
Sono-assisted-thrombolysis by three-dimensional diagnostic ultrasound improves epicardial recanalization and microvascular perfusion in acute myocardial infarction
Introduction
Thrombolysis and percutaneous coronary intervention (PCI) have been used to enhance the prognosis of patients with ST-segment elevation myocardial infarction (STEMI). However, two important clinical difficulties posed by this treatment need to be addressed. First, patient-side factors, such as delays to their transfer to a PCI-capable center and the initiation of PCI, can make it difficult to achieve early reperfusion (1). The size and span of the infarct zone are proportional to the time it is in an ischemic state (2,3). Several measures have been taken to reduce the ischemic time, such as promoting early recognition of STEMI and pre-hospital cardiac catheterization laboratory initiation. However, the overall ischemic time for patients with STEMI is still very long (4). Second, even with adequate epicardial revascularization, up to 50% of patients develop a microvascular injury, which leads to a larger infarct size, negative left ventricular remodeling, and a poorer prognosis (5).
Owing to the lower recanalization rate and a higher rate of hemorrhagic complications with thrombolysis compared to PCI, efforts have been made to develop a simple, non-invasive treatment technique that would allow for higher recanalization rates with lower complication rates. The combination of ultrasound (US) with microbubbles (MBs), which is known as sonothrombolysis, has been used to improve thrombus breakdown, and more selective targeting tactics are currently under development (6). However, sonothrombolysis mostly improves microvascular blood flow without epicardial recanalization (7), and its feasibility and safety still require further study. Conventional two-dimensional (2D) US also has drawbacks such as low spatial resolution, limited coverage, and continuous sliding of the probe during operation.
Therefore, there is an urgent need to develop innovative and more effective treatment strategies for STEMI that promote myocardial microcirculation and epicardial coronary artery recanalization as soon as possible following diagnosis. Furthermore, treatment should be applied immediately if microvascular injury can be prevented or treated. Depending on feasibility, microvascular damage should be addressed or avoided altogether.
This study aimed to investigate the various interactions that may occur between therapeutic US (TUS), MBs, and thrombolytic drugs. This study explored these interactions by using three-dimensional (3D) ultrasonography to assess the effects of thrombolysis with half-dose recombinant tissue plasminogen activator (r-tPA) on epicardial recanalization and microcirculation in patients with acute STEMI. We present the following article in accordance with the ARRIVE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-21-1247/rc).
Methods
Preparation of MBs
Perfluoropropane (C3F8, purity 99.9%, purchased from Guangdong Foshan Gas Chemical Co., Ltd., China), glycerol, propylene glycol, sodium chloride, and glycerol were dissolved in distilled water. Then, dipalmitoylphosphatidylcholine (DPPC), dipalmitoylphosphatidic acid (DPPA), and dipalmitoylphosphatidylethanolamine-polyethylene glycol 5000 (DPPE-PEG5000), which were all obtained from Avanti Polar-Lipids (Alabaster, AL, USA), were added at a ratio of 10:8:82 and dissolved together in distilled water as previously reported (8,9). To create an MB suspension, 10 mL of MBs were mixed with 100 mL of 0.9% sodium chloride. According to results obtained with a Coulter counter, the final MB concentration was 3.89×109 bubbles/mL and the mean MB diameter was 2.68±0.37 µm (Multisizer III, Beckman Coulter, Pasadena, CA, USA).
Ultrasound parameters and protocol
An EPIQ 7C US probe with an X5-1 3D transducer (Philips Medical Systems, Best, Netherlands) was used for ultrasound imaging and ultrasound therapy. Two imaging modes are available: (I) a TUS mode in which the predefined therapeutic region (similar to the box in color Doppler imaging) is superimposed on the anatomical imaging (B mode); and (II) a real-time low mechanical index (MI) contrast-only imaging mode (MI <0.3) for monitoring the presence (and replenishment) of MBs within individual heart segments. Depending on the image quality, three or four apical windows were employed in the TUS mode to guide intermittent high MI 3D US pulses (frequency 1.6 to 2.1 MHz, MI =1.19, with a 2-s duration and 5-s intervals) in the thoracic region. During the high MI pulse treatment interval, low MI imaging was used to identify MBs in the at-risk area at the same frame rate as in the high MI treatment period. The total duration of the US procedure was 30 min.
As indicated by the risk zone during the contrast supplementation period, the area that revealed contrast deficiencies following TUS was considered at risk.
Animal preparation
Animal experiments were performed under a project license (No. AAS161203P) granted by the institutional board of the Ethics Committee of Shenzhen Advanced Animal Study Service Center. All experiments compliance with the National Institutes of Health guidelines for the care and use of animals (NIH Publication No. 85-23, as updated in 2011) guidelines for the care and use of animals.
Thirty-three Bama pigs (17 males and 16 females) were procured from Guangzhou Feed Research Institute. The pigs had an average age of 56 weeks and an average weight of 43 kg. They were fed an acclimation diet with 15% fat and 2% cholesterol before starting on a 4-week high-cholesterol diet. The experimental pigs were given unlimited access to food and water, and were fed from a single compartment. They were housed in a high-humidity (60–75%) chamber that had a suitable temperature (15–23 ℃) and sufficient air circulation.
One day before modeling, a combination of 50 mg of prednisone, 325 mg of Plavix, and 300 mg of aspirin was provided in the pigs’ feed. Fasting started 8 hours before modeling, and at 1 hour before modeling, the pigs were fed 50 mg of prednisone.
A 30 mg/kg injectable mixture of 3% pentobarbital and 10% xylazine was administered pre-intubation to sedate the animals before the procedure. The oxygen content in the mixture was kept constant at 24% throughout the treatment. Two catheters were inserted into the pigs’ femurs to monitor their hemodynamics and deliver MBs as needed. During the procedure, an 8F guide catheter was utilized for digital angiography and balloon catheter placement in the coronary artery. The pigs’ heart rates and oxygen saturation (SpO2) were continually monitored and recorded throughout the procedure. The pigs were given low doses of intravenous dobutamine (1 to 3 µg/min) to keep their systolic blood pressure (SBP) above 80 mmHg. Lidocaine boluses (40 mg intravenously followed by 20 mg three times) and continuous lidocaine infusions (2 to 4 mg/min intravenously) were administered to reduce arrhythmias in all the animals (7).
An acute left anterior descending (LAD) thrombotic blockage was created in each of the pigs. One of the pigs (male) died of unresponsive ventricular fibrillation before commencing therapy, which reduced the sample size to 32 (16 males and 16 females). All of the pigs were subjected to the same procedure: replication of the Virchow triad in their coronary arteries (10). The development of endothelial damage, staleness, and hypercoagulability was required to achieve this. Endothelial injury was induced by inserting a balloon catheter into the LAD coronary artery after the second diagonal branch and inflating the balloon to a maximum diameter of 130% of the measured coronary artery three times, for 30 s each time with 2-min intervals. Subsequently, the balloon catheter proximal to the injury site was removed and partially inflated to limit the blood flow near the wound. Then, a sufficient amount (0.5–1.0 mL) of coagulated arterial blood was administered to the injury site through the balloon catheter to establish a hypercoagulable condition. Ten milliliters of arterial blood was drawn through the sheath, and then heparin (80 U/kg) was injected intravenously, followed by another 40 U/kg intravenously every hour, to form a small thrombus.
Once an occlusion of the LAD had been verified by angiogram, it needed to be present for at least 20 min before therapy could be administered. The balloon catheter was used to repeatedly provide tiny thrombus injections until a persistent occlusion was detected. If spontaneous recanalization occurred, the catheter was used to administer tiny thrombus injections repeatedly until a persistent occlusion was observed. If no persistent occlusion was found, the procedure was repeated.
Animal protocol
The pigs were subjected to baseline heart rate, oxygen saturation, arterial blood pressure (BP), myocardial blood flow (MBF), and perfusion defect size (final infarct size) assessments using contrast-low-MI imaging and ST-segment elevation from a 12-lead electrocardiogram (ECG).
The STEMI pigs (n=32) were randomized into four groups: (I) a 3D-sono-assisted-thrombolysis group (the 3D/TUS + MB + r-tPA group, n=8), with a continuous intravenous infusion of non-targeted MBs and a half dose of r-tPA (0.5 mg/kg) and intermittent high MI impulses from a transthoracic-guided 3D transducer (3D/TUS impulses); (II) a 3D-sonothrombolysis group (the 3D/TUS + MB group, n=8), with a continuous intravenous infusion of MBs with the same 3D/TUS impulses without r-tPA; (III) a full-dose r-tPA group (the r-tPA group, n=8), with full-dose (1 mg/kg) r-tPA administered over 90 min without US or MB; and (IV) a 3D/TUS alone group (the 3D/TUS group, n=8), with the same 3D/TUS impulses but no r-tPA or MB.
For r-tPA-treated pigs, 50% of the total dose was loaded during the first 30 min and the rest during the next 60 min. The duration of all treatments was 30 min.
Measurement of MBF
Assessment of epicardial coronary recanalization
Coronary angiography was performed at three time points: baseline, post occlusion, and post treatment. The flow in the distal segment of the LAD was then observed to assess the occurrence of recanalization of the epicardial coronary artery. The flow in the LAD was estimated visually using the thrombolysis in myocardial infarction (TIMI) criteria by experienced reviewers who were blinded to treatment protocol (11). To calculate the recanalization rate, a TIMI flow grade ≥2 was defined as revascularization.
Microcirculatory changes in the at-risk myocardium
Myocardial contrast echocardiography was performed at three different time points: baseline, post occlusion, and post treatment. All images were stored on a CD-ROM drive and analyzed offline using myocardial contrast echocardiography software (MCE 2.9, Oregon Health and Science University, Portland, OR, USA) (12).
The high-risk myocardial perfusion region was identified as the area of interest. The following function was fitted to time-versus-background-subtracted video intensity data (background-subtracted color-coded images) from the skeletal muscle:
where the rate constant β is the microvascular flux rate and MBV is the microvascular blood volume. Myocardial blood flow was calculated as the product of MBV and β, and was used to measure the microcirculation of patients at risk of myocardial infarction (13). Acoustic intensity quantitatively reflects MBF.
Changes in ST-segment elevation on ECG
Standard 12-lead ECGs were obtained at baseline, post occlusion and at 60 min post treatment, and the maximal ST-segment elevation in V3 was compared.
Changes in at-risk and infarcted myocardial areas
Evans blue staining
Twenty-four hours after the various treatments, the femoral arteries of each pig were re-punctured bilaterally to obtain left and right coronary angiograms. The balloon was expanded to 130% of the coronary artery diameter to block blood flow, and then 1% Evans blue (45 mL total; 30 mL for the left main coronary and 15 mL for the right coronary) was administered through coronary catheters placed in the left and right major coronary arteries to identify the high-risk region. The pigs were sacrificed 10 min later, and the balloon catheters were removed from their bodies.
2,3,5-triphenyltetrazolium chloride (TTC) staining
After rinsing, the excised hearts were evenly cut into five pieces along the short axis. The myocardium from each pig was then placed in a foil-covered container with 2% 2,3,5-TTC solution. The containers were placed in an incubator for staining at 37 ℃ for 15–20 min, and the coloration of the stained myocardium was evaluated.
Assessment of changes in the at-risk myocardium and infarcted myocardial areas
Since Evans blue can enter various tissues through the blood, the myocardium will be colored blue where there is blood flow, while areas of the myocardium without blood flow will be left uncolored.
In the normal myocardium, TTC, a proton acceptor in the respiratory chain with a pyridine-nucleoside structural system, reacts with dehydrogenase and appears red, whereas in the ischemic myocardium, which has decreased dehydrogenase activity, it cannot react and thereby appears white.
Considering the above, after successful staining, the area without Evans blue staining was the myocardial area at risk, and the pale area without Evans blue staining or TTC staining was the infarcted myocardial area. The ratio of the infarcted myocardial area divided by the at-risk myocardial area (IM/RM) was used as an indicator of myocardial perfusion after treatment; the lower the ratio, the better the myocardial perfusion recovery. Differences in perfusion recovery between two groups were examined by comparing the IM/RM.
Statistical analysis
The data were displayed as the mean ± standard deviation (mean ± SD). After Bonferroni correction, Fisher’s exact probability method was used to compare the results of count data reported as percentage rates. Student’s t-test was used to compare data between two groups. Changes between groups were analyzed by one-way analysis of variance (ANOVA). When differences between groups were statistically significant (P<0.05), Fisher’s least significant difference test was used for post-hoc comparison of paired (pre versus post occlusion or treatment) or unpaired (comparisons between the four groups) data. The correlation coefficient between two independent variables was calculated using Pearson’s correlation coefficient. All statistical analyses were performed using IBM SPSS version 21.0 software (IBM Corp., Armonk, NY, USA). All analyses were two-tailed, and a P value of less than 0.05 was considered statistically significant in all cases.
Results
Coronary recanalization of the epicardium
Before treatment, the average amounts of coagulated arterial blood administered to the pigs in the four groups were 0.83±0.05, 0.78±0.09, 0.80±0.07, and 0.75±0.11 mL in the 3D/TUS + MB + r-tPA, 3D/TUS + MB, r-tPA and 3D/TUS groups, respectively, and there was no statistical difference between the groups (all P>0.05). After treatment, there were seven cases of recanalization of the distal LAD in the 3D/TUS + MB + r-tPA group (recanalization rate =87.5%. The 3D/TUS + MB group and the r-tPA group had a further 3 (37.5%), and 4 (50.0%) cases, respectively. However, there were no cases of recanalization in the 3D/TUS group (0%) (Figure 1).
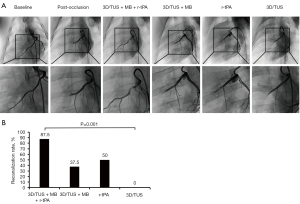
Except for the 3D/TUS group, the recanalization rate in the groups was statistically significant compared to baseline (P=0.005). After treatment, there was a marked difference in the recanalization rate between the 3D/TUS + MB + r-tPA and 3D/TUS groups (P=0.001). However, for the recanalization rate, the 3D/TUS + MB + r-tPA group showed no significant differences when compared to the 3D/TUS + MB and r-tPA groups (both P>0.05); the 3D/TUS + MB and r-tPA groups also failed to exhibit a statistically significant difference (P>0.05).
Microcirculatory changes in the at-risk myocardium
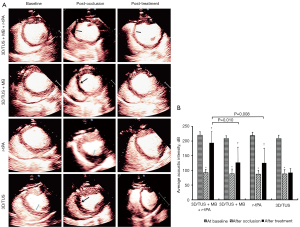
Although all four groups of pigs had notably reduced microvascular perfusion following LAD blockage (all P<0.001), there was no statistically significant difference between them (all P>0.05). A scatterplot showing the average acoustic intensity of the myocardium in each group is displayed in Figure S1.
Following treatment, microvascular perfusion was markedly greater in the 3D/TUS + MB + r-tPA, 3D/TUS + MB, and r-tPA groups than in the 3D/TUS group (P<0.001, P=0.047, and P=0.034, respectively). There was a statistically significant difference between the 3D/TUS + MB + r-tPA and 3D/TUS + MB groups (P=0.010) and between the 3D/TUS + MB + r-tPA and r-tPA groups (P=0.008). However, the 3D/TUS + MB and r-tPA groups did not show any statistically significant differences (P>0.05) (Figure 2).
Changes in ST-segment elevation on ECG
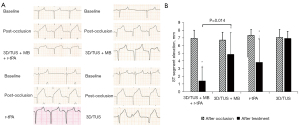
Post occlusion, the ST-segment on ECG elevated significantly compared to baseline in all four groups (all P<0.05), although there were no statistical differences between the groups (all P>0.05). A scatterplot showing the ST-segment elevation on ECG in V3 in each group is shown in Figure S2.
Compared to that post occlusion, the ST-segment elevation in three groups (the 3D/TUS + MB + r-tPA, 3D/TUS + MB, and r-tPA groups) decreased considerably after treatment (P<0.001, P=0.034, and P=0.025, respectively), but there was no statistically significant post-treatment difference in ST-segment elevation in the 3D/TUS group (P>0.05). There was a notable reduction in ST-segment elevation in the 3D/TUS + MB + r-tPA group compared to the r-tPA group (P=0.014), but there was no significant difference between the 3D/TUS + MB + r-tPA and 3D/TUS + MB groups (P>0.05) or between the 3D/TUS + MB and r-tPA groups (P>0.05) (Figure 3). Comparisons of the ST-segment elevation on 12-lead ECG in each group at baseline, post occlusion, and post treatment are shown in Figure S3.
Changes in the at-risk myocardial and infarcted myocardial areas
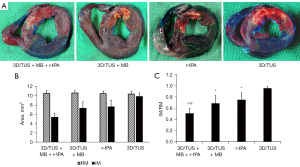
After treatment, the risk areas were similar in all four groups and there were no statistical differences between them (10.51±0.60 vs. 10.61±0.52 vs. 10.46±0.57 vs. 10.39±0.59 mm2 in the 3D/TUS + MB + r-tPA, 3D/TUS + MB, r-tPA and 3D/TUS groups, respectively, all P>0.05). A scatterplot showing the IM, RM, and IM/RM in each group is displayed in Figure S4.
There were significant differences in the infarcted myocardial area between the four groups (all P<0.05), except for between the 3D/TUS + MB and r-tPA groups (P=0.665). The IM/RM in three groups (the 3D/TUS + MB + r-tPA, 3D/TUS + MB, and r-tPA groups) was substantially lower than that in the 3D/TUS group (P<0.001, P=0.044, and P=0.008, respectively). The IM/RM was considerably lower in the 3D/TUS + MB + r-tPA group than in the 3D/TUS + MB and r-tPA groups (P=0.015 and P=0.048, respectively), but no significant difference was observed between the 3D/TUS + MB and the r-tPA groups (P>0.05) (Figure 4).
Discussion
According to the findings of this study, US-guided injection of non-targeted MBs and half-dose r-tPA administered by guided high MI impulses from a 3D diagnostic US probe could improve microvascular flow and function in the at-risk region following acute LAD embolism in high-cholesterol diet pigs. Compared to sonothrombolysis and thrombolysis, 3D-sono-assisted-thrombolysis can more effectively treat acute LAD thrombotic occlusions, with better rates of recanalization of the epicardial coronary arteries and smaller final infarct sizes.
Enhancing the microcirculation by using better techniques
To our knowledge, the cardiovascular magnetic resonance indices of microcirculatory resistance and microvascular obstruction have been linked to poor long-term outcomes in patients with reperfused STEMI (14). The magnitude of myocardial infarction (15), the degree of myocardial salvages (16), and the degree of microvascular obstruction (17) have all been associated with poorer outcomes. It has been shown that 65% of patients with acute STEMI still had microvascular obstruction following advanced percutaneous coronary procedures (18). The results of the cardiovascular magnetic resonance survey showed that microvascular obstruction was associated with a larger infarct size (19) and a poorer prognosis (20), confirming the close link between infarct size and microvascular obstruction.
In rural areas around the world, thrombolytic therapy is the most frequently used treatment for patients with STEMI, despite having lower recanalization rates and higher hemorrhagic complications than PCI. Furthermore, thrombolytic therapy does not appear to have any significant effect on enhancing microcirculation (6,21). As a result, efforts have been made to develop a non-invasive therapeutic technique that is easily adaptable and can achieve higher recanalization rates while minimizing the risk of complications (6).
Ultrasound-guided thrombolysis is one of the most specific targeted tactics to improve thrombus dissolution. Previous studies have shown that the addition of sonothrombolysis before and after emergency PCI for acute STEMI can immediately improve microvascular flow (22,23). Microbubble cavitation induced by US has both catalytic and flow-inducing properties, and this approach takes advantage of these properties to create flow. The administration of high MI ultrasonic impulses causes the compression, expansion, and collapse of contrast microspheres during the simultaneous infusion of MB resulting in a localized microflow that breaks up the thrombus (24,25). The present study used non-targeted intravenous MBs similar to those that are currently commercially available and comparable to erythrocytes, allowing them to quickly reach the microcirculation and treat patients (26,27). Sonothrombolysis has emerged as a viable supplementary therapy to treat cardiovascular ischemic illness in recent years, and can enhance microvascular blood flow without requiring epicardial coronary recanalization (7) and may provide more satisfying outcomes.
In our investigation, 3D-sono-assisted-thrombolysis outperformed 3D-sonothrombolysis in increasing microvascular flow and function within the area at risk following acute LAD thrombotic occlusion and half-dose r-tPA-assisted epicardial coronary recanalization. Comparing 3D-sono-assisted-thrombolysis to 3D-sonothrombolysis, the IM/RM ratio was 0.51±0.14 vs. 0.69±0.28, P<0.05; the ST-segment elevation decrease was 1.40±1.80 vs. 4.84±2.84 mm (P=0.014).
More insights into the underlying mechanics
According to Belcik et al. (28,29), limb perfusion is enhanced by blocking endothelial nitric oxide synthase (eNOS) and by adenosine triphosphate (ATP) and purinergic signaling in mice with chronic ischemia. A previous study performed by our team also confirmed that sonothrombolysis had a transient effect on microcirculatory perfusion in acute ischemic tissues from rats, with the eNOS/nitric oxide signaling pathway being the primary mediator (30). However, additional studies are needed to determine the exact nature of the other underlying mechanisms.
The benefits of 3D US
In this study, the guided 3D high MI impulses came from a clinical diagnostic US system, which is more accurate, efficient, and convenient than conventional 2D US. Three-dimensional diagnostic probes can scan tissue in 3D without manual movement because the probes can be moved mechanically, and the 3D images are then assembled (31). Therefore, the high MI impulses from the 3D probe can be administered to the whole at-risk area without probe manipulation. Compared with 2D US, 3D US overcomes the drawbacks of low spatial resolution, restricted coverage, and continuous sliding of the probe during operation. Furthermore, 3D cavitation exposure has been found to improve the microcirculation in muscles and the left ventricular cavity. A significant number of vasodilators in the right upper coronary arteries can be released during this exposure to further promote enhanced blood flow and increase the amount of blood accessible for circulation (32).
Studies have also shown that the increase in blood lipid levels in patients with STEMI is associated with increased levels of endogenous fibrinolytic activator (33,34). The effect of 3D US combined with MB-guided inertial cavitation to dissolve the thrombus may increase the adhesion of the plasminogen activator to the thrombus.
Thus, 3D diagnostic US combined with inertial cavitation guided by intravenous drip MBs can directly improve the infarct-related recanalization rate and enhance microcirculation in pigs with acute STEMI. Three-dimensional sono-assisted-thrombolysis is a novel, safe, and effective technique for treating cardiovascular thromboembolic disease.
Limitations of the study
Firstly, due to the small sample size, the recanalization rate was higher in the 3D-sono-assisted-thrombolysis group than in the 3D/TUS + MB and r-tPA groups but did not reach statistical significance. Therefore, future studies with larger sample sizes are needed. Also, further exploration of the optimal pulsing interval and the optimal overall dimensions of the 3D transducer is needed to achieve optimal 3D exposure regardless of whether cavitation is used to increase flow or to enhance thrombolysis (32). Finally, when sonothrombolysis is applied, the optimal strategy (2D or 3D) will depend on additional mechanistic insights, such as whether thrombolysis should target coronary or distal microcirculatory recanalization (35,36).
Prognosis and future directions
Over the long term, research has found that using 3D high MI impulses and injectable MB effectively decreases microvascular obstruction, lowers the IM/RM, improves microcirculation, and delays the unfavorable remodeling that leads to reduced left ventricular ejection fraction and the development of congestive heart failure (22). Therefore, this safe and efficient therapy may prevent severe problems in patients with STEMI and reduce the enormous long-term expense related to these complications (7).
It is possible to deliver contrast US to patients with STEMI at the point of care because it is portable, widely accessible, and non-invasive. Sonothrombolysis is more likely to succeed if it is delivered immediately rather than later. It is therefore essential to apply unique treatment options for STEMI and to treat or prevent microvascular damage as soon as feasible after diagnosis. Patients may benefit from more successful treatment when 3D-sono-assisted-thrombolysis is performed at the initial point of interaction between the physician and patient.
Intra-ambulance sonothrombolysis for STEMI has shown promising results in the first clinical trial (23), and the delivery of 3D-sono-assisted-thrombolysis in the ambulance may provide a convenient means of achieving rapid reperfusion while also treating microvascular injury and preventing systemic delays in the event of STEMI. The clinical feasibility and efficacy of 3D-sono-assisted-thrombolysis in the treatment of STEMI in the clinical context need to be studied further. In the future, with the help of image software such as ImageJ image software (version 1.51, open source, Bethesda, Maryland, USA), the efficacy of 3D-sono-assisted-thrombolysis can be better quantitatively assessed (37).
Conclusions
Three-dimensional diagnostic US combined with inertial cavitation guided by intravenous drip MB can directly improve the infarct-related recanalization rate and improve microcirculation in acute porcine STEMI. In addition, 3D-assisted sono-thrombolysis can improve the thrombolytic effect of r-tPA on acute STEMI in pigs, enhance the improvement of microcirculation by r-tPA, and reduce the r-tPA dosage.
Acknowledgments
Funding: This study was supported by grants from the Guangdong Natural Science Foundation for Distinguished Young Scholars (No. 2016A030306028), the Guangzhou Science and Technology Program (No. 201506010021), and the Foundation of the President of Nanfang Hospital (No. 2020Z006 and No. 2018Z018).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-21-1247/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-21-1247/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský PESC Scientific Document Group. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018;39:119-77. [Crossref] [PubMed]
- Reimer KA, Lowe JE, Rasmussen MM, Jennings RB. The wavefront phenomenon of ischemic cell death. 1. Myocardial infarct size vs duration of coronary occlusion in dogs. Circulation 1977;56:786-94. [Crossref] [PubMed]
- Reimer KA, Jennings RB. The "wavefront phenomenon" of myocardial ischemic cell death. II. Transmural progression of necrosis within the framework of ischemic bed size (myocardium at risk) and collateral flow. Lab Invest 1979;40:633-44. [PubMed]
- Vos NS, Amoroso G, Grundeken MJ, Ijsselmuiden AJ, van Geuns RJ, Spaargaren R, Tijssen JG, Koch KT. Pre-hospital management, procedural performance and outcomes for primary percutaneous coronary intervention in ST-elevation myocardial infarction in the Netherlands: Insights from the Dutch cohort of the APPOSITION-III trial. Neth Heart J 2016;24:730-9. [Crossref] [PubMed]
- van Kranenburg M, Magro M, Thiele H, de Waha S, Eitel I, Cochet A, Cottin Y, Atar D, Buser P, Wu E, Lee D, Bodi V, Klug G, Metzler B, Delewi R, Bernhardt P, Rottbauer W, Boersma E, Zijlstra F, van Geuns RJ. Prognostic value of microvascular obstruction and infarct size, as measured by CMR in STEMI patients. JACC Cardiovasc Imaging 2014;7:930-9. [Crossref] [PubMed]
- Slikkerveer J, Juffermans LJ, van Royen N, Appelman Y, Porter TR, Kamp O. Therapeutic application of contrast ultrasound in ST elevation myocardial infarction: Role in coronary thrombosis and microvascular obstruction. Eur Heart J Acute Cardiovasc Care 2019;8:45-53. [Crossref] [PubMed]
- Xie F, Slikkerveer J, Gao S, Lof J, Kamp O, Unger E, Radio S, Matsunaga T, Porter TR. Coronary and microvascular thrombolysis with guided diagnostic ultrasound and microbubbles in acute ST segment elevation myocardial infarction. J Am Soc Echocardiogr 2011;24:1400-8. [Crossref] [PubMed]
- Wu J, Leong-Poi H, Bin J, Yang L, Liao Y, Liu Y, Cai J, Xie J, Liu Y. Efficacy of contrast-enhanced US and magnetic microbubbles targeted to vascular cell adhesion molecule-1 for molecular imaging of atherosclerosis. Radiology 2011;260:463-71. [Crossref] [PubMed]
- Xie F, Everbach EC, Gao S, Drvol LK, Shi WT, Vignon F, Powers JE, Lof J, Porter TR. Effects of attenuation and thrombus age on the success of ultrasound and microbubble-mediated thrombus dissolution. Ultrasound Med Biol 2011;37:280-8. [Crossref] [PubMed]
- Bagot CN, Arya R. Virchow and his triad: a question of attribution. Br J Haematol 2008;143:180-90. [Crossref] [PubMed]
- Ganz W. The thrombolysis in myocardial infarction (TIMI) trial. N Engl J Med 1985;313:1018. [Crossref] [PubMed]
- Wei K. Assessment of myocardial blood flow and volume using myocardial contrast echocardiography. Echocardiography 2002;19:409-16. [Crossref] [PubMed]
- Wei K, Jayaweera AR, Firoozan S, Linka A, Skyba DM, Kaul S. Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a constant venous infusion. Circulation 1998;97:473-83. [Crossref] [PubMed]
- McAlindon E, Pufulete M, Harris J, Lawton C, Johnson T, Strange J, Baumbach A, Bucciarelli-Ducci C. Microvascular dysfunction determines infarct characteristics in patients with reperfused ST-segment elevation myocardial infarction: The MICROcirculation in Acute Myocardial Infarction (MICRO-AMI) study. PLoS One 2018;13:e0203750. [Crossref] [PubMed]
- Bello D, Einhorn A, Kaushal R, Kenchaiah S, Raney A, Fieno D, Narula J, Goldberger J, Shivkumar K, Subacius H, Kadish A. Cardiac magnetic resonance imaging: infarct size is an independent predictor of mortality in patients with coronary artery disease. Magn Reson Imaging 2011;29:50-6. [Crossref] [PubMed]
- Eitel I, Desch S, Fuernau G, Hildebrand L, Gutberlet M, Schuler G, Thiele H. Prognostic significance and determinants of myocardial salvage assessed by cardiovascular magnetic resonance in acute reperfused myocardial infarction. J Am Coll Cardiol 2010;55:2470-9. [Crossref] [PubMed]
- Cochet AA, Lorgis L, Lalande A, Zeller M, Beer JC, Walker PM, Touzery C, Wolf JE, Brunotte F, Cottin Y. Major prognostic impact of persistent microvascular obstruction as assessed by contrast-enhanced cardiac magnetic resonance in reperfused acute myocardial infarction. Eur Radiol 2009;19:2117-26. [Crossref] [PubMed]
- Niccoli G, Burzotta F, Galiuto L, Crea F. Myocardial no-reflow in humans. J Am Coll Cardiol 2009;54:281-92. [Crossref] [PubMed]
- de Waha S, Desch S, Eitel I, Fuernau G, Zachrau J, Leuschner A, Gutberlet M, Schuler G, Thiele H. Impact of early vs. late microvascular obstruction assessed by magnetic resonance imaging on long-term outcome after ST-elevation myocardial infarction: a comparison with traditional prognostic markers. Eur Heart J 2010;31:2660-8. [Crossref] [PubMed]
- Ogasawara S, Mukawa H, Sone T, Tsuboi H, Morishima I, Uesugi M, Matsushita E, Morita Y, Okumura K, Murohara T. Presence of myocardial hypoenhancement on multidetector computed tomography after primary percutaneous coronary intervention in acute myocardial infarction predicts poor prognosis. Int J Cardiol 2015;184:101-7. [Crossref] [PubMed]
- Padro T, Manfrini O, Bugiardini R, Canty J, Cenko E, De Luca G, Duncker DJ, Eringa EC, Koller A, Tousoulis D, Trifunovic D, Vavlukis M, de Wit C, Badimon L. ESC Working Group on Coronary Pathophysiology and Microcirculation position paper on 'coronary microvascular dysfunction in cardiovascular disease'. Cardiovasc Res 2020;116:741-55. [Crossref] [PubMed]
- Aguiar MOD, Tavares BG, Tsutsui JM, Fava AM, Borges BC, Oliveira MT Jr, Soeiro A, Nicolau JC, Ribeiro HB, Chiang HP, Sbano JCN, Goldsweig A, Rochitte CE, Lopes BBC, Ramirez JAF, Kalil Filho R, Porter TR, Mathias W Jr. Sonothrombolysis Improves Myocardial Dynamics and Microvascular Obstruction Preventing Left Ventricular Remodeling in Patients With ST Elevation Myocardial Infarction. Circ Cardiovasc Imaging 2020;13:e009536. [Crossref] [PubMed]
- El Kadi S, Porter TR, van Rossum AC, Kamp O. Sonothrombolysis in the ambulance for ST-elevation myocardial infarction: rationale and protocol. Neth Heart J 2021;29:330-7. [Crossref] [PubMed]
- Chen X, Leeman JE, Wang J, Pacella JJ, Villanueva FS. New insights into mechanisms of sonothrombolysis using ultra-high-speed imaging. Ultrasound Med Biol 2014;40:258-62. [Crossref] [PubMed]
- Marmottant P, Hilgenfeldt S. Controlled vesicle deformation and lysis by single oscillating bubbles. Nature 2003;423:153-6. [Crossref] [PubMed]
- Porter TR. The utilization of ultrasound and microbubbles for therapy in acute coronary syndromes. Cardiovasc Res 2009;83:636-42. [Crossref] [PubMed]
- Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M, Hochman JS, Krumholz HM, Kushner FG, Lamas GA, Mullany CJ, Ornato JP, Pearle DL, Sloan MA, Smith SC JrCanadian Cardiovascular Society. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction--executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1999 guidelines for the management of patients with acute myocardial infarction). J Am Coll Cardiol 2004;44:671-719. [Crossref] [PubMed]
- Belcik JT, Mott BH, Xie A, Zhao Y, Kim S, Lindner NJ, Ammi A, Linden JM, Lindner JR. Augmentation of limb perfusion and reversal of tissue ischemia produced by ultrasound-mediated microbubble cavitation. Circ Cardiovasc Imaging 2015;8:e002979. [Crossref] [PubMed]
- Belcik JT, Davidson BP, Xie A, Wu MD, Yadava M, Qi Y, Liang S, Chon CR, Ammi AY, Field J, Harmann L, Chilian WM, Linden J, Lindner JR. Augmentation of Muscle Blood Flow by Ultrasound Cavitation Is Mediated by ATP and Purinergic Signaling. Circulation 2017;135:1240-52. [Crossref] [PubMed]
- Qiu S, Li D, Wang Y, Xiu J, Lyu C, Kutty S, Zha D, Wu J. Ultrasound-Mediated Microbubble Cavitation Transiently Reverses Acute Hindlimb Tissue Ischemia through Augmentation of Microcirculation Perfusion via the eNOS/NO Pathway. Ultrasound Med Biol 2021;47:1014-23. [Crossref] [PubMed]
- Acar P, Battle L, Dulac Y, Peyre M, Dubourdieu H, Hascoet S, Groussolles M, Vayssière C. Real-time three-dimensional foetal echocardiography using a new transabdominal xMATRIX array transducer. Arch Cardiovasc Dis 2014;107:4-9. [Crossref] [PubMed]
- Muller MA, Belcik T, Hodovan J, Ozawa K, Brown E, Powers J, Sheeran PS, Lindner JR. Augmentation of Tissue Perfusion with Contrast Ultrasound: Influence of Three-Dimensional Beam Geometry and Conducted Vasodilation. J Am Soc Echocardiogr 2021;34:887-95. [Crossref] [PubMed]
- Porter TR, LeVeen RF, Fox R, Kricsfeld A, Xie F. Thrombolytic enhancement with perfluorocarbon-exposed sonicated dextrose albumin microbubbles. Am Heart J 1996;132:964-8. [Crossref] [PubMed]
- Tsutsui JM, Xie F, Johanning J, Lof J, Cory B, He A, Thomas L, Matsunaga T, Unger E, Porter TR. Treatment of deeply located acute intravascular thrombi with therapeutic ultrasound guided by diagnostic ultrasound and intravenous microbubbles. J Ultrasound Med 2006;25:1161-8. [Crossref] [PubMed]
- Mathias W Jr, Tsutsui JM, Tavares BG, Fava AM, Aguiar MOD, Borges BC, Oliveira MT Jr, Soeiro A, Nicolau JC, Ribeiro HB, Chiang HP, Sbano JCN, Morad A, Goldsweig A, Rochitte CE, Lopes BBC, Ramirez JAF, Kalil Filho R, Porter TR. MRUSMI Investigators. Sonothrombolysis in ST-Segment Elevation Myocardial Infarction Treated With Primary Percutaneous Coronary Intervention. J Am Coll Cardiol 2019;73:2832-42. [Crossref] [PubMed]
- Lindner JR. Therapeutic Contrast Echocardiography: Bubbles Become Medicine. J Am Coll Cardiol 2019;73:2843-5. [Crossref] [PubMed]
- Kratzer W, Güthle M, Dobler F, Seufferlein T, Graeter T, Schmidberger J, Barth TF, Klaus J. Comparison of superb microvascular imaging (SMI) quantified with ImageJ to quantified contrast-enhanced ultrasound (qCEUS) in liver metastases-a pilot study. Quant Imaging Med Surg 2022;12:1762-74. [Crossref] [PubMed]


