
Imaging findings of inflammatory myofibroblastic tumors in the breast: a case description and literature analysis
Introduction
Inflammatory myofibroblastic tumors (IMTs) of the breast originate from mesenchymal tissue and are mainly composed of myofibroblast and multiple inflammatory cells (lymphocyte, plasmacyte and eosinophil). IMT is a rare borderline mesenchymal neoplasm that arises most commonly in the lung, peritoneum, and liver. The incidence of IMT is 0.04–0.7% (1). It occurs rarely in the breast. To date, fewer than 100 cases of breast IMT have been reported (2). Because the histopathological examination of IMT in beast is similar to inflammatory reaction fibrous tissue hyperplasia, it is easy to be misdiagnosed such as inflammatory fibrosarcoma and breast cancer or fibroadenoma (3). Here, we report a case of breast IMT in a 71-year-old woman.
Case presentation
A 71-year-old female was admitted to our hospital after suffering from a lump in her right breast for 2 months. She suffered a lump in her right breast at home, but experienced no pain or nipple retraction. She had not been admitted to the hospital and did not undergo examination. Three days ago, she came to our hospital for further treatment. She had a history of hypertension for 8 years with long-term regular use of drugs (no exact details), and it was effectively controlled. She had no history of breast cancer in her family, other infectious and chronic diseases. This study was approved by the Ethics Committee of Lishui Central Hospital (No. 2021-278). All procedures performed in this study were in accordance with the ethical guidelines of the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Physical examination
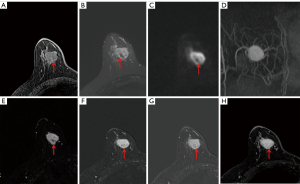
Physical examination showed an approximately 30 mm × 20 mm mass with no clear boundary, and non-mobile in the right upper quadrant of the breast. Enlarged lymph nodes were not palpable in the axilla. The results of tumor markers were normal (AFP, CEA, CA199, CA242 and CA50). In addition, there were no specific findings of the other laboratory examinations. Mammography showed high density irregular mass measuring 22 mm × 20 mm in the right breast upper inner quadrant (Figure 1A,1B). Sonography showed irregular hypoechoic mass with posterior acoustic enhancement and associated increased vascularity (Figure 1C, arrow). Color-Doppler flow imaging (CDFI): abundant blood supply was found in the tumor (Figure 1D). Resistance Index (RI): 0.82, elasticity score: 2–3. Ultrasonographic diagnosis: hypoechoic mass in the upper quadrant of the right inner breast, Breast Imaging Reporting and Data System (BI-RADS) classification: 4B. Magnetic resonance (MR) examination showed irregular T1 hyopintense and T2 hyperintense mass with persistent type enhancement, hyperintense on diffusion-weighted imaging (DWI). In some areas, the mass showed an iso-high signal on the T1-weighted sequence (Figure 2A, arrow), while it showed a low signal on T2-weighted and DWI (Figure 2B,2C, arrow). Multiple blood vessels are seen around the tumor (Figure 2D). On MR enhancement images, the mass showed strong continuous enhancement (Figure 2E-2H), and the low-signal areas on T2-weighted imaging (T2WI) and DWI showed gradual filling enhancement (Figure 2E-2H, arrow). MR diagnosis: breast mass on the right upper quadrant, BI-RADS classification 4C.

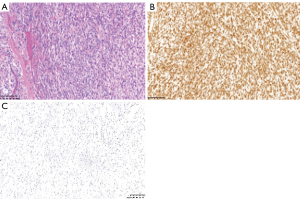
Under general anesthesia, the lumpectomy of right breast was performed. During the operation, a lesion with a size of 25 mm × 20 mm was detected in the upper quadrant of the right breast with an intact capsule and a hard and clear boundary. Postoperative pathological examination showed that the tumor cells were fusiform or hypertrophic fusiform, with heaps of lymphocytes and plasma cell infiltration (HE, ×200) (Figure 3A). Immunohistochemistry: the spindle cells were positive for CD68 and Vimentin (VIM) and negative for Creatine Kinase (CK), Anaplastic Lymphoma Kinase (ALK), smooth muscles actin (SMA), S100, estrogen receptor (ER) and Desmin (Figure 3B,3C). Pathological diagnosis: mammary gland IMT. There was no evidence of local recurrence or distant metastasis at the 3-month, 6-month, 1-year, and 4-year follow-ups.
Discussion
IMT is a special type of mesenchymal tumor that has been confusedly declared as plasma cell granuloma, inflammatory pseudotumor, and inflammatory myofibroblastic hyperplasia. In 2002, it was officially named IMT by the World Health Organization and consisted of differential myofibroblastic spindle cells admixed with a large number of plasma cells and/or lymphocytes, intermediate biological potential (low-grade malignant), and rare metastasis. The first case that occurred in the breast was reported by Pettinato in 1988 (4). Breast IMT is a rare mesenchymal tumor with myofibroblast proliferation as the background of inflammation. Its biological behavior is unstable and has the potential of recurrence and malignant transformation. IMT of mammary gland is diagnosed by excluding other spindle cell lesions according to the characteristics of Pathomorphology and the expression results of VIM, actin, SMA, Desmin and ALK in immunohistochemistry. Part of IMT does not have ALK ectopic, which belongs to ALK negative, and has c-ros oncogene receptor kinase (ROS). Platelet-derived growth factor receptor (PDGFRB) can be used for immunohistochemical or molecular detection of ROS1, fluorescence in situ hybridization (FISH) or second-generation sequencing can also be used (5). FSIH or second-generation sequencing will be performed in our case.
The etiology of IMT is unknown, and it is initially thought to be related to trauma, infection, surgery and other stimuli, which eventually activate myofibroblasts with proliferative potential to proliferate and form tumors (6,7). However, the genetic changes are already known. Fifty to sixty percent of IMTs have clonal rearrangement of the ALK gene on chromosome 2P23, resulting in overexpression of ALK protein, and a small number of cases involved rearrangements of ROS1, NTRK3, RET, PDGFRβ, IGF1R and other genes. the ALK immunostaining pattern varies depending on the ALK fusion pattern, for example RANBP2-ALK is associated with a nuclear membranous pattern, RRBP-1 ALK with a perinuclear accentuated cytoplasmic pattern, and clathrin heavy chain-anaplastic lymphoma kinase (CLTC)-ALK with a granular cytoplasmic pattern, many other ALK fusion variants show a diffuse cytoplasmic pattern (most commonly seen in IMT) (8-12). IMT in the breast is mostly spontaneous and rarely caused by trauma, surgical stimulation, or autoimmune diseases.
Clinical features
Previous studies have reported that rare disease occurs in people at all age stages with a major female preponderance, and the average age of onset is 44.6 years old (age range: 13–86 years) (13,14). There are no specificity in the clinical symptoms. Most of the patients with IMT admitted to the hospital owing to the breast mass found by themselves or physical examination, and a few of them may have symptoms such as fever and weight loss. This case we reported was a 71-year-old woman who was admitted by physical examination without any clinical symptoms.
Imaging features
Imaging examination methods of the breast mainly include ultrasound, mammogram, and dynamic contrast-enhanced MR. Due to the extremely low incidence rate, IMTs of the breast are mostly reported as individual cases (15-18). Therefore, there is no systematic description or summary of its imaging features in the literature, and most of them are reported on the imaging performance of ultrasound and mammograms (17-20). There is also no characteristic imaging appearance of the disease on ultrasound and mammography. However, there are certain imaging features in MR images in our case. By summarizing the previous literature (Table 1) and combining our case, we concluded that the main imaging features of MR were a single mass, unclear boundary, lobulated or microlobulated, with or without calcification. Most of the lesions presented as fast-in and fast-out enhancement on MR images (2,15,16,21,22). The enhancement mode on MR images is mainly related to the ratio of blood vessels and inflammatory cells to scar fibrous tissue. If the lesion is abundant in blood vessels and dominated by inflammatory cells, the enhancement mode on MR images is a fast-in and fast-exit type, and if scar fibrous tissue is dominant, the enhancement mode is the continuous enhancement type. Of course, different tissue components can exist in the same lesion, so the imaging findings of the lesion are heterogeneous, which has not been reported in the previous literature. In the case we reported, the lesion showed mainly low signal intensity on T1-weighted images, with small patches of slightly high signal areas in the interior. The lesion presented as high signal intensity on T2-weighted and DWI images, and the high signal area on T1WI was a low signal. After enhancement, the whole lesion showed continuous and obvious enhancement, but the low-signal regions on T2-weighted imaging and DWI showed delayed filling enhancement, indicating that this area was dominated by fibrous scar tissue. Postoperative microscopic examination also confirmed the presence of fibrous scar tissue. The above imaging features have not been reported in previous literature. In addition, compared with malignant tumors such as breast cancer, this case has no characteristic imaging features on ultrasound and mammography images. Of course, even though the combination utilization of three imaging methods cannot provide a definitive diagnosis of breast IMT, the final diagnosis still depends on the pathology.
Table 1
| Reference | Age, years | Sex | Location | Size | MRI features | |
|---|---|---|---|---|---|---|
| DWI | Enhancement characteristics | |||||
| Mao (2) | 43 | Female | Left upper quadrant | 12 mm | Increased signal intensity | Washout pattern |
| Li (15) | 39 | Female | Upper inner quadrant | 48 mm | NA | NA |
| Khanafshar (16) | 47 | Female | Right upper quadrant | NA | NA | NA |
| Bosse (21) | 23 | Female | Left upper quadrant | 20 mm | Increased signal intensity | Washout pattern |
| Choi (22) | 27 | Female | Right upper quadrant | 30 mm | Increased signal intensity | Heterogeneous (washout pattern) |
MRI, magnetic resonance imaging; DWI, diffusion weighted imaging; NA, not available.
Differential diagnosis
Due to the atypical clinical and imaging features of IMT, it is commonly misdiagnosed as other benign and malignant breast tumors, such as fibromatosis, nodal fasciitis, scar carcinoma of skin, breast cancer, inflammatory fibrosarcoma, and fibromyxoid sarcoma. Among the above tumor, IMT is most likely to be misdiagnosed as breast cancer, which is the most common malignant tumor of the breast. On the enhancement MR images, it manifests the characteristics of fast-in fast-out without local delayed enhancement and other features. Among the tumor-like lesions or benign tumors, IMT is most likely to be misdiagnosed as nodal fasciitis. Nodular fasciitis often occurs in the superficial parts of the upper limb and trunk and is characterized by rapid growth, which can be distinguished from IMT. In addition, IMT should be differentiated from these diseases in soft tissue pathology such as IMT, IgG4-related disease, epithelioid inflammatory myofibroblastic sarcoma, inflammatory pseudotumor, inflammatory well-differentiated liposarcoma, inflammatory dedifferentiated liposarcoma. IgG4-related disease is a rare chronic inflammatory disease with fibrosis, characterized by a large number of lymphocytes in the affected tissues, especially IGG4-positive plasma cells. Serum IgG4 levels contribute to the diagnosis of IGG4-related disease (RD). Most patients can achieve significant remission after hormone therapy (23). On gross pathology, epithelioid inflammatory myofibroblastic sarcoma present as a large nodular, myxoid appearance on the cut surface, and may be accompanied by necrosis. The tumor is characterized by a myxoid background in which inflammatory cells can be seen such as neutrophils, lymphocytes, plasma cells and eosinophils, especially neutrophils. Inflammatory pseudotumor is characterized by myofibroblast proliferation and accompanied by lymphocytes and plasma cells. Histopathologically, inflammatory well-differentiated liposarcoma are mainly composed of mature adipose tissue and irregular fibrous septa, with atypical hyper-stained stromal cells, less commonly and adipogenic cells, including some inflammatory cells. The spindle cells and adipocytes are often mixed in inflammatory well-differentiated liposarcoma, suggesting adipogenic tumors. Inflammatory dedifferentiated liposarcoma is rare, inflammatory cells are more than mucinous matrix in the stroma. Although neoplastic spindle cells are easy to be neglected, these cells still showed some atypia (24).
Conclusions
In summary, IMT of the breast is a rare disease and genetics are known, with no specific clinical symptoms or signs. In this case, there are still specific MR imaging findings that can be used for the preliminary diagnosis of the disease, and it is helpful to judge the extent of the lesion and its relationship with adjacent tissues, but the final diagnosis still depends on pathology by puncture biopsy [automatic biopsy needle (VPA14/10)] or modified mastectomy (lumpectomy). Imaging examinations, especially MR, will play an important role in the diagnosis of breast IMT.
Acknowledgments
We would thank the staff of the Department of Breast Surgery and Ultrasonography at Lishui Central Hospital for providing valuable clinical and ultrasonic support. In the meantime, we would like to thank all of our friends.
Funding: This study was supported by the Lishui Public Welfare Research Project (No. 2020GYX17).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-144/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Panagiotopoulos N, Patrini D, Gvinianidze L, Woo WL, Borg E, Lawrence D. Inflammatory myofibroblastic tumour of the lung: a reactive lesion or a true neoplasm? J Thorac Dis 2015;7:908-11. [PubMed]
- Mao X, Liu H, Du J, Yu N, Chen L, Zhang L. Imaging findings of inflammatory myofibroblastic tumor in breast: A case report. Medicine (Baltimore) 2018;97:e11804. [Crossref] [PubMed]
- Tan MH, Chen W, Zou QC, Guo JH, Zhou YJ, Ou RF, Deng CH, Hu ZX, Lei WH. Inflammatory myofibroblastic tumor of the breast: a clinicopathologic study of one case. J Diag 2020;27:472-5.
- Pettinato G, Manivel JC, Insabato L, De Chiara A, Petrella G. Plasma cell granuloma (inflammatory pseudotumor) of the breast. Am J Clin Pathol 1988;90:627-32. [Crossref] [PubMed]
- Chang JC, Zhang L, Drilon AE, Chi P, Alaggio R, Borsu L, Benayed R, Travis WD, Ladanyi M, Antonescu CR. Expanding the Molecular Characterization of Thoracic Inflammatory Myofibroblastic Tumors beyond ALK Gene Rearrangements. J Thorac Oncol 2019;14:825-34. [Crossref] [PubMed]
- Vecchio GM, Amico P, Grasso G, Vasquez E, La Greca G, Magro G. Post-traumatic inflammatory pseudotumor of the breast with atypical morphological features: A potential diagnostic pitfall. Report of a case and a critical review of the literature. Pathol Res Pract 2011;207:322-6. [Crossref] [PubMed]
- Zen Y, Kasahara Y, Horita K, Miyayama S, Miura S, Kitagawa S, Nakanuma Y. Inflammatory pseudotumor of the breast in a patient with a high serum IgG4 level: histologic similarity to sclerosing pancreatitis. Am J Surg Pathol 2005;29:275-8. [Crossref] [PubMed]
- WHO Classification of Tumours Editorial Board. WHO classification of tumours. Soft tissue and bone tumours. 5th ed. Lyon: IARC Press, 2020:109-11.
- Ding R, Sheng SJ, Gong QX. Research progress on the molecular genetics of inflammatory myofibroblastic tumor. Zhonghua Bing Li Xue Za Zhi 2021;50:1415-8. [PubMed]
- Bridge JA, Kanamori M, Ma Z, Pickering D, Hill DA, Lydiatt W, Lui MY, Colleoni GW, Antonescu CR, Ladanyi M, Morris SW. Fusion of the ALK gene to the clathrin heavy chain gene, CLTC, in inflammatory myofibroblastic tumor. Am J Pathol 2001;159:411-5. [Crossref] [PubMed]
- Lee JC, Li CF, Huang HY, Zhu MJ, Mariño-Enríquez A, Lee CT, Ou WB, Hornick JL, Fletcher JA. ALK oncoproteins in atypical inflammatory myofibroblastic tumours: novel RRBP1-ALK fusions in epithelioid inflammatory myofibroblastic sarcoma. J Pathol 2017;241:316-23. [Crossref] [PubMed]
- Mariño-Enríquez A, Wang WL, Roy A, Lopez-Terrada D, Lazar AJ, Fletcher CD, Coffin CM, Hornick JL. Epithelioid inflammatory myofibroblastic sarcoma: An aggressive intra-abdominal variant of inflammatory myofibroblastic tumor with nuclear membrane or perinuclear ALK. Am J Surg Pathol 2011;35:135-44. [Crossref] [PubMed]
- Inoue M, Ohta T, Shioya H, Sato S, Takahashi H, Nakata N, Taniguchi C, Hirano M, Nishioka M, Yamakawa H. Inflammatory myofibroblastic tumors of the breast with simultaneous intracranial, lung, and pancreas involvement: ultrasonographic findings and a review of the literature. J Med Ultrason (2001) 2018;45:331-5. [PubMed]
- Zhou P, Chen YH, Lu JH, Jin CC, Xu XH, Gong XH. Inflammatory myofibroblastic tumor after breast prosthesis: A case report and literature review. World J Clin Cases 2022;10:1432-40. [PubMed]
- Li J, Yun W, Qin J, Zhao J, Liu X, Wu J, Ji M, Tang J. Inflammatory myofibroblastic tumor of the breast coexisting with breast cancer: a case report. Breast Care (Basel) 2013;8:290-2. [Crossref] [PubMed]
- Khanafshar E, Phillipson J, Schammel DP, Minobe L, Cymerman J, Weidner N. Inflammatory myofibroblastic tumor of the breast. Ann Diagn Pathol 2005;9:123-9. [Crossref] [PubMed]
- Talu CK, Çakır Y, Hacıhasanoğlu E, Leblebici C, Aksoy Ş, Nazlı MA. Inflammatory Myofibroblastic Tumor of the Breast Coexisting with Pseudoangiomatous Stromal Hyperplasia. J Breast Health 2016;12:171-3. [Crossref] [PubMed]
- Siraj F, Kaur M, Dalal V, Sonam J. Inflammatory Myofibroblastic Tumor of the Breast Mimicking Malignancy in an Elderly Male. Ochsner J 2017;17:277-9. [PubMed]
- Zhao HD, Wu T, Wang JQ, Zhang WD, He XL, Bao GQ, Li Y, Gong L, Wang Q. Primary inflammatory myofibroblastic tumor of the breast with rapid recurrence and metastasis: A case report. Oncol Lett 2013;5:97-100. [Crossref] [PubMed]
- Markopoulos C, Charalampoudis P, Karagiannis E, Antonopoulou Z, Mantas D. Inflammatory myofibroblastic tumor of the breast. Case Rep Surg 2015;2015:705127. [Crossref] [PubMed]
- Bosse K, Ott C, Biegner T, Fend F, Siegmann-Luz K, Wallwiener D, Hahn M. 23-Year-Old Female with an Inflammatory Myofibroblastic Tumour of the Breast: A Case Report and a Review of the Literature. Geburtshilfe Frauenheilkd 2014;74:167-70. [Crossref] [PubMed]
- Choi EJ, Jin GY, Chung MJ, Moon WS, Youn HJ. Primary Inflammatory Myofibroblastic Tumors of the Breast with Metastasis: Radiographic and Histopathologic Predictive Factors. J Breast Cancer 2015;18:200-5. [Crossref] [PubMed]
- Takahashi H, Yamamoto M, Suzuki C, Naishiro Y, Shinomura Y, Imai K. The birthday of a new syndrome: IgG4-related diseases constitute a clinical entity. Autoimmun Rev 2010;9:591-4. [Crossref] [PubMed]
- Si HP, Wang Z, Fan QH, Zhang YF, Yang DQ, Zhang ZH, Gong QX. Dedifferentiated liposarcoma with inflammatory myofibroblastic tumor-like features: a clinicopathological analysis of five cases. Zhonghua Bing Li Xue Za Zhi 2019;48:282-7. [PubMed]


