
Retinal microvasculature alteration in patients with systemic sclerosis and chloroquine treatment
Introduction
Systemic sclerosis (SSc) is an immune-mediated chronic multisystem connective tissue disease with extensive tissue fibrosis and microangiopathy (1,2). Vascular involvement is a prominent feature of SSc, and can be seen in fundus microvessels (MIR) in addition to the peripheral microcirculation of the skin. Retinal vascular abnormality is a very important part of ocular SSc (3,4). In addition, although SSc is a rare disease, it has a higher mortality rate than any other rheumatic disease (5). Monitoring of retinal microvascular changes will help to gauge SSc progression and implement effective treatment strategies in a timely fashion. Optical coherence tomography angiography (OCTA) is a good choice to generate real-time, slab-segmented and high-quality angiographic images in a non-invasive setting to evaluate the vascular network of retina and choroid from a functional and dynamic point of view.
Chloroquine and hydroxychloroquine are antimalarial drugs, but they have immunosuppressive effects and can be used to treat systemic lupus erythematosus, rheumatoid arthritis and other rheumatic diseases (6-8). As a derivative of chloroquine, hydroxychloroquine is more widely used in the clinical treatment of SSc. It regulates the immune response by inhibiting Toll-like receptors in dendritic cells, inhibiting extracellular oxidants in neutrophils, interfering with T cell signal transduction and affecting lysosome function (9-12). However, long-term use of chloroquine and hydroxychloroquine can cause retinal toxicity, which in turn leads to irreversible visual impairment (13). Among patients who took hydroxychloroquine for more than 5 years, the incidence of retinal toxicity was 7.5%, increasing to nearly 20% after 20 years of treatment (14,15).
OCTA can detect both superficial and deep retinal vessels, aiding observation of abnormal retinal vascular changes (16). Compared with fundus fluorescein angiography, OCTA is not only noninvasive, but also has higher resolution and takes less time, so is advantageous as a means to observe and study fundus changes in patients (17). Previous studies have used OCTA to quantitatively study retinal MIR in patients with SSc and those on long-term chloroquine treatment (18,19). The purpose of our study is to evaluate changes in retinal MIR density in patients with SSc and the additional effects of chloroquine on these changes. We present the following article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-21-1166/rc).
Methods
Research subjects
In this cross-sectional study, 30 patients with SSc and 15 healthy controls (30 eyes) were recruited from the First Affiliated Hospital of Nanchang University. Patients diagnosed with SSc were recruited from the Outpatient Department of Rheumatism Immunology; and healthy normal subjects were recruited from the Ocular Disease Clinical Research Center. Among the SSc patients, 15 (30 eyes) did not take chloroquine and 15 (30 eyes) had a history of chloroquine treatment. The healthy control group consisted of individuals without ocular or systemic disease. An ophthalmologist from the First Affiliated Hospital of Nanchang University evaluated the absence of abnormalities in the eyes of these subjects through clinical examination and OCTA imaging between May and September 2020.
Recruitment criteria
Participants with SSc all met the following criteria: 2013 classification criteria for systemic sclerosis: an American College of Rheumatology (ACR)/European League against Rheumatism collaborative initiative (20). All patients with SSc had cutaneous involvement and were in the indurative stage.
Patients in the SSc group did not take chloroquine, while patients in the chloroquine group had SSc and had been treated with chloroquine for more than 7 years. The mean dose of chloroquine was 400 mg/day (200 mg bid). The control group was comprised of 15 healthy subjects with no ocular abnormalities evaluated by clinical examination and OCTA imaging at the ophthalmology division of the medical center. All subjects had blood pressure measurements and OCTA imaging performed by the same examiner in the same place after sitting for 10 minutes. In addition, all patients underwent ophthalmic evaluation and clinical examination, including binocular vision, intraocular pressure, C-reactive protein, erythrocyte sedimentation rate, antibodies, injury index, blood pressure, disease course, and Hospital Anxiety and Depression Scale (21). Table 1 summarizes the patients’ demographic and clinical data. We used the Systemic Lupus Erythematosus Disease Activity Index-2000 (SLEDAI-2K) to assess disease activity. A score of 0–4 means basically no activity, a score of 5–9 means light activity, a score of 10–14 means moderate activity, and ≥15 means heavy activity. The Systemic Lupus Erythematosus International Collaborating Clinics (SLICC)/ACR Injury Index (SDI) can be used to evaluate organ damage.
Table 1
| Parameters | Control group (n=15) | SSc group (n=15) | Chloroquine group (n=15) | P value |
|---|---|---|---|---|
| Age (years) | ||||
| Mean ± SD | 39.67±6.13 | 40.27±9.48 | 44.47±3.04 | 0.118a |
| Range | 28–48 | 26–57 | 38–48 | |
| Gender (male/female), n | 6/9 | 6/9 | 6/9 | 1.000b |
| Log MAR | 0.07±0.04 | 0.14±0.13 | 0.21±0.15 | 0.008a* |
| Average IOP (mmHg) | 15.47±1.03 | 15.37±1.32 | 15.70±1.33 | 0.751a |
| ESR (mm) | 3.80±1.21 | 14.67±4.59 | 11.13±2.62 | <0.001a* |
| CRP (10 mg/L) | 1.36±0.71 | 3.13±2.23 | 1.68±0.59 | 0.002a* |
| ANA+ | 1/15 | 15/15 | 15/15 | <0.001b* |
| APA+ | 1/15 | 1/15 | 1/15 | 1.000b |
| SLEDAI-2K | 0 | 3.73±1.67 | 2.33±1.68 | <0.001a* |
| SDI | 0 | 0.47±0.52 | 0.20±0.41 | 0.007a* |
| Blood pressure | ||||
| SBP (mmHg) | 125.73±16.49 | 129.20±15.53 | 126.33±16.33 | 0.821a |
| DBP (mmHg) | 82.00±7.46 | 81.87±10.19 | 75.53±11.70 | 0.138a |
| Disease duration (years) | N/A | 8.60±2.41 | 9.80±1.82 | 0.136c |
| Chloroquine treatment time (years) | N/A | N/A | 8.20±1.66 | |
| HADS | 2.40±1.06 | 9.53±3.04 | 10.13±2.62 | <0.001a* |
*, P<0.05; a, F test; b, Pearson chi-square; c, t-test. SD, standard deviation; SSc, systemic sclerosis; log MAR, logarithm of the minimum angle of resolution; IOP, intraocular pressure; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; ANA, antinuclear antibody; APA, antiphospholipid antibody; SLEDAI-2K, Systemic Lupus Erythematosus Disease Activity Index-2000; SDI, Systemic Lupus Erythematosus International Collaborating Clinics/American College of Rheumatology Injury Index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HADS, Hospital Anxiety and Depression Scale; N/A, not available.
Exclusion conditions
Patients with any of the following conditions were excluded: (I) Ophthalmic surgery performed in the preceding 6 months, such as vitrectomy and laser photocoagulation; (II) glaucoma, severe cataract, nystagmus, significant media opacity, poor eye fixation, refractive spherical and cylindrical error ≥2 diopters that could possibly confound the observation and patients with other retinal abnormalities on fundus images, blue autofluorescence and optical coherence tomography (OCT); (III) systemic autoimmune diseases such as systemic lupus erythematosus; (IV) circulatory diseases with ocular effects, such as diabetes; (V) cardiovascular diseases, such as ischemic heart disease and vascular disease; (VI) contraindications for pupil dilation or intolerance to topical anesthetic or mydriasis; (VII) use of other drugs that may damage the retina, such as tamoxifen; (VIII) pregnancy or lactation; or (IX) long-term smokers or alcoholics.
Ethical considerations
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the First Affiliated Hospital of Nanchang University and informed consent was taken from all the participants.
OCTA
The RTVue Avanti XR system (Optovue, Fremont, CA, USA) was used for angiography, and all examinations were performed by the same physician, performing at the same time interval (2–4 p.m.) of the day. The retinal cross sections and microvasculature were displayed simultaneously. OCTA parameters were as follows: scanning speed 70,000 A-scans/s, bandwidth 45 nm, central wavelength 840 nm, horizontal resolution 22 µm, and axial resolution 5 mm. Five angiographs were performed for a total of 216 A-scans along the X-axis. Each scan focused on the foveal center, located at 216 raster positions along the Y-axis, and the acquisition time was 3.9 s. En-face OCTA images of 3 mm × 3 mm were obtained through four volumetric scans of two vertical and two horizontal scans (933,120 A-scans). B scans (216 y-positions × 5 positions; 1,080 in total) were captured at 270 frames per second. Finally, the 3 mm × 3 mm en-face OCTA images of each eye were calculated (22-24).
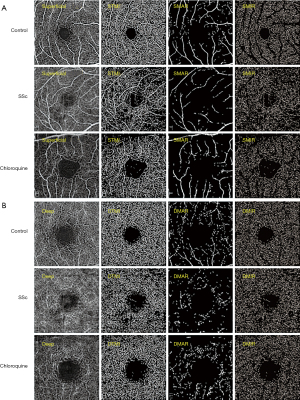
The retinal capillary network was artificially divided into two different physiological layers: the superficial retinal layer (SRL, internal limiting membrane to anterior boundary of ganglion cell layer) (Figure 1A) and the deep retinal layer (DRL, inner boundary of the inner plexiform layer to outer boundary of the outer plexiform layer) (Figure 1B). In the two layers, macrovessels (MAR), MIR and the total microvessels (TMI) were each analyzed. Blood vessel density was defined as the ratio of perfusion vessel area to measured area. All subjects used the right (R) eye first. Data from the left (L) eye were flipped to obtain a mirror image of the R eye. L and R eye data were averaged and analyzed together. Macular retinal images were segmented using two methods: (I) hemispheric segmentation, which divides the image into diagonal quadrants, superior right (SR), superior left (SL), inferior left (IL) and inferior right (IR); (II) early treatment of diabetic retinopathy study (ETDRS) method, in which images were divided into 4 quadrants by the diagonal of the 2 quadrants, followed by superior (S), inferior (I), R and L. The corresponding vascular densities of the control, SSc and chloroquine groups were each calculated, and data from the SSc group were compared with those of the control group, and with those of the chloroquine group.
Statistical analysis
The data were statistically analyzed using GraphPad Prism 8 (GraphPad Software, La Jolla, CA, USA) and SPSS 22.0 (IBM, Armonk, NY, USA) and expressed as mean ± standard deviation (SD). Continuous data were analyzed by one-way analysis of variance (ANOVA), and then a pairwise comparison was made between groups using a post-hoc comparison test. P values less than 0.05 were considered statistically significant. Categorical data including sex, antinuclear antibody and antiphospholipid antibody were compared using chi-square test, while the disease duration was compared between the SSc and chloroquine groups using an independent-samples t-test. The prognostic values of the MIR densities of the three groups were compared using the receiver operating characteristic (ROC) curve.
Results
Clinical and demographic characteristics of participants
Figure 2 summarizes the recruitment of participants. There were 15 participants (30 eyes) in each group. In addition to their demographic characteristics (including age, gender, blood pressure and disease duration), their clinical parameters related to SSc and chloroquine use (including visual acuity, erythrocyte sedimentation rate, C-reactive protein, antinuclear antibodies SLEDAI-2K, SLICC/ACR Injury Index and Hospital Anxiety and Depression Scale) were recorded. Among them, age, intraocular pressure, antiphospholipid antibody, blood pressure and disease duration were statistically similar between groups, but other parameters were statistically significantly different. The specific data are shown in Table 1 and results of the post-hoc comparison test are shown in Table 2.
Table 2
| Parameters | P value of intergroup comparison | ||
|---|---|---|---|
| Control group vs. SSc group | Control group vs. chloroquine group | SSc group vs. chloroquine group | |
| Log MAR | 0.163 | 0.010* | 0.443 |
| ESR (mm) | <0.001* | <0.001* | 0.049* |
| CRP (10 mg/L) | 0.027* | 0.466 | 0.077 |
| SLEDAI-2K | <0.001* | 0.001* | 0.086 |
| SDI | 0.011* | 0.227 | 0.342 |
| HADS | <0.001* | <0.001* | 0.919 |
*, P<0.05. SSc, systemic sclerosis; log MAR, logarithm of the minimum angle of resolution; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; SLEDAI-2K, Systemic Lupus Erythematosus Disease Activity Index-2000; SDI, Systemic Lupus Erythematosus International Collaborating Clinics/American College of Rheumatology Injury Index; HADS, Hospital Anxiety and Depression Scale.
Comparison of superficial vessel density at different retinal locations among the three groups
In the superficial macular region, MIR, TMI and MAR differed significantly between the three groups (P<0.05, Table 3). Post-hoc comparisons (Table 4), showed significant differences in MIR and TMI between all groups, and in MAR between SSc and chloroquine and between control and chloroquine but not between the control and SSc groups. The hemispheric segmentation method showed lower superficial density of retinal capillaries in SSc than in controls in all areas except IR (P<0.05). The ETDRS method showed that retinal superficial capillary density in all retinal regions was significantly lower than in controls (P<0.05). Similarly, using the hemispheric segmentation method, we found that retinal superficial capillary density in the IL and IR superficial regions were significantly lower in the chloroquine group than in the SSc group (P<0.05). The ETDRS method showed significantly lower retinal superficial capillary density in the I and R regions in the chloroquine group than the SSc group (P<0.05). The comparisons between groups using different segmentation methods are shown in Figure 3.
Table 3
| Location | Control group (n=15, 30 eyes) | SSc group (n=15, 30 eyes) | Chloroquine group (n=15, 30 eyes) | P value |
|---|---|---|---|---|
| TMI | 1.78±0.29 | 1.70±0.47 | 1.62±0.47 | <0.001* |
| MIR | 1.87±0.23 | 1.70±0.12 | 1.60±0.13 | <0.001* |
| MAR | 1.11±0.04 | 1.10±0.03 | 1.04±0.06 | <0.001* |
| SR | 1.68±0.02 | 1.48±0.17 | 1.44±0.14 | <0.001* |
| SL | 1.67±0.03 | 1.54±0.15 | 1.46±0.19 | <0.001* |
| IL | 1.69±0.02 | 1.61±0.05 | 1.54±0.08 | <0.001* |
| IR | 1.66±0.04 | 1.62±0.07 | 1.45±0.09 | <0.001* |
| S | 1.68±0.02 | 1.52±0.15 | 1.45±0.14 | <0.001* |
| I | 1.67±0.02 | 1.64±0.03 | 1.47±0.09 | <0.001* |
| R | 1.72±0.04 | 1.67±0.09 | 1.51±0.11 | <0.001* |
| L | 1.66±0.03 | 1.56±0.06 | 1.54±0.13 | <0.001* |
*, P<0.05. SSc, systemic sclerosis; TMI, total microvascular; MIR, microvascular; MAR, macrovascular; SR, superior right; SL, superior left; IL, inferior left; IR, inferior right; S, superior; I, inferior; R, right; L, left.
Table 4
| Location | P value of intergroup comparison | ||
|---|---|---|---|
| Control group vs. SSc group | Control group vs. chloroquine group | SSc group vs. chloroquine group | |
| TMI | <0.001* | <0.001* | <0.001* |
| MIR | <0.001* | <0.001* | 0.007* |
| MAR | 0.316 | <0.001* | 0.001* |
| SR | <0.001* | <0.001* | 0.768 |
| SL | <0.001* | <0.001* | 0.268 |
| IL | <0.001* | <0.001* | 0.001* |
| IR | 0.056 | <0.001* | <0.001* |
| S | <0.001* | <0.001* | 0.179 |
| I | 0.007* | <0.001* | <0.001* |
| R | 0.009* | <0.001* | <0.001* |
| L | <0.001* | <0.001* | 0.815 |
*, P<0.05. SSc, systemic sclerosis; TMI, total microvascular; MIR, microvascular; MAR, macrovascular; SR, superior right; SL, superior left; IL, inferior left; IR, inferior right; S, superior; I, inferior; R, right; L, left.
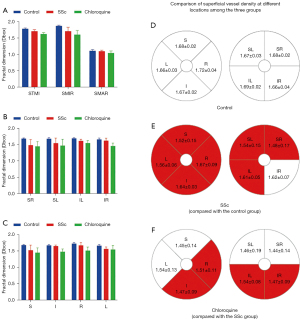
Comparison of deep vessel density in different retinal regions between the three groups
Results of deep macular retinal vascular density analysis in each retinal zone are shown in Table 5. Significant changes in superficial MIR and TMI (P<0.05) but not MAR (P>0.05) were found between the three groups. Post-hoc tests (Table 6) showed significant differences in deep TMI and MIR between the groups. Using the hemispheric segmentation method, capillary density in the deep layer of retina (except the IR area) in patients with SSc was significantly lower than that in healthy controls (P<0.05). Using the ETDRS method, the retinal deep capillary density in S and L regions were significantly lower in SSc than in controls (P<0.05). Similarly, using the hemispheric segmentation method, capillary density in the IL region of the superficial retinal vessels was significantly lower in the chloroquine group than in the SSc group (P<0.05). Using the ETDRS method, the retinal deep capillary density in the I and R regions were significantly lower in the chloroquine group than in the SSc group (P<0.05). Comparisons between groups under different segmentation methods are shown in Figure 4.
Table 5
| Location | Control group (n=15, 30 eyes) | SSc group (n=15, 30 eyes) | Chloroquine group (n=15, 30 eyes) | P value |
|---|---|---|---|---|
| TMI | 1.82±0.05 | 1.73±0.06 | 1.56±0.14 | <0.001* |
| MIR | 1.68±0.06 | 1.57±0.04 | 1.41±0.10 | <0.001* |
| MAR | 1.01±0.07 | 1.00±0.09 | 0.97±0.07 | 0.125 |
| SR | 1.62±0.05 | 1.52±0.11 | 1.44±0.15 | <0.001* |
| SL | 1.60±0.06 | 1.44±0.14 | 1.37±0.19 | <0.001* |
| IL | 1.63±0.04 | 1.58±0.05 | 1.51±0.09 | <0.001* |
| IR | 1.59±0.08 | 1.56±0.05 | 1.50±0.15 | 0.002* |
| S | 1.60±0.04 | 1.44±0.14 | 1.36±0.18 | <0.001* |
| I | 1.61±0.07 | 1.60±0.05 | 1.52±0.16 | 0.003* |
| R | 1.66±0.06 | 1.64±0.06 | 1.49±0.14 | <0.001* |
| L | 1.57±0.08 | 1.48±0.07 | 1.46±0.15 | <0.001* |
*, P<0.05. SSc, systemic sclerosis; TMI, total microvascular; MIR, microvascular; MAR, macrovascular; SR, superior right; SL, superior left; IL, inferior left; IR, inferior right; S, superior; I, inferior; R, right; L, left.
Table 6
| Location | P value of intergroup comparison | ||
|---|---|---|---|
| Control group vs. SSc group | Control group vs. chloroquine group | SSc group vs. chloroquine group | |
| TMI | <0.001* | <0.001* | <0.001* |
| MIR | <0.001* | <0.001* | 0.007* |
| SR | <0.001* | <0.001* | 0.086 |
| SL | <0.001* | <0.001* | 0.305 |
| IL | <0.001* | <0.001* | 0.002* |
| IR | 0.366 | 0.012* | 0.068 |
| S | <0.001* | <0.001* | 0.206 |
| I | 0.924 | 0.026* | 0.042* |
| R | 0.568 | <0.001* | <0.001* |
| L | <0.001* | 0.002* | 0.904 |
*, P<0.05. SSc, systemic sclerosis; TMI, total microvascular; MIR, microvascular; SR, superior right; SL, superior left; IL, inferior left; IR, inferior right; S, superior; I, inferior; R, right; L, left.
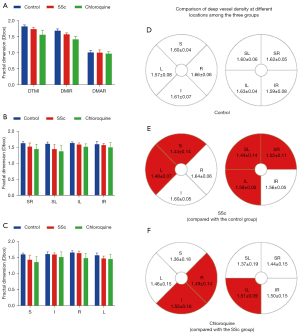
ROC curve analysis
OCTA showed high specificity and sensitivity in superficial retinal vascular density, indicating that this method can distinguish between the groups included here (Figure 5). In the superficial layer of the retina, the capillary density was significantly different between the control and SSc groups in the TMI (P<0.001), MIR (P<0.001), SR (P<0.001), SL (P<0.001), IL (P<0.001), S (P<0.001), I (P=0.003), R (P=0.02) and L (P<0.001) retinal areas. The highest area under the ROC curve was for superficial MIR, at 0.993 [95% confidence interval (CI): 0.980–1.000], indicating high accuracy of SSc diagnosis based on superficial MIR (Figure 5A). Significant differences in TMI (P<0.001), MIR (P<0.001), MAR (P<0.001), IL (P=0.001), IR (P<0.001), I (P<0.001) and R (P<0.001) superficial retinal regions were found between the SSc and chloroquine groups. Among them, the highest area under the ROC curve was for the superficial layer of the IR at 0.967 (95% CI: 0.929–1.000), indicating that that this region and layer offer accuracy in the diagnosis of retinal damage caused by chloroquine (Figure 5B).
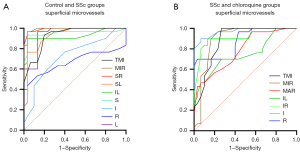
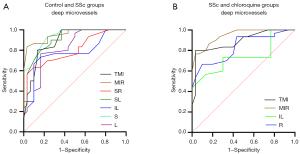
OCTA also shows high sensitivity and specificity for deep retinal capillary density, with the potential to distinguish patients with SSc patients from controls and from SSc patients on long-term chloroquine therapy (Figure 6). In the DRL, significant differences between SSc and controls were found in the TMI (P<0.001) and MIR (P<0.001) in the SR (P<0.001), SL (P<0.001), IL (P<0.001), S (P<0.001) and L (P<0.001) regions of the retina. The area under the ROC curve was highest for superficial MIR, at 0.954 (95% CI: 0.908–1.000), indicating that diagnosis of SSc based on superficial MIR has high accuracy (Figure 6A). Similarly, significant differences were found between the SSc and chloroquine groups in TMI (P<0.001) and MIR (P<0.001) in the IL (P=0.001) and R (P<0.001) regions. Among them, the area under the ROC curve was highest for superficial MIR, at 0.948 (95% CI: 0.900–0.997), indicating high accuracy in the diagnosis of retinal damage caused by chloroquine (Figure 6B).
Discussion
We used OCTA to investigate changes in retinal capillary density in patients with SSc with and without long-term chloroquine use. Compared with the healthy control group, capillary density in the deep and particularly the superficial macular regions in SSc patients were decreased. Long-term use of chloroquine in patients with SSc will further aggravate retinal damage and further reduce the retinal capillary density in the macular area, especially the superficial layer in the I region.
SSc is a complex disease involving interaction between vascular abnormality, abnormal activation of the immune system and tissue fibrosis. Vascular disease plays a central role in the pathogenesis of SSc, which is characterized by abnormal microcirculation and local tissue ischemia (25). Macular retinal capillaries are affected and previous study has quantitatively measured the thickness, density and perfusion of retinal and choroidal capillaries in patients with SSc using OCTA (26), and confirmed that retinal microvascular changes occur in early SSc before clinical evidence of retinopathy (27). In our study, hemispheric segmentation and ETDRS methods were used to identify regions for measurement of retinal capillary density in the superficial and deep macular areas. The results further verified a decrease in retinal capillary density caused by SSc. At the same time, it was found that the area of decreased vascular density was relatively concentrated in the S part. The mechanism by which retinal capillary changes occur in SSc patients has not been clearly described, but many studies have found that choroidal thickness is decreased in SSc patients without apparent ophthalmopathy (28-30), and there is a mutual regulation mechanism between retinal and choroidal blood circulation (31,32). The long-term course of disease may lead to interaction between them and reduce the overall blood vessel density.
Chloroquine and hydroxychloroquine are immunomodulatory antimalarial drugs that can be used to treat rheumatic diseases (8), but their long-term use may lead to retinal damage (14,33,34). According to the suggestion of Marmor et al. (15), the chloroquine group we recruited were SSc patients who had been treated with chloroquine for more than 7 years. Compared with the SSc group, capillary density was reduced in parts of the retina in the chloroquine group. The American Academy of Ophthalmology recommends annual screening after taking hydroxychloroquine for 5 years (15), but none of the screening tests are gold standard. Therefore, some people have used electrophysiological methods and OCTA to study and compare the retinal damage caused by chloroquine and to explore the appropriate method to evaluate the retinal damage caused by chloroquine (19). In this study, we focused on retinal capillary density of SSc patients with and without long-term chloroquine treatment using hemispheric segmentation and ETDRS methods of retinal region definition, and then further eliminated the influence of retinal damage caused by SSc itself on the experimental results. At the same time, it was found that the area of decreased vascular density was relatively concentrated in the I part. S versus I retinal differences in the progression of other retinal diseases have been reported in both humans (35) and mouse models (36). In many of these cases the more rapid progression of disease in I retina is attributed to the modifying effects of light.
It can be seen from Table 1 that chloroquine does have a therapeutic effect on SSc and can effectively reduce SDI. However, long-term use of chloroquine for SSc will lead to vision loss, and our research shows that there is an association with retinal capillary damage, which is likely to be irreversible, consistent with previous research results (37,38). As can be seen from Figures 5,6, macular capillary scanning and vascular density measurement with OCTA can effectively identify retinal damage in patients with SSc and further aggravation caused by chloroquine treatment. Rommel et al. confirmed that early impairment of retinal and choroidal microperfusion in patients with SSc could be found by OCTA analysis, and with the progression of the disease, the vascular damage of the two tissues showed a significant correlation (26). Therefore, regular OCTA tests in SSc patients with a long course of disease or treatment with chloroquine may allow early identification of anomaly, allowing changes to be made to avoid or minimize damage.
As a cross-sectional study with a small sample size, our study has some limitations. To quantitatively analyze the retinal damage in patients with different stages of SSc and patients with SSc treated with chloroquine for different periods, a longitudinal cohort study with a larger sample size should be conducted. In addition, comprehensive analysis should be combined with other diagnostic criteria of SSc to form a more complete monitoring system. OCT, electrophysiological and visual fields tests between subjects should be compared to explore whether vascular changes are associated with functional or other structural changes.
Acknowledgments
We gratefully thank the reviewers for their constructive comments.
Funding: This study was supported by the National Natural Science Foundation (No. 82160195), Central Government Guides Local Science and Technology Development Foundation (No. 20211ZDG02003), Key Research Foundation of Jiangxi Province (No. 20181BBG70004, 20203BBG73059), Excellent Talents Development Project of Jiangxi Province (No. 20192BCBL23020), Natural Science Foundation of Jiangxi Province (No. 20181BAB205034), Grassroots Health Appropriate Technology “Spark Promotion Plan” Project of Jiangxi Province (No. 20188003), Health Development Planning Commission Science Foundation of Jiangxi Province (Nos. 20201032 and 202130210), and Health Development Planning Commission Science TCM Foundation of Jiangxi Province (Nos. 2018A060 and 2020A0087).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-21-1166/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-21-1166/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the First Affiliated Hospital of Nanchang University and informed consent was taken from all the participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Denton CP, Khanna D. Systemic sclerosis. Lancet 2017;390:1685-99. [Crossref] [PubMed]
- Allanore Y, Simms R, Distler O, Trojanowska M, Pope J, Denton CP, Varga J. Systemic sclerosis. Nat Rev Dis Primers 2015;1:15002. [Crossref] [PubMed]
- Ushiyama O, Ushiyama K, Yamada T, Koarada S, Tada Y, Suzuki N, Ohta A, Nagasawa K. Retinal findings in systemic sclerosis: a comparison with nailfold capillaroscopic patterns. Ann Rheum Dis 2003;62:204-7. [Crossref] [PubMed]
- Waszczykowska A, Goś R, Waszczykowska E, Dziankowska-Bartkowiak B, Jurowski P. Prevalence of ocular manifestations in systemic sclerosis patients. Arch Med Sci 2013;9:1107-13. [Crossref] [PubMed]
- Tyndall AJ, Bannert B, Vonk M, Airò P, Cozzi F, Carreira PE, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis 2010;69:1809-15. [Crossref] [PubMed]
- Plantone D, Koudriavtseva T. Current and Future Use of Chloroquine and Hydroxychloroquine in Infectious, Immune, Neoplastic, and Neurological Diseases: A Mini-Review. Clin Drug Investig 2018;38:653-71. [Crossref] [PubMed]
- Nishiguchi M, Yamamoto Y, Jinnin M. Novel disease-modifying drugs against skin fibrosis of systemic sclerosis. Trends in Immunotherapy 2020;4:105-14. [Crossref]
- Rainsford KD, Parke AL, Clifford-Rashotte M, Kean WF. Therapy and pharmacological properties of hydroxychloroquine and chloroquine in treatment of systemic lupus erythematosus, rheumatoid arthritis and related diseases. Inflammopharmacology 2015;23:231-69. [Crossref] [PubMed]
- Costedoat-Chalumeau N, Dunogué B, Morel N, Le Guern V, Guettrot-Imbert G. Hydroxychloroquine: a multifaceted treatment in lupus. Presse Med 2014;43:e167-80. [Crossref] [PubMed]
- Jančinová V, Pažoureková S, Lucová M, Perečko T, Mihalová D, Bauerová K, Nosáľ R, Drábiková K. Selective inhibition of extracellular oxidants liberated from human neutrophils--A new mechanism potentially involved in the anti-inflammatory activity of hydroxychloroquine. Int Immunopharmacol 2015;28:175-81. [Crossref] [PubMed]
- Goldman FD, Gilman AL, Hollenback C, Kato RM, Premack BA, Rawlings DJ. Hydroxychloroquine inhibits calcium signals in T cells: a new mechanism to explain its immunomodulatory properties. Blood 2000;95:3460-6. [Crossref] [PubMed]
- van Loosdregt J, Spreafico R, Rossetti M, Prakken BJ, Lotz M, Albani S. Hydroxychloroquine preferentially induces apoptosis of CD45RO+ effector T cells by inhibiting autophagy: a possible mechanism for therapeutic modulation of T cells. J Allergy Clin Immunol 2013;131:1443-6.e1. [Crossref] [PubMed]
- Melles RB, Marmor MF. The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapy. JAMA Ophthalmol 2014;132:1453-60. [Crossref] [PubMed]
- Yusuf IH, Sharma S, Luqmani R, Downes SM. Hydroxychloroquine retinopathy. Eye (Lond) 2017;31:828-45. [Crossref] [PubMed]
- Marmor MF, Kellner U, Lai TY, Melles RB, Mieler WFAmerican Academy of Ophthalmology. Recommendations on Screening for Chloroquine and Hydroxychloroquine Retinopathy (2016 Revision). Ophthalmology 2016;123:1386-94. [Crossref] [PubMed]
- Savastano MC, Lumbroso B, Rispoli M. In vivo characterization of retinal vascularization morphology using optical coherence tomography angiography. Retina 2015;35:2196-203. [Crossref] [PubMed]
- Mo S, Krawitz B, Efstathiadis E, Geyman L, Weitz R, Chui TY, Carroll J, Dubra A, Rosen RB. Imaging Foveal Microvasculature: Optical Coherence Tomography Angiography Versus Adaptive Optics Scanning Light Ophthalmoscope Fluorescein Angiography. Invest Ophthalmol Vis Sci 2016;57:OCT130-40. [Crossref] [PubMed]
- Rothe M, Rommel F, Klapa S, Humrich JY, Nieberding R, Lange T, Sochurek JAM, Plöttner P, Grisanti S, Riemekasten G, Ranjbar M. Evaluation of retinal microvascular perfusion in systemic sclerosis: a case-control study. Ann Rheum Dis 2019;78:857-8. [Crossref] [PubMed]
- Akhlaghi M, Kianersi F, Radmehr H, Dehghani A, Naderi Beni A, Noorshargh P. Evaluation of optical coherence tomography angiography parameters in patients treated with Hydroxychloroquine. BMC Ophthalmol 2021;21:209. [Crossref] [PubMed]
- van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum 2013;65:2737-47. [Crossref] [PubMed]
- Yu Y, Feng L, Shao Y, Tu P, Wu HP, Ding X, Xiao WH. Quality of life and emotional change for middle-aged and elderly patients with diabetic retinopathy. Int J Ophthalmol 2013;6:71-4. [PubMed]
- Ye L, Zhou SS, Yang WL, Bao J, Jiang N, Min YL, Yuan Q, Tan G, Shen M, Shao Y. Retinal microvasculature alteration in active thyroid-associated ophthalmopathy. Endocr Pract 2018;24:658-67. [Crossref] [PubMed]
- Hormel TT, Huang D, Jia Y. Artifacts and artifact removal in optical coherence tomographic angiography. Quant Imaging Med Surg 2021;11:1120-33. [Crossref] [PubMed]
- Mehta N, Cheng Y, Alibhai AY, Duker JS, Wang RK, Waheed NK. Optical coherence tomography angiography distortion correction in widefield montage images. Quant Imaging Med Surg 2021;11:928-38. [Crossref] [PubMed]
- Hughes M, Herrick AL. Systemic sclerosis. Br J Hosp Med (Lond) 2019;80:530-6. [Crossref] [PubMed]
- Rommel F, Prangel D, Prasuhn M, Grisanti S, Ranjbar M. Correlation of retinal and choroidal microvascular impairment in systemic sclerosis. Orphanet J Rare Dis 2021;16:27. [Crossref] [PubMed]
- Kılınç Hekimsoy H, Şekeroğlu MA, Koçer AM, Akdoğan A. Analysis of retinal and choroidal microvasculature in systemic sclerosis: an optical coherence tomography angiography study. Eye (Lond) 2020;34:763-70. [Crossref] [PubMed]
- Ingegnoli F, Gualtierotti R, Pierro L, Del Turco C, Miserocchi E, Schioppo T, Meroni PLACUTE study group. Choroidal impairment and macular thinning in patients with systemic sclerosis: the acute study. Microvasc Res 2015;97:31-6. [Crossref] [PubMed]
- Coşkun E, Zengin O, Kenan S, Kimyon G, Erdogan Er K, Okumus S, Mesut Onat A, Erbagcı I, Kısacık B. Evaluation of choroidal thickness in patients with scleroderma. Eye (Lond) 2016;30:588-92. [Crossref] [PubMed]
- Esen E, Tas DA, Sizmaz S, Turk I, Unal I, Demircan N. Evaluating Choroidal Characteristics in Systemic Sclerosis Using Enhanced Depth Imaging Optical Coherence Tomography. Ocul Immunol Inflamm 2017;25:356-62. [Crossref] [PubMed]
- Luo X, Shen YM, Jiang MN, Lou XF, Shen Y. Ocular Blood Flow Autoregulation Mechanisms and Methods. J Ophthalmol 2015;2015:864871. [Crossref] [PubMed]
- Delaey C, Van De Voorde J. Regulatory mechanisms in the retinal and choroidal circulation. Ophthalmic Res 2000;32:249-56. [Crossref] [PubMed]
- Geamănu Pancă A, Popa-Cherecheanu A, Marinescu B, Geamănu CD, Voinea LM. Retinal toxicity associated with chronic exposure to hydroxychloroquine and its ocular screening. J Med Life 2014;7:322-6. Review. [PubMed]
- Jover JA, Leon L, Pato E, Loza E, Rosales Z, Matias MA, Mendez-Fernandez R, Díaz-Valle D, Benitez-Del-Castillo JM, Abasolo L. Long-term use of antimalarial drugs in rheumatic diseases. Clin Exp Rheumatol 2012;30:380-7. [PubMed]
- Greenstein VC, Lima de Carvalho JR Jr, Parmann R, Amaro-Quireza L, Lee W, Hood DC, Tsang SH, Sparrow JR. Quantitative Fundus Autofluorescence in HCQ Retinopathy. Invest Ophthalmol Vis Sci 2020;61:41. [Crossref] [PubMed]
- Zhao J, Ueda K, Riera M, Kim HJ, Sparrow JR. Bisretinoids mediate light sensitivity resulting in photoreceptor cell degeneration in mice lacking the receptor tyrosine kinase Mer. J Biol Chem 2018;293:19400-10. [Crossref] [PubMed]
- Ruamviboonsuk P, Lai TYY, Chang A, Lai CC, Mieler WF, Lam DSC. for Asia-Pacific Vitreo-Retina Society. Chloroquine and Hydroxychloroquine Retinal Toxicity Consideration in the Treatment of COVID-19. Asia Pac J Ophthalmol (Phila) 2020;9:85-7. [Crossref] [PubMed]
- Stokkermans TJ, Goyal A, Trichonas G. Chloroquine and Hydroxychloroquine Toxicity. In: StatPearls. Treasure Island (FL): StatPearls Publishing; April 21, 2022.



