The imaging of osteomyelitis
Introduction
Osteomyelitis is inflammation of the bone marrow secondary to infection, which can progress to osteonecrosis, bone destruction and septic arthritis. It is an important cause of permanent disability in both children and adults worldwide (1). Osteomyelitis has a bimodal age distribution with peak incidences in children under 5 and adults over 50 years of age (2). Two epidemiological studies conducted in the United States within the last decade have demonstrated an increase in the incidence and severity of acute osteomyelitis in children, linked to the increasing prevalence of methicillin-resistant Staphylococcus Aureus (MRSA) (3,4).
The typical clinical presentation of osteomyelitis with pain, erythema and oedema of the affected part is non-specific and can be caused by a multitude of other diseases (5). Poor feeding and irritability may be the only symptoms present in infants. Serum inflammatory markers may be normal, especially in neonates and patients with chronic osteomyelitis (6). For these reasons, imaging plays an integral role in establishing the diagnosis of osteomyelitis and characterising the extent of disease spread. The importance of imaging goes beyond making the initial diagnosis as radiologists are able to perform image-guided abscess aspirations and bone biopsies to direct further management, and follow-up scans are often required during the course of treatment to ensure resolution of infection (7).
This article provides an overview of the imaging of osteomyelitis, focusing on the correlation between radiological features and the underlying pathological processes. The pathogenesis of acute and chronic osteomyelitis will be described, together with a summary of key age-related differences in the patterns of disease. This is followed by a review of the imaging features of osteomyelitis on plain radiography, magnetic resonance imaging (MRI), nuclear medicine, computed tomography (CT) and ultrasound. There will be a particular emphasis on MRI because it is the imaging modality of choice for the investigation of suspected osteomyelitis in current evidence-based guidelines (8).
Pathogenesis
An understanding of the pathogenesis of osteomyelitis is essential for recognition and interpretation of its imaging findings. Osteomyelitis arises from infection with a variety of microorganisms via different mechanisms. The progression of disease from acute to chronic stages produces a constellation of pathological features that can vary according to the age of the patient.
Staphylococcus aureus is the causative organism in up to 80% of cases of osteomyelitis. Most of these cases involve community-acquired MRSA strains. MRSA infection is associated with an increased incidence of extra-osseous disease, greater number of surgical interventions and longer hospital stays (4). One postulated cause for the increased severity of osteomyelitis in these patients is the production of a toxin known as Panto-Valentine leukocidin (PVL) by MRSA strains (3).
Other common pathogens include Staphylococcus epidermidis and Enterobacter species. Certain organisms predominate in specific clinical settings, such as Salmonella species in sickle-cell patients and Pseudomonas or Klebsiella in intravenous drug users. Fungal osteomyelitis most commonly occurs in immunocompromised patients (9).
It is important to be aware of an aseptic form of osteomyelitis known as chronic recurrent multifocal osteomyelitis (CRMO) that primarily affects children and adolescents. Blood cultures and bone biopsies in CRMO do not yield any microbial growth and there is no response to antibiotics. While the aetiology of CRMO has not been firmly established, an autoimmune cause has been postulated and there is a known association with inflammatory bowel disease (10).
Routes of disease spread
Three main routes for spread of osteomyelitis have been described; these are haematogenous, contiguous and direct inoculation (11).
Haematogenous spread
Blood-borne organisms, usually bacteria, are deposited in the medullary cavity and form a nidus of infection. In long bones, the region which is most predisposed to infection is the metaphysis, because it has a large supply of slow-flowing blood. This creates an ideal environment for bacteria to accumulate and proliferate (2). The metaphysis is also prone to infection because there is discontinuity in the endothelial lining of the metaphyseal vessel walls. The gaps in the metaphyseal vessels allow bacteria to escape from the bloodstream into the medullary cavity. In flat bones, the equivalent regions where infection tends to originate are the bony-cartilaginous junctions (12).
Contiguous spread
Infections originating from soft tissues and joints can spread contiguously to bone. This often occurs in the context of vascular insufficiency, such as in patients with diabetes mellitus or peripheral vascular disease. There is a diminished immune response secondary to poor perfusion of the infected region. In these patients, the lower extremities are most commonly affected as there is associated peripheral neuropathy, which predisposes to repeated microtrauma (13).
Direct inoculation
Direct seeding of bacteria into bone can occur as a result of open fractures, insertion of metallic implants or joint prostheses, human or animal bites and puncture wounds (13).
Acute and chronic osteomyelitis
Osteomyelitis can be divided into acute and chronic stages (Figure 1). The duration of disease determines what imaging findings are seen in osteomyelitis.
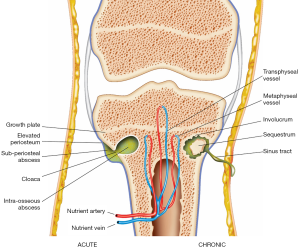
Acute osteomyelitis
In osteomyelitis secondary to haematogenous spread or direct inoculation, bacterial proliferation within the bone induces an acute suppurative response. There is accumulation of pus within the medullary cavity leading to raised intramedullary pressure and vascular congestion, which can disrupt the intraosseous blood supply. Reactive bone and hypervascular granulation tissue may form around the intramedullary pus, giving rise to a well-circumscribed intraosseous abscess, also known as a Brodie’s abscess (14).
The rise in intramedullary pressure may eventually lead to rupture of the bony cortex, producing a cortical defect known as a cloaca, the Latin term for ‘sewer’. Intramedullary pus can spread outward through the cloaca and form a subperiosteal abscess. This causes elevation of the periosteum and disrupts the periosteal blood supply to the bone (14). Continual accumulation of pus in the subperiosteal space leads to rupture of the periosteum and spread of infection to soft tissues through a channel between the bone and skin surface known as a sinus tract. In up to 1% of patients who have persistent draining sinus tracts, squamous cell carcinoma may develop in the epithelial lining of the tract (15).
In osteomyelitis secondary to contiguous spread from soft tissue infections, the direction of infection is essentially the reverse to that of haematogenous osteomyelitis.
Chronic osteomyelitis
If the acute infection is inadequately treated, there will be progression of disease to chronic osteomyelitis. The pathological features of chronic osteomyelitis are a result of osteonecrosis, caused by disruption of the intraosseous and periosteal blood supply during the acute stage of disease. A fragment of dead infected bone becomes separated from viable bone and is known as a sequestrum. The bacteria within the devascularised sequestrum are protected from antibiotics and the endogenous immune response, thus forming a nidus for chronic infection which may persist for many years (1). In an attempt to wall off the sequestrum, an inflammatory reaction characterised by osteoclastic resorption and periosteal new bone formation occurs. The sequestrum becomes surrounded by pus, granulation tissue and a reactive shell of new bone known as an involucrum. The involucrum may have a cloaca through which the pus or sequestrum can be discharged (14).
Age-dependent differences
There are several important age-dependent differences in the pathophysiology of osteomyelitis that explain the imaging findings seen in infants, children and adults.
Mechanism of infection
Haematogenous spread is the predominant mechanism of infection in children and usually causes long bone osteomyelitis (2). In adults, haematogenous spread is less common and when it does occur, usually leads to vertebral osteomyelitis. Adult osteomyelitis is most commonly caused by contiguous spread from soft tissue infections or direct inoculation (13).
Intraosseous vascular anatomy
During skeletal maturation, there are changes in intraosseous vascular anatomy that determine the pattern of osteomyelitis spread in different age groups. In infants below 18 months of age, metaphyseal and epiphyseal vessels anastomose via transphyseal vessels that perforate the growth plate. These transphyseal vessels allow infection to spread from the metaphysis, where osteomyelitis commonly originates, to the growth plate, epiphysis and joint space. This may result in slipped epiphyses, growth impairment and joint destruction. In children older than 18 months of age, the growth plate ossifies and forms a barrier between the metaphysis and epiphysis, limiting the spread of infection from the metaphysis (12). In adulthood, the growth plate is reabsorbed, removing the barrier between the metaphyseal and epiphyseal vessels. These vessels reanastomose, once again allowing spread of infection into the epiphysis and joint space (14).
Subperiosteal abscess formation
Subperiosteal abscesses are more common in children than in adults for two main reasons. In children, the cortical bone is thinner and more easily ruptured, leading to spread of infection from the medullary cavity to the subperiosteal space (16). The periosteum in children is also more loosely attached to the surface of the cortex and is easily separated, allowing accumulation of pus beneath the periosteal layer as a subperiosteal abscess (2).
Plain radiography
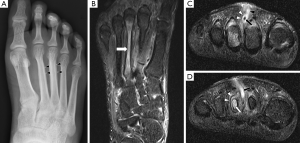
Plain radiography has low sensitivity and specificity for detecting acute osteomyelitis. As many as 80% of patients who present in the first two weeks of infection onset will have a normal radiograph (2). Bone marrow oedema, which is the earliest pathological feature, is not visible on plain films. The features of acute osteomyelitis that may be visible include a periosteal reaction secondary to elevation of the periosteum (Figure 2), a well-circumscribed bony lucency representing an intraosseous abscess (Figure 3) and soft tissue swelling. However, none of these findings are specific to osteomyelitis and can also be seen in stress fractures, bone tumours or soft tissue infections (5).
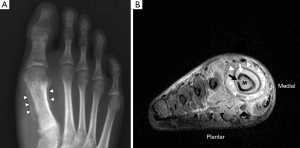
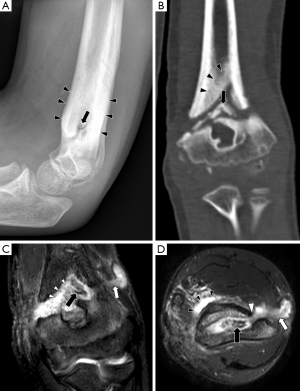
In chronic osteomyelitis, a sequestrum may be visible on plain radiographs as a focal sclerotic lesion with a lucent rim (Figure 4). An involucrum can be seen as thickened and sclerotic bone surrounding the sequestrum. There can also be marked cortical destruction, a disorganised trabecular pattern and ill-defined bony lucencies. These findings of chronic osteomyelitis are best demonstrated with CT (17).
Despite its limitations, plain radiography should still be the first-line imaging test in suspected osteomyelitis, as it is useful for excluding other differentials such as fractures. Plain radiographs are also useful for assessing the progression of disease, by comparing changes seen on follow-up films with the initial radiograph (6).
Magnetic resonance imaging
MRI has emerged as the imaging modality of choice for diagnosing osteomyelitis because of its excellent anatomical detail, high sensitivity for detecting early infection and lack of ionising radiation (17). The protocols and pulse sequences used in the evaluation of osteomyelitis will be described, followed by the MRI findings in acute and chronic osteomyelitis (Table 1).

Full table
Magnetic resonance imaging (MRI) protocols
In suspected osteomyelitis, the affected area is imaged in axial, sagittal and coronal planes using multiple pulse sequences. A pulse sequence is a set of parameters that highlights different tissue characteristics. The typical sequences used in the evaluation of osteomyelitis are as follows:
- T1-weighted (T1W) sequences provide good anatomical detail and enable delineation of the medulla, cortex, periosteum and soft tissues. On T1W images, fluid has low signal (appears dark), abscesses have low to intermediate signal and fat has high signal;
- Fluid-sensitive sequences include T2-weighted (T2W), fat-suppressed (FS) and short-tau inversion recovery (STIR) sequences. These all display fluid as high signal and are useful for detecting infection and inflammation, which cause an increase in tissue fluid content. Fat on T2W images has variable signal but is generally less bright than on T1W images. In fat-suppressed and STIR sequences, the signal from fat is decreased, increasing the visibility of inflammatory changes and fluid collections. Fat suppression can be applied to T1, T2 or proton density-weighted sequences. STIR sequences are more commonly used as the fluid-sensitive sequence in an osteomyelitis MRI protocol, as they are generally more sensitive than fat-suppressed sequences in demonstrating fluid;
- Proton density-weighted (PD) sequences are intermediately weighted between T1 and T2. PD images provide good anatomical detail but with less tissue contrast compared to T1W images (9).
Indications for intravenous gadolinium contrast
Gadolinium is a contrast agent that causes enhancement of tissues according to their degree of vascularity. This enhancement is best assessed on FS-T1 sequences (17). When investigating suspected osteomyelitis, there are various clinical contexts in which gadolinium is useful. If a possible abscess or sinus tract is seen, post-contrast FS-T1 sequences will allow further characterisation, as will be described later in the article (2). Contrast is also indicated in suspected epiphyseal infection because the unenhanced images may appear normal. Contrast administration is essential for differentiating an abscess from a phlegmon, which is a solid inflammatory mass (5). Overall, there is a low threshold for gadolinium administration and we routinely obtain post-contrast sequences for patients with suspected osteomyelitis at our institution. However, it is important to note that intravenous gadolinium is contraindicated in patients with impaired renal function because of the risk of nephrogenic systemic fibrosis.
Magnetic resonance imaging (MRI) findings in acute osteomyelitis
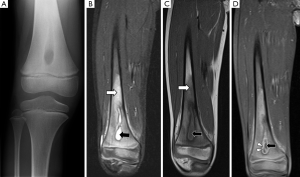
Bone marrow oedema is the earliest feature of acute osteomyelitis seen on MRI and can be detected as early as 1 to 2 days after the onset of infection (2). The normal marrow has high T1 signal due to fat within the medulla. In acute osteomyelitis, the bone marrow becomes congested with fluid and pus, producing low signal on T1W images and high signal on fluid-sensitive and post-contrast sequences (Figures 2,3,5,6). Comparison with marrow signal in adjacent or contralateral bones can be useful for detecting oedema (5).
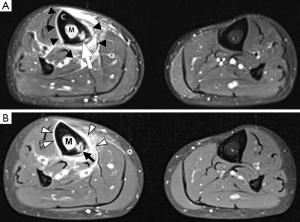
Intraosseous and subperiosteal abscesses will have low signal on T1W images and high signal on fluid-sensitive sequences. A thin rim of intermediate T1 signal is seen surrounding the abscess, representing hypervascular granulation tissue. On post-contrast FS-T1 images, the peripheral granulation tissue will enhance while the central pus-filled cavity remains low in signal intensity (Figures 3,5). This pattern of peripheral enhancement is known as the penumbra sign and can help differentiate an abscess from a phlegmon (17). A phlegmon, which is a solid inflammatory mass, would demonstrate more heterogeneous enhancement instead of the discrete peripheral enhancement seen with abscesses (18). This distinction is important because an abscess usually requires more immediate intervention with aspiration or surgical decompression (19).
The sinus tract is seen as a linear fluid-filled structure extending from bone to the skin surface (Figures 4,6). As with abscesses, sinus tracts are lined by hypervascular granulation tissue and will also demonstrate peripheral enhancement after intravenous contrast (17).
An additional finding to note in acute osteomyelitis is periostitis, which is seen as elevation of the low-signal periosteum off the cortical surface and corresponds to the periosteal reaction seen on plain radiographs (Figures 2,5,6).
Magnetic resonance imaging (MRI) findings in chronic osteomyelitis
The sequestrum can be difficult to visualise on MRI. It appears dark on all sequences because it is a fragment of necrotic bone that has very few protons available to produce an MR signal. However, the sequestrum is surrounded by hypervascular granulation tissue so it will have peripheral enhancement on post-contrast sequences, making it more conspicuous (Figure 4) (17). The involucrum is seen as a thickened shell of bone around the sequestrum which displays either normal signal or oedema (9).
A cloaca can be seen in both acute and chronic osteomyelitis as a cortical defect that drains pus from within the medulla to the surrounding soft tissues. It is most easily seen on fluid-sensitive sequences because the draining pus within it will have high signal (Figures 2,4) (9).
Magnetic resonance imaging (MRI) sensitivity and specificity
MRI has very high sensitivity for the detection of osteomyelitis; a normal MRI virtually excludes osteomyelitis (5). However, this high sensitivity means that MRI can overestimate the severity of infection. Abnormalities on MRI may also persist even after the infection has begun to resolve (20). The MRI findings should always be correlated with the clinical and biochemical picture to avoid unnecessary or overly aggressive treatment.
The specificity of MRI for diagnosis of osteomyelitis, as quoted in the literature, is usually less than the sensitivity (5). This is because the MRI appearances of osteomyelitis may be similar to other pathologies such as neuropathic arthropathy, malignancy and trauma. As always, the clinical presentation and biochemical findings should be taken into consideration. However, there are several key imaging features which can help distinguish osteomyelitis from other pathologies; these are discussed in the following section on differential diagnosis.
Differential diagnoses on magnetic resonance imaging (MRI)
Highly suggestive features of osteomyelitis on MRI are a peripherally enhancing intraosseous lesion, a non-enhancing sequestrum and a sinus tract. The presence of intra and extramedullary fat globules, seen as foci of high T1 signal, is a less common finding of acute osteomyelitis but which is nonetheless highly suggestive (21). A proposed aetiology for the presence of these fat globules is increased intramedullary pressure causing extrusion of medullary fat. Bone marrow oedema and periostitis are more equivocal features which are often seen in other pathologies.
Depending on the clinical context, the following differentials may be considered when investigating suspected osteomyelitis:
- Reactive osteitis—reactive osteitis occurs secondary to trauma, cellulitis, pressure sores or inflammatory arthropathy and produces high marrow signal on fluid-sensitive sequences. To distinguish between reactive osteitis and osteomyelitis, the corresponding T1W images should be carefully scrutinised. In reactive osteitis, the marrow can have intermediate T1 signal or poorly demarcated areas of low T1 signal in a subcortical distribution. In acute osteomyelitis, the marrow is invariably of low T1 signal and appears darker and more well-demarcated compared to reactive osteitis, with an intramedullary distribution (18,19);
- Neuropathic arthropathy—neuropathic arthropathy, or Charcot’s joint, can cause soft tissue and marrow changes which mimic osteomyelitis. The distribution of the abnormalities is key to differentiating these two entities. Neuropathic arthropathy usually affects multiple bones in a periarticular distribution, while osteomyelitis typically affects single bones in weight-bearing areas such as the 1st metatarsophalangeal joint and calcaneus. Marrow oedema adjacent to soft tissue inflammation is also typical of osteomyelitis (18,22);
- Malignancy—on serial MRIs, osteomyelitis tends to cause more rapid destructive change compared to malignant bone tumours (17). Abscesses demonstrate peripheral rim enhancement whereas tumours usually enhance heterogeneously (23);
- Langerhans cell histiocytosis (LCH)—when it affects long bones, LCH tends to be centered on the diaphysis while haematogenous osteomyelitis tends to originate in the metaphysis (5);
- Osteoid osteoma—an osteoid osteoma is a benign tumour which is seen as an oval lucent lesion with a densely sclerotic center. It may appear similar to a sequestrum. Osteoid osteomas are usually round whereas sequestra are irregularly shaped. On post-contrast sequences, osteoid osteomas will enhance avidly while sequestra do not enhance. Osteoid osteomas are not associated with bone destruction or soft tissue inflammation (17);
- Stress injuries—bones which undergo repetitive stress may demonstrate marrow oedema with periosteal reaction, similar to osteomyelitis. However, unlike osteomyelitis, the signal abnormality is confined to bone in stress injuries and there is no inflammatory change in the surrounding soft tissue (17).
Once osteomyelitis has been established as the most likely diagnosis based on the MRI findings and clinical history, treatment with empirical antibiotics would be commenced. If the patient fails to respond to antibiotics, a bone biopsy specimen may be required so that a definitive diagnosis can be made on microbiology and histology.
Magnetic resonance imaging (MRI) contraindications and limitations
Despite the many advantages of MRI, there are circumstances where it may not be feasible. The presence of a permanent pacemaker or intracranial aneurysm coils is an absolute contraindication. In patients with metallic prostheses, the usefulness of MRI is decreased because of susceptibility artefact, although metallic artefact suppression techniques are now available for reducing this limitation (9). Infants and young children may require sedation or general anaesthesia before they can undergo an MRI scan. Cost and lack of accessibility remain important considerations in many parts of the world. Hence, it is important to have an understanding of alternative modalities for assessing suspected osteomyelitis.
Nuclear medicine
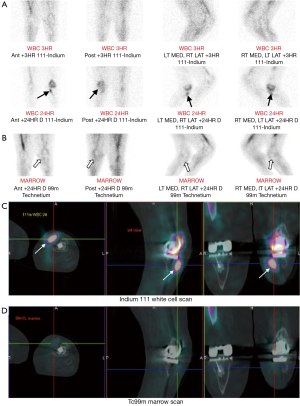
Nuclear medicine studies involve intravenous administration of a radionuclide, which emits radiation that is detected by a gamma camera. This allows assessment of abnormal bone metabolism, which in osteomyelitis manifests as areas of increased radionuclide uptake. The most commonly performed radionuclide studies for diagnosing osteomyelitis are the triple-phase, gallium and white cell scans, which are described individually in this section together with a newer technique, 18F-fluorodeoxyglucose positron emission tomography (FDG-PET). In general, nuclear medicine studies have very high sensitivity in the detection of osteomyelitis and allow imaging of the whole skeleton to look for multiple sites of infection (24). However, nuclear medicine studies are limited by poor specificity and anatomical localisation. If there is an abnormal result, further confirmation with MRI or bone biopsy is usually required before a diagnosis of osteomyelitis can be established. Newer targeted radionuclides can increase the specificity of nuclear medicine studies and hybrid imaging techniques such as single photon emission computed tomography-CT (SPECT-CT) provide more anatomical information than conventional techniques (Figure 7) (25,26).
Triple-phase bone scan
In a triple-phase bone scan, technetium-99m-labelled MDP (Tc99m-MDP) is injected intravenously followed by image acquisition in three phases: the angiographic, tissue and osseous phases (27). Tc99m-MDP is a radiopharmaceutical that localises to areas of increased osteoblastic activity and is useful for differentiating osteomyelitis from cellulitis. In osteomyelitis, there is high tracer uptake in all three phases. In cellulitis, there is high uptake only in the first two phases (24).
Triple-phase bone scans have high sensitivity for detecting osteomyelitis in non-violated bone, even in the early stages of infection. However, their specificity is lower when bone has been violated—for instance in trauma, malignancy or previous surgery. These can all cause increased osseous uptake, making differentiation from osteomyelitis difficult. A combined white cell and marrow scan, described later in this section, is a better test for investigating suspected osteomyelitis in violated bone. Triple-phase scans are also difficult to interpret in suspected vertebral osteomyelitis because of overlying vascular structures. If these scans are equivocal, further imaging with other tracers such as gallium and labelled white cells can be performed to obtain a more definitive diagnosis (24).
Gallium scan
Gallium-67 is a radionuclide that binds to acute phase reactants such as transferrin and accumulates in areas of infection and inflammation. Gallium scans have higher specificity than triple-phase scans and these two tests are often combined when investigating suspected vertebral osteomyelitis. Osteomyelitis is likely if there is greater tracer uptake in the gallium scan compared to the triple-phase scan. Conversely, if the gallium scan is normal, then osteomyelitis is unlikely, regardless of the bone scan findings (24,26). The main limitation of gallium scans are that they take 48–72 hours to complete, necessitating multiple visits to the nuclear medicine department (22).
White cell scans
The combined white cell and marrow scan is the current study of choice for investigating suspected osteomyelitis in violated bone. In white cell scans, the patient’s white blood cells are labelled with a radionuclide, either Indium-111 or Tc99m-HMPAO, then returned to the patient intravenously. Increased white cell uptake is seen in areas of infection. However, normal bone marrow also takes up white cells in a variable distribution. To differentiate between infection and physiological marrow uptake, the white cell scan is combined with a bone marrow scan that uses Tc99m-labelled colloid. The bone marrow scan provides a map of physiological white cell uptake that is then compared to the white cell scan. Any discordance in white cell uptake between the two studies indicates a focus of infection (Figure 7) (24).
Fluorodeoxyglucose positron emission tomography (FDG-PET)
Fluorine-18 fluorodeoxyglucose (18F-FDG) is a positron-emitting radiopharmaceutical that localises to hypermetabolic tissues that have high glucose uptake. FDG-PET has been shown to have the highest sensitivity of all the radionuclide techniques in the detection of chronic osteomyelitis. This is because FDG accumulates in activated macrophages, which are the predominant cell type found in chronic infection (28).
Computed tomography
Computed tomography is more widely available than MRI and image acquisition is less time-consuming. CT has good spatial resolution and can demonstrate clearly the anatomical relationship between areas of infection and important structures such as the spinal cord or major vessels. Hence, percutaneous aspirations and biopsies are often performed under CT guidance to avoid damage to these structures. CT has superior bony resolution to MRI and is better at demonstrating osseous changes such as cortical destruction, periosteal reactions and sequestrum formation. As with plain radiographs, the sequestrum on CT appears as a sclerotic lesion with a lucent rim (Figure 4). Intramedullary gas is an ancillary sign of osteomyelitis that is also best seen on CT (9).
However, the evaluation of osteomyelitis with CT is limited by its poorer soft tissue resolution compared to MRI. CT is unable to demonstrate bone marrow oedema, which means that a normal CT does not exclude early osteomyelitis. Other limitations of CT are ionizing radiation exposure and image degradation by streak artefact when metallic implants are present (7). Despite these limitations, CT remains a useful alternative when MRI is unavailable or contraindicated.
Ultrasound
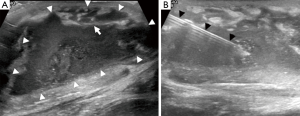
Ultrasound is of limited use in the diagnosis of osteomyelitis, as it cannot assess bone. Ultrasound is also an operator-dependent technique and can be challenging with larger patients. However, it can be useful for detecting soft tissue or subperiosteal collections, especially in children, although an MRI will still be required for a more thorough assessment. Subperiosteal abscesses are seen on ultrasound as periosteal elevation with an underlying fluid collection. Soft tissue oedema is seen as areas of hypervascularity around the affected bone on colour Doppler (2). If a collection is seen, the dynamic nature of ultrasonography makes it useful for guiding needle aspiration (Figure 8) (29).
Summary
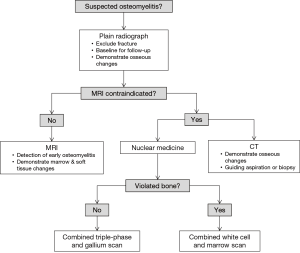
Imaging plays a central role in the diagnosis and management of osteomyelitis; a summary flow chart for imaging modality choice is provided in Figure 9. Plain radiographs should ideally be obtained first to exclude other pathologies such as fractures. MRI is the best imaging modality for establishing the diagnosis of osteomyelitis as it can demonstrate bone marrow oedema, confirm the presence of abscesses and delineate extraosseous disease spread. If MRI is contraindicated or unavailable, nuclear medicine studies and CT are useful alternatives. The triple phase bone scan has high sensitivity for detecting acute osteomyelitis in non-violated bone. For violated bone, a combined white cell and bone marrow scan is the current study of choice. CT allows visualisation of osseous changes such as sequestrum formation and also for guiding aspiration and biopsy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Lew DP, Waldvogel FA. Osteomyelitis. Lancet 2004;364:369-79. [Crossref] [PubMed]
- Jaramillo D. Infection: musculoskeletal. Pediatr Radiol 2011;41 Suppl 1:S127-34. [Crossref] [PubMed]
- Arnold SR, Elias D, Buckingham SC, Thomas ED, Novais E, Arkader A, Howard C. Changing patterns of acute hematogenous osteomyelitis and septic arthritis: emergence of community-associated methicillin-resistant Staphylococcus aureus. J Pediatr Orthop 2006;26:703-8. [Crossref] [PubMed]
- Gafur OA, Copley LA, Hollmig ST, Browne RH, Thornton LA, Crawford SE. The impact of the current epidemiology of pediatric musculoskeletal infection on evaluation and treatment guidelines. J Pediatr Orthop 2008;28:777-85. [Crossref] [PubMed]
- Pugmire BS, Shailam R, Gee MS. Role of MRI in the diagnosis and treatment of osteomyelitis in pediatric patients. World J Radiol 2014;6:530-7. [Crossref] [PubMed]
- Offiah AC. Acute osteomyelitis, septic arthritis and discitis: differences between neonates and older children. Eur J Radiol 2006;60:221-32. [Crossref] [PubMed]
- Pineda C, Espinosa R, Pena A. Radiographic imaging in osteomyelitis: the role of plain radiography, computed tomography, ultrasonography, magnetic resonance imaging, and scintigraphy. Semin Plast Surg 2009;23:80-9. [Crossref] [PubMed]
- iRefer Guidelines. Making the Best Use of Clinical Radiology version 7.0.2. Accessed 21st June 2015.
- Rajashanker B, Whitehouse RW. Chapter 53: Bone, joint and spinal Infection. In: Adam A, Dixon AK, Gillard JH, et al. editors. Grainger & Allison's Diagnostic Radiology, 6th ed. New York, NY: Churchill Livingstone, 2015:1241-2.
- Audu GK, Nikaki K, Crespi D, Spray C, Epstein J. Chronic recurrent multifocal osteomyelitis and inflammatory bowel disease. J Pediatr Gastroenterol Nutr 2015;60:586-91. [Crossref] [PubMed]
- Lew DP, Waldvogel FA. Osteomyelitis. N Engl J Med 1997;336:999-1007. [Crossref] [PubMed]
- Stephen RF, Benson MK, Nade S. Misconceptions about childhood acute osteomyelitis. J Child Orthop 2012;6:353-6. [Crossref] [PubMed]
- Calhoun JH, Manring MM. Adult osteomyelitis. Infect Dis Clin North Am 2005;19:765-86. [Crossref] [PubMed]
- Rosenberg AE. Chapter 26: Bones, joints and soft tissue tumors. In: Kumar V, Abbas AK, Fausto N, et al. editors. Robbins and Cotran Pathologic Basis of Disease. 8th ed. Philadelphia, PA: Saunders Elsevier, 2010:1221-2.
- Luchs JS, Hines J, Katz DS, Athanasian EA. MR imaging of squamous cell carcinoma complicating chronic osteomyelitis of the femur. AJR Am J Roentgenol 2002;178:512-3. [Crossref] [PubMed]
- De Boeck H. Osteomyelitis and septic arthritis in children. Acta Orthop Belg 2005;71:505-15. [PubMed]
- Manaster BJ. Musculoskeletal Imaging: The Requisites, 3rd ed. Philadelphia, PA: Mosby Elsevier, 2007:545-64.
- Donovan A, Schweitzer ME. Use of MR imaging in diagnosing diabetes-related pedal osteomyelitis. Radiographics 2010;30:723-36. [Crossref] [PubMed]
- Collins MS, Schaar MM, Wenger DE, Mandrekar JN. T1-weighted MRI characteristics of pedal osteomyelitis. AJR Am J Roentgenol 2005;185:386-93. [Crossref] [PubMed]
- Helms C, Major N, Anderson M. Chapter 5: Musculoskeletal Infections. In: Helms C, Major N, Anderson M, editors. Musculoskeletal MRI, 2nd Ed. Philadelphia, PA: Saunders Elsevier, 2009:92-110.
- Davies AM, Hughes DE, Grimer RJ. Intramedullary and extramedullary fat globules on magnetic resonance imaging as a diagnostic sign for osteomyelitis. Eur Radiol 2005;15:2194-9. [Crossref] [PubMed]
- Christian S, Kraas J, Conway WF. Musculoskeletal infections. Semin Roentgenol 2007;42:92-101. [Crossref] [PubMed]
- Shimose S, Sugita T, Kubo T, Matsuo T, Nobuto H, Ochi M. Differential diagnosis between osteomyelitis and bone tumors. Acta Radiol 2008;49:928-33. [Crossref] [PubMed]
- Mettler F, Guiberteau M. Chapter 8: Skeletal System. In: Mettler F, Guiberteau M, editors. Essentials of Nuclear Medicine Imaging, 6th ed. Philadelphia, PA: Saunders Elsevier, 2012:296-300.
- Santiago Restrepo C, Giménez CR, McCarthy K. Imaging of osteomyelitis and musculoskeletal soft tissue infections: current concepts. Rheum Dis Clin North Am 2003;29:89-109. [Crossref] [PubMed]
- El-Maghraby TA, Moustafa HM, Pauwels EK. Nuclear medicine methods for evaluation of skeletal infection among other diagnostic modalities. Q J Nucl Med Mol Imaging 2006;50:167-92. [PubMed]
- DiPoce J, Jbara ME, Brenner AI. Pediatric osteomyelitis: a scintigraphic case-based review. Radiographics 2012;32:865-78. [Crossref] [PubMed]
- Palestro CJ. FDG-PET in musculoskeletal infections. Semin Nucl Med 2013;43:367-76. [Crossref] [PubMed]
- Cardinal E, Bureau NJ, Aubin B, Chhem RK. Role of ultrasound in musculoskeletal infections. Radiol Clin North Am 2001;39:191-201. [Crossref] [PubMed]


