip US studies performed from January 2018 to December 2019 to evaluate DDH in infants younger than 6 months in West China Hospital, Sichuan University. Participants were included in the study if they (I) had positive physical examinations, including unequal length of legs, asymmetric hip and leg creases, abnormal hip bounce, and limited hip abduction; (II) were people with a potentially high risk, such as a breech delivery and DDH family history; and (III) had a routine physical examination in 4- to 6-week-old infants. Participants were excluded if they had a pathological hip dislocation, hip trauma, congenital multiple joint contractures with hip dislocation, or involvement of the hip due to familial genetic disease.
The sample size of this study was determined to be at least 1,400 according to our previous pilot sampling and the following sample size formula of the cross-sectional study:
(where N = sample size, Z1-α/2 = confidence interval, SD = standard deviation, d = desired precision).
Initially, 1,450 infants were identified in our US databases, and 46 infants were excluded because of the exclusion criteria. The standard longitudinal and transverse section US images of the hip were collected for Graf type classification and femoral head ossification analysis, respectively. Finally, a total of 1,037 normal participants with 2,074 mature hips and 367 DDH participants with 456 dysplastic hips were included in this study (Figure 1).
Hip US examination
The Philips iU22 US system (Philips Healthcare, Bothell, WA, USA) with an L12-5 linear array transducer (5–12 MHz) was used. The participants were placed into a lateral position, with the hip joint slightly flexed. The transducer was placed on the hip joint to obtain the standard coronal section image of the hip joint with 3 essential landmarks according to the Graf method: the inferior border of the ilium, the osseous acetabular roof, and the labrum, (14). The α angle is the osseous acetabular roof angle, and the β angle is the acetabular cartilaginous angle (Figure 2). According to the Graf US classification, infant hips can be categorized into the following types: I, IIa, IIb, IIc, and D, III or IV (14). When we measured the femoral head coverage, the FHD was recorded as D (Figure 2).
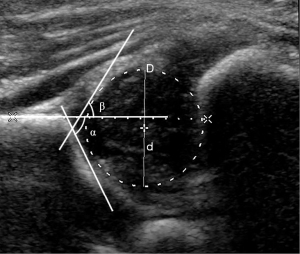
Graf type I hips were considered mature hips or the control group. All affected hips that were staged as Graf type IIa–IV were placed into the DDH group and were classified into 5 categories according to disease severity: Graf IIa (+), physiologically immature hip; Graf IIa (–), maturational deficit hip; Graf IIb, dysplastic hip; Graf IIc, heavily dysplastic hip; and Graf D, III or IV, decentered hip (5,8).
Femoral head ossification center type
All US standard transverse section images of the hip were read by 2 pediatric radiologists (reader 1 J.L., with 7 years of experience, and reader 2 H.L., with 5 years of experience) to classify the ossification center of the femoral head.
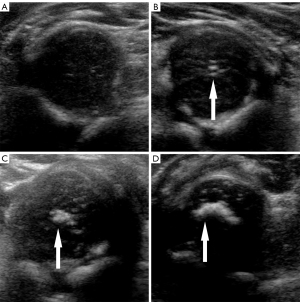
Maturity of the femoral head ossification center was classified into 4 categories according to US morphology and echo intensity: type I, no ossification; type II, punctate ossification; type III, patchy ossification without acoustic shadow; and type IV, crescent ossification with acoustic shadow (Figure 3) (15).
Tests of intraobserver and interobserver reliability
Forty participants were randomly selected to test the intraobserver and interobserver reliability of the measurement of the α angle, the β angle, FHD, Graf classification, and femoral head ossification center type. The 2 pediatric radiologists were independent in their interpretation of US images.
Statistical analysis
SPSS v. 25.0 software (IBM Corp., Armonk, NY, USA) was used for statistical analysis. Propensity score matching for age and sex was used to match the different DDH type patients with the normal participants (1:1). Continuous variables were compared using Student’s t test or 1-way analysis of variance, categorical variables were compared using the χ2 test, and ranked variables were compared using the Mann-Whitney test or the Kruskal-Wallis test. For the intraobserver and interobserver variability tests, the intraclass correlation coefficient (ICC), weighted kappa test, and Bland-Altman plots were used. P<0.05 was considered statistically significant.
Results
Participant characteristics
After hip US examination, a total of 1,037 normal participants (607 females aged 4.0±1.2 months; 430 males aged 3.9±1.2 months) with 2,074 mature hips were included in this study (Table 1). Among them, 578 participants had positive physical examinations, and 459 participants were scheduled for a routine physical examination at 4- to 6-week-old All participants were outpatients. The age stratification of the participants is shown in Table 1. The femoral head ossification center of males occurred significantly later (i.e., a lower percentage of ossification types II–IV) than that of females (P<0.001; Table 1). A total of 367 DDH participants (301 females aged 3.7±1.6 months; 66 males aged 3.3±1.8 months) with 456 dysplastic hips were included in this study (Table 2). Among them, 267 participants had positive physical examinations, and 100 participants were scheduled for a routine physical examination at 4–6 weeks. All participants were outpatients except for 2 participants who were inpatients with exomphalos. There was a total of 278 cases of unilaterally affected DDH and 89 cases of bilaterally affected DDH. The femoral head ossification center type and mean FHD of each DDH type are shown in Table 2.
Table 1
| Sex | Age/month† | Femoral head ossification center type (hips)/numbers‡ | Total participants/numbers | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–<1 | 1–<2 | 2–<3 | 3–<4 | 4–<5 | 5–6 | I | II | III | IV | |||
| Female | 4 | 41 | 63 | 174 | 174 | 151 | 411 (33.9) | 101 (8.3) | 327 (26.9) | 375 (30.9) | 607 | |
| Male | 2 | 22 | 74 | 126 | 121 | 85 | 484 (56.3) | 81 (9.4) | 152 (17.7) | 143 (16.6) | 430 | |
†, data are the number of participants. ‡, data are the number of hips, with percentages in parentheses. The comparison of female and male ossification type stratification used the Mann-Whitney test.
Table 2
| Graf type | Unilateral: bilateral† | Age, month‡ | Femoral head ossification center type§ | FHD, cm‡ | Total hips | |||
|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | |||||
| IIa | 75:39 | 2.0±0.7 | 133 (91.7) | 3 (2.1) | 5 (3.4) | 4 (2.8) | 1.62±0.15 | 145 |
| IIb | 160:45 | 4.6±1.2 | 108 (45.8) | 24 (10.2) | 43 (18.2) | 61 (25.8) | 1.75±0.15 | 236 |
| IIc | 19:17 | 3.4±1.6 | 26 (70.3) | 2 (5.4) | 6 (16.2) | 3 (8.1) | 1.64±0.18 | 37 |
| D | 3:2 | 3.3±1.9 | 4 (80.0) | 0 | 1 (20.0) | 0 | 1.65±0.25 | 5 |
| III | 10:0 | 4.2±1.5 | 9 (90.0) | 0 | 0 | 1 (10.0) | 1.59±0.14 | 10 |
| IV | 11:9 | 3.1±1.4 | 19 (82.6) | 2 (8.7) | 2 (8.7) | 0 | 1.44±0.18 | 23 |
†, the unilateral affected DDH indicates the affected DDH type hip and another Graf type I hip. The bilateral affected DDH includes the same DDH types and the different DDH type hips. ‡, data are means ± standard deviations. §, data are number of hips, with percentages in parentheses. FHD, femoral head diameter; DDH, developmental dysplasia of the hip.
Intraobserver and interobserver reliability
There was good intraobserver reproducibility of the measurement of the α angle, the β angle, and FHD, with ICCs of 0.99, 0.99, and 0.92, respectively (all P values <0.001). There was good agreement between the 2 operators in the measurement of the α angle, the β angle, and FHD, with ICCs of 0.99, 0.95, and 0.91, respectively (all P values <0.001). The weighted kappa values of the measurements of the Graf classification and femoral head ossification type were 0.90 and 0.85, respectively (both P values <0.001), which showed good reliability. The Bland-Altman plots also showed good agreement between the 2 operators in measuring the α angle, the β angle, and FHD (Figures S1-S3).
The developmental FHD and femoral head ossification center data in mature hips
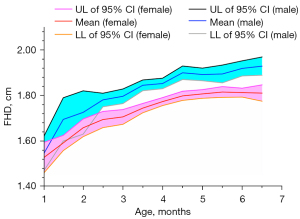
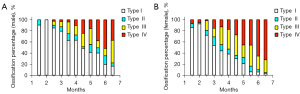
We analyzed the developmental data of mature hips (Graf type I). The mean FHD and the corresponding 95% confidence interval of every age stratification are shown in Figure 4 and Table S1. As expected, the FHD of mature male hips was significantly larger than that of female hips from the age of 2 months to 6 months (all P values <0.01; Table S1). The femoral head ossification center occurred at 2–3 months in females and 3–4 months in males (Table S2; Figure 5). From 3 months to 6 months, the femoral head ossification center of males occurred significantly later (i.e., a lower percentage of ossification types II–IV) than that of females of the same age (all P values <0.05; Table S2; Figure 5). There was no significant difference in femoral head ossification center types between males and females at less than 3 months of age (all P values >0.05; Table S2).
The impact of different DDH types on FHD development
There was no significant difference in FHD between Graf IIa (+) and age- and sex-matched mature hips (P=0.186; Figure 6A). However, the FHD of Graf IIa (–) was significantly smaller than that of the matched mature hips (1.64±0.15 vs. 1.72±0.13 cm; P=0.006; Figure 6A). The FHD of Graf IIb was also smaller than that of the matched mature hips (1.75±0.15 vs. 1.79±0.15 cm; P=0.006; Figure 6A). The FHD of Graf IIc was significantly smaller than that of the matched mature hips (1.65±0.18 vs. 1.73±0.15 cm; P=0.035; Figure 6A). The FHD of Graf D, III or IV was also markedly smaller than that of the matched mature hips (1.51±0.19 vs. 1.71±0.18 cm; P<0.001; Figure 6A). The results indicated marked developmental retardation of the femoral head in Graf IIa (–), IIb, IIc, D, III or IV.
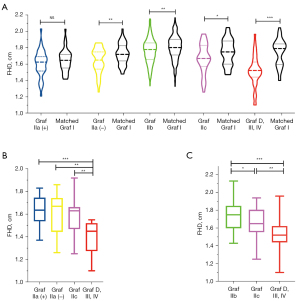
After age- and sex-matching and intercomparison of different DDH types, the developmental retardation of the femoral head in Graf D, III or IV was the worst among all the DDH types (the FHD was smaller than that of Graf IIa (+), IIa (–), IIb, or IIc; all P values <0.01; Figures 6B,6C). The FHD of Graf IIc was smaller than that of the matched Graf IIb (P=0.027; Figure 6C). Because of the mismatched age of Graf IIa and IIb, we did not compare the FHDs of these hips.
The impact of different DDH types on femoral head ossification center development
The occurrence of femoral head ossification in Graf IIa (–) was significantly delayed compared with that in matched mature hips (P=0.045; Figure 7A). There was no significant difference in femoral head ossification center types between Graf IIa (+)/IIb/IIc and the matched mature hips (Figure 7A). However, the occurrence of the femoral head ossification center in Graf D, III or IV was markedly later than that in matched mature hips (P=0.027; Figure 7A).
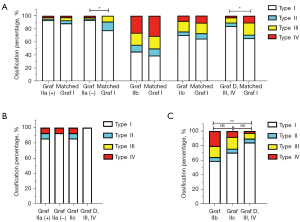
After age- and sex-matching and intercomparison of different DDH types, no significant difference in femoral head ossification center types was found among Graf IIa (+), IIa (–), IIc, and D, III or IV (Figure 7B). However, the occurrence of the femoral head ossification center in Graf D, III or IV was significantly delayed compared with that in matched Graf IIb hips (P=0.008; Figure 7C). Because of the mismatched age of Graf IIa and IIb, we did not compare the femoral head ossification center types of these hips.
Discussion
DDH is a developmental disorder and not a congenital disease. A relatively normal range of the development of the femoral head will provide more reference for the evaluation of the development of infants’ hips and as a risk factor for the surveillance of DDH. This study provided a relatively normal range of FHD of male and female infants from 1 to 6 months old. From 2 months old, the FHD of males was significantly larger than that of females at the same age. From 3 months old, the femoral head ossification center of males occurred significantly later than that of females of the same age. According to the latest World Health Organization child growth standard, boys develop later than girls at the same age, and the first-year growth rate of boys is faster than that of girls of the same age (16). The peak in growth of boys (14–15 years) is later than that of girls (12–13 years), which fits the occurrence time of the femoral head ossification center (17).
We found that the femoral head ossification center occurred at 2–3 months in females and 3–4 months in males. The ossific nucleus of the femoral head appears radiographically by age 4–6 months in normal hips. Compared with the reported ossification rate curve (18), the femoral head ossification in our study occurred much earlier. The ossification center can be seen 6–8 weeks earlier with sonography than with radiography (19), and the better feeding and nutrition condition currently also accounts for these results (20).
This study revealed the developmental retardation of the femoral head in DDH. Compared with the matched mature hips, the FHD of Graf IIa (–) hips was significantly smaller, and the occurrence of the femoral head ossification center was delayed. However, no significant developmental retardation was found in Graf IIa (+) hips. The development retardation of FHD was also found in Graf IIb, IIc, and D, III or IV hips. The occurrence of the femoral head ossification center was delayed in Graf D, III or IV hips.
A right anatomic relationship and located femoral head are necessary for acetabular and femoral head development (21). Persistent lateral hip subluxation and dislocation result in a shallow acetabulum and a mismatched femoral head. A smaller femoral head and delayed appearance of the ossific nucleus in radiographic DDH were also found in the study of Wu et al. (22) and Sugawara et al. (23). More importantly, a smaller femoral head was significantly associated with the incidence of avascular necrosis (22). The surveillance of femoral head size in DDH by US and appropriate treatment are necessary to avoid avascular necrosis or other poor outcomes. Closed reduction and subsequent spica casting are alternative, important, and widespread treatment methods for DDH (24). However, if the diagnosis of DDH is too late, closed reduction is no longer possible, as the femoral head continues growing, making repositioning into the acetabulum mechanically impossible or aggravated.
Management of type IIa/IIa (–) hips remains controversial, and these hips always carry a risk of either overtreatment or development of true hip dysplasia (25,26). If untreated, 95.3% and 84.4% of type IIa (+) and IIa (–) hips, respectively, become normal (27). Therefore, sonographic surveillance for these immature hips is conducted in some areas (10). However, according to the latest international DDH guideline, a Pavlik harness is recommended to treat Graf IIa (–) hips from 6 weeks of age (5,8). Bilgili et al. (25) found that, compared with Graf type IIa (+) hips, Graf type IIa (–) hips were less improved during natural progression and needed more treatment with a hip abduction orthosis. Omeroglu et al. (28) found that Graf Type IIa (–) had a 93% rate of treatment success by the Pavlik harness. Our study showed significant developmental retardation of Graf IIa (–) hips, which supports the appropriate treatment for these hips.
Our results showed that US is a useful and easy method for screening and early diagnosis of DDH. However, to evaluate the therapeutic effect following open and closed reduction, magnetic resonance imaging (MRI) is typically performed to confirm the concentric reduction and predict prognosis (24,29). At this point, for severe DDH that needs reduction, the US results of femoral head size and femoral head ossification may be reproduced by MRI images. We would like to continue further studying this topic.
There are several limitations of this study. First, we employed a retrospective design with no follow-up. The classification of DDH may change over time. Second, we used the Graf type I mature group as the control group. However, there was selection bias in our study. The participants in the Graf I group might have had risk factors or abnormal physical examinations. We will continue future hip studies for healthy infants. Third, we did not record the FHD or the femoral head ossification center type after treatment. Successful treatment should result in the improvement or catch-up of the femoral head. Finally, the present study was conducted in 1 medical center, and validation in different centers is needed.
In summary, we provided a relatively normal range for the development of infants’ hips from 1 to 6 months old and found significant developmental retardation of the femoral head; that is, delayed ossification and smaller size FHD, in Graf IIa (–), IIb, IIc, and D, III or IV hips. This is a preliminary study of the developmental impact of DDH on the femoral head. We will continue the follow-up study after treatment and compare outcomes of different therapeutic strategies.
Acknowledgments
The authors are grateful to Professor Li Qiu and the musculoskeletal ultrasound group in the Department of Medical Ultrasound, West China Hospital, Sichuan University, for their help in ultrasound examinations.
Funding: This study was supported by the National Natural Science Foundation of China (No. 82071940) and the Post-Doctor Research Project, West China Hospital, Sichuan University (No. 2018HXBH035).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-22-513/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-513/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of West China Hospital, Sichuan University (No. 2022-1372), and the informed consent for this retrospective study was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Patel HCanadian Task Force on Preventive Health Care. Preventive health care, 2001 update: screening and management of developmental dysplasia of the hip in newborns. CMAJ 2001;164:1669-77. [PubMed]
- Xu N, Xia B, Tao H, Sun K, Liu Q, Chen W, Wang D, Gao H, Guo Y, Liu Y, Gao J, Teng J, Li T, He Q, Wu Z. Epidemiological investigation and ultrasonic diagnosis of developmental dysplasia of the hip in Chinese infants: A large multi-center cohort study. Medicine (Baltimore) 2022;101:e28320. [Crossref] [PubMed]
- Graf R. Hip sonography: background; technique and common mistakes; results; debate and politics; challenges. Hip Int 2017;27:215-9. [Crossref] [PubMed]
- Writing Group of the Pediatric Ultrasound Committee of Ultrasound Branch of Chinese Medical Education Association. Xu N, Xia B, Tao HW, Sun K, Liu QH, Chen WQ, Wang D, Gao H, Guo Y, Liu Y, Gao J, Teng JB. Chinese Expert Consensus on Ultrasonographic Acquisition, Measurement, and Reporting System for Developmental Dysplasia of the Hip. Advanced Ultrasound in Diagnosis and Therapy 2020;4:1-8. [Crossref]
- O'Beirne JG, Chlapoutakis K, Alshryda S, Aydingoz U, Baumann T, Casini C, et al. International Interdisciplinary Consensus Meeting on the Evaluation of Developmental Dysplasia of the Hip. Ultraschall Med 2019;40:454-64. [Crossref] [PubMed]
- Nguyen JC, Dorfman SR, Rigsby CK, Iyer RS, Alazraki AL, Anupindi SA, Bardo DME, Brown BP, Chan SS, Chandra T, Garber MD, Moore MM, Pandya NK, Shet NS, Siegel A, Karmazyn B. ACR Appropriateness Criteria® Developmental Dysplasia of the Hip-Child. J Am Coll Radiol 2019;16:S94-S103. [Crossref] [PubMed]
- Swarup I, Penny CL, Dodwell ER. Developmental dysplasia of the hip: an update on diagnosis and management from birth to 6 months. Curr Opin Pediatr 2018;30:84-92. [Crossref] [PubMed]
- Chinese Medical Association Pediatric Surgery Branch Orthopaedic Group, Chinese Medical Association Orthopaedic Branch Pediatric Trauma and Orthopaedic Group. Detection and treatment of pediatric developmental dysplasia of the hip in children up to two year of age:clinical practice guideline. Zhonghua Gu Ke Za Zhi 2017;37:641-50.
- Bonsel JM, Gielis WP, Pollet V, Weinans HH, Sakkers RJB. Statistical Shape Modeling of US Images to Predict Hip Dysplasia Development in Infants. Radiology 2022;303:425-32. [Crossref] [PubMed]
- Rosendahl K, Dezateux C, Fosse KR, Aase H, Aukland SM, Reigstad H, Alsaker T, Moster D, Lie RT, Markestad T. Immediate treatment versus sonographic surveillance for mild hip dysplasia in newborns. Pediatrics 2010;125:e9-16. [Crossref] [PubMed]
- Wood MK, Conboy V, Benson MK. Does early treatment by abduction splintage improve the development of dysplastic but stable neonatal hips? J Pediatr Orthop 2000;20:302-5. [Crossref] [PubMed]
- Wanner MR, Loder RT, Jennings SG, Ouyang F, Karmazyn B. Changes in femoral head size and growth rate in young children with severe developmental dysplasia of the hip. Pediatr Radiol 2017;47:1787-92. [Crossref] [PubMed]
- Barrera CA, Cohen SA, Sankar WN, Ho-Fung VM, Sze RW, Nguyen JC. Imaging of developmental dysplasia of the hip: ultrasound, radiography and magnetic resonance imaging. Pediatr Radiol 2019;49:1652-68. [Crossref] [PubMed]
- Graf R, Scott S, Lercher K, Baumgartner F, Benaroya A. Hip Sonography: Diagnosis and Management of Infant Hip Dysplasia. Springer Berlin Heidelberg; 2006.
- Harcke HT, Lee MS, Sinning L, Clarke NM, Borns PF, MacEwen GD. Ossification center of the infant hip: sonographic and radiographic correlation. AJR Am J Roentgenol 1986;147:317-21. [Crossref] [PubMed]
- The World Health Organization. WHO child growth standards: length/height-for-age; c2006 [cited 2022 May 1]. Available online: https://www.who.int/tools/child-growth-standards/standards/length-height-for-age
- de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ 2007;85:660-7. [Crossref] [PubMed]
- Stewart RJ, Patterson CC, Mollan RA. Ossification of the normal femoral capital epiphysis. J Bone Joint Surg Br 1986;68:653. [Crossref] [PubMed]
- Paranjape M, Cziger A, Katz K. Ossification of femoral head: normal sonographic standards. J Pediatr Orthop 2002;22:217-8. [Crossref] [PubMed]
- Narese F, Puccio G, Mazzucco W, Falzone A, Venturella V, Narese D, Capra E. Earlier appearance of the ossification center of the femoral head in breast-fed versus formula-fed infants. Nutrition 2011;27:1108-11. [Crossref] [PubMed]
- Lee MC, Eberson CP. Growth and development of the child's hip. Orthop Clin North Am 2006;37:119-32. v. [Crossref] [PubMed]
- Wu J, Yuan Z, Li J, Zhu M, Canavese F, Xun F, Li Y, Xu H. Does the size of the femoral head correlate with the incidence of avascular necrosis of the proximal femoral epiphysis in children with developmental dysplasia of the hip treated by closed reduction? J Child Orthop 2020;14:175-83. [Crossref] [PubMed]
- Sugawara R, Watanabe H, Taki N, Aihara T, Furukawa R, Nakata W, Takeshita K, Kikkawa I. New radiographic standards for age at appearance of the ossification center of the femoral head in Japanese: Appearance at ≤12 months of age is normal in Japanese infants. J Orthop Sci 2019;24:166-9. [Crossref] [PubMed]
- Walter SG, Bornemann R, Koob S, Ossendorff R, Placzek R. Closed Reduction as Therapeutic Gold Standard for Treatment of Congenital Hip Dislocation. Z Orthop Unfall 2020;158:475-80. [Crossref] [PubMed]
- Bilgili F, Sağlam Y, Göksan SB, Hürmeydan ÖM, Birişik F, Demirel M. Treatment of Graf Type IIa Hip Dysplasia: A Cut-off Value for Decision Making Balkan Med J 2018;35:427-30.
- Alves C, Truong WH, Thompson MV, Suryavanshi JR, Penny CL, Do HT, Dodwell ER. Diagnostic and treatment preferences for developmental dysplasia of the hip: a survey of EPOS and POSNA members. J Child Orthop 2018;12:236-44. [Crossref] [PubMed]
- Roovers EA, Boere-Boonekamp MM, Mostert AK, Castelein RM, Zielhuis GA, Kerkhoff TH. The natural history of developmental dysplasia of the hip: sonographic findings in infants of 1-3 months of age. J Pediatr Orthop B 2005;14:325-30. [Crossref] [PubMed]
- Ömeroğlu H, Köse N, Akceylan A. Success of Pavlik Harness Treatment Decreases in Patients ≥ 4 Months and in Ultrasonographically Dislocated Hips in Developmental Dysplasia of the Hip. Clin Orthop Relat Res 2016;474:1146-52. [Crossref] [PubMed]
- Johnson MA, Gohel S, Nguyen JC, Sankar WN. MRI Predictors of Residual Dysplasia in Developmental Dysplasia of the Hip Following Open and Closed Reduction. J Pediatr Orthop 2022;42:179-85. [Crossref] [PubMed]



