
Ultra-low dose CT for scaphoid fracture detection—a simulational approach to quantify the capability of radiation exposure reduction without diagnostic limitation
Introduction
The scaphoid plays a central role in the wrist movement by stabilizing the proximal carpal row (1). It is most commonly affected by fractures upon falls. Patients often present with localized pain, soft tissue swelling and restricted range of movement. In 1980, Cooney et al. postulated that for good fracture consolidation an early diagnosis including fragmentation pattern, sufficient blood supply and immobilization with stabilization are the most important factors that contribute to scaphoid fracture healing (2). Depending on the type of fracture a conservative or an operative approach is indicated to prevent nonunion and pseudarthrosis (3). Complications such as scaphoid nonunion or osteonecrosis of the proximal pole due to its retrograde blood supply or consequent instability is seen in 5% after fractures (4), thus accurate diagnosis and imaging is essential.
Scaphoid fractures have the reputation of being difficult to diagnose and often affecting young patients that may arguably suffer greater form unnecessary immobilization or misdiagnosis. Typically, when a scaphoid injury is suspected, several X-rays are taken including anterior-posterior, lateral view and Stecher method (or formerly often used scaphoid series comprising of a posterior-anterior, oblique, lateral and angled posterior-anterior projection) with each approximately 1 µSv effective dose (5). However, controversy exists in the literature on how to proceed consequently when and which cross sectional imaging technique to be best used in but it is clear that MRI or CT has improved diagnosis and classification of fractures (6,7). CT has shown higher sensitivity (ranging from 64–100%) in fracture detection especially in radiographically occult fractures (sensitivity of conventional radiography ranging from 50–94%) and enables the correct Herbert classification (7,8). Especially for the quantification of intra-articular displacements of fracture fragments (such as at the radius), CT scanning offers more reliability than conventional plain radiography (9).
CT diagnostic is not only initially important for surgeons in therapy planning (10) but arguably as important during the healing process in both conservatively treated and operated patients to allow early action and minimization of complications.
The role of MRI and spectroscopy certainly have their place in diagnosis especially in occult, missed and more complex cases. MRI is used in indistinct fractures with the highest imaging sensitivity (range, 67–100%), however the advantages of CT with its broad availability, speed and generally limited contraindications explain its central role in musculoskeletal (MSK) trauma (7,9,11).
A reduction of radiation dosage according to the ALARA principle (“as low as reasonably achievable) is essential in CT imaging whilst maintaining an acceptable image quality for the assessment of osseous structures. To generally reduce the radiation, scan parameters have been altered or post-processing software used (12-15). The aim of this study was to evaluate the effect of simulation of dose reduction on fracture detection and classification at the scaphoid, and diagnostic confidence (DC), whilst correlating this to the readers’ experience in MSK radiology. The original CT scans of the patients were considered as the reference standard and the simulated ultra-low dose (ULD)-CT images assessed inter-individually. We present the following article in accordance with the STARD reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-21-1196/rc).
Methods
This study was approved by the institutional review board of the Eberhard Karls University of Tuebingen (No. 644/2020BO) and the need for written informed consent was waived for this retrospective analysis of clinically acquired CT data. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Study population
From the patient population of a level 1 trauma center (BG trauma center Tuebingen) a convenient sample size of 30 consecutive patients was selected who had received a clinically indicated CT of the wrist for scaphoid fracture assessment between April 1st 2018 and July 31st and whose raw CT data files were still available. Exclusion criteria were metal implants in the scan field.
Technical parameters of the high quality CT study protocol
CT image acquisition was performed on a 128-slice, single source CT (SOMATOM Definition Edge, Siemens Healthineers) using a high quality study protocol, which is eligible for patients with or without metal implants (see Table 1). No iterative image reconstruction was used. All patients were examined head first with the arm elevated lying next to the head, the elbow in extension and due to the patient’s condition preferably in prone position (superman position).
Table 1
| Tube voltage: 120 kV |
| Tube current: 180 mAs |
| Pitch: 0.85 |
| Rotation time: 1.0 s (flying focal spot) |
| Collimation: 16 mm × 0.3 mm |
| Slice thickness: 0.4 mm |
Simulation of ULD-CT
ULD-CT simulations were generated using the raw CT data sets from the initial high quality CT with the dedicated software package ReconCT (Version 14.2.0.40998, Siemens Healthineers) similar to previous studies (14-17). Simulations were made at 20%, 10%, and 5% of the original dosage and were then compared to the original data sets (100% dose level). The software ReconCT essentially generates images with reduced effective radiation dose by adding noise to the raw data prior to the reconstruction process. Axial, parasagittal and paracoronal reconstructions were made at every dose level according to the current guidelines (18) with a field of view (FOV) of 50.0 mm × 50.0 mm, slice thickness 0.4 mm, increment 0.3 mm, edge-enhancing reconstruction kernel (B60), bone window with center/ width of 1,200/3,000 Hounsfield units (HU), respectively (Figure 1).
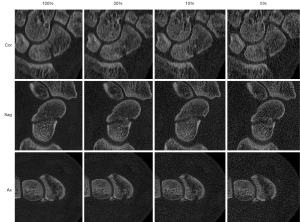
Estimation of the effective radiation dose
According to the AAPM (American Association of Physicists in Medicine) Report 204 (19) to account for size differences of the CT phantom and patient volume the size specific dose estimates (SSDE) were calculated from computed tomography dose index (CTDIvol) and the mean diameters of the scanned area, both anterior-posterior and lateral. Standardized measuring points were the distal end of the distal radio-ulnar joint, the distal end of the scapho-lunate joint, and the distal end of the carpo-metacarpal 1 joint. A conversion factor was calculated for effective dose per local dose (SSDE) and volume using CT-Expo (Version 2.7, Georg Stamm and Hans Dieter Nagel, Hannover, Germany) in order to estimate the effective radiation exposure at the lower extremities. Assuming that the tissue structure of the wrist would be similar to that at the ankle conversion is also valid for upper extremities when solely the extremity is in the scan volume. Saltybaeva et al. (20) use a similar approach for the estimation of effective dose at lower extremities using the dose length product (DLP). In this study, the SSDE was used instead to account for the differences in diameter between the CT phantoms (32 and 16 cm) of the wrist. To account for differences in volume between the simulations at the ankles and the wrist inside the scan volume the calculation was performed per volume.
Evaluation of high quality CT and ULD-CT regarding scaphoid fracture assessment
The original high quality CT data sets (100%) and the simulated ULD-CT (dose levels at 20%, 10%, 5%) were in random order and pseudonymized with numbers from 001 to 100. Blinded to patient data, acquisition time and dose level, three readers with varying experience in radiology assessed the CT independently in 4 reading sessions each at least 2 weeks apart from one another (incorporating 25 randomly selected CT/ULD-CT data): one expert reader (EXP; MSK fellowship trained radiology specialist), one moderately experienced reader (MOD; final year of radiology residency), and one inexperienced reader (BEG; after approximately one year of radiology residency).
The readers evaluated the presence of a scaphoid fracture and relevant fracture features according to the widely used Krimmer classification and Herbert classification (21,22) as follows: fracture (yes vs. no); fracture age estimation (acute/subacute vs. old/pseudarthrosis); localization of the fracture (proximal vs. middle vs. distal third vs. only tubercle of the scaphoid—defined by the most proximal part of the fracture); displacement (mm); fracture morphology (avulsion vs. transverse vs. long oblique fracture vs. longitudinal or multidirectional fracture); fragment count (n); perilunate displacement (yes vs. no); hump back deformity (yes vs. no).
Cut offs with non-dichotomous variables were set as the following: localization of the fracture (proximal/middle third vs. distal third/tubercle), displacement (cortical malalignment ≥1 mm vs. no cortical malalignment), fracture morphology (avulsion/transverse vs. long oblique/longitudinal/multidirectional), and fragment count (2-part fracture vs. n>2). The reading results of EXP on the original CT data sets were set as the reference standard.
In addition, the DC of all readers regarding the detection of a scaphoid fracture was assessed on a 5-point-Likert-scale of the original CT images and all ULD-CT reconstructions from 1 “non-diagnostic quality” to 5 “excellent image quality”.
Statistical analysis
The Shapiro-Wilk-W-test was used to assess the distribution of quantification data. Normally distributed variables were analyzed using Student’s t test and are presented as arithmetic mean and standard deviation. Data that did not follow normal distribution were analyzed using Wilcoxon-test and is reported as median and range. Correlations of ordinal variables were analyzed by Likelihood-ratio. P values <0.05 indicate statistical significance. Sensitivity and specificity were calculated for fracture detection and classification of fracture features. Sensitivity and specificity were assessed according to the reference standard (vide supra). Fleiss-Kappa was used for the agreement between the readers at the different dose levels and the reference standard regarding scaphoid fracture detection and classification. Fleiss-Kappa values of 0.00–0.20 were considered as slight, 0.21–0.40 as fair, 0.41–0.60 as moderate, 0.61–0.80 as substantial, and 0.81–1.00 as almost perfect/perfect levels of agreement (23).
Results
Study population
The final study collective included 25 patients (7 female, 17 male; 37±16.34 years; Figure 2). The prevalence of scaphoid fracture in the study collective was 56.0% (Table 2); 71.4% of the fractures were morphologically acute/subacute fractures. Most frequent fracture localization was the middle third (64.3%). Most of the fractures were non-displaced (42.9%) or had only a slight (1 mm) cortical malalignment (28.6%) and were transverse fractures (50.0%); 85.7% of the scaphoid fracture were 2-part fractures. Hump back deformity was present in 14.3% of the scaphoid fractures. There was no perilunate fracture. According to Krimmer classification 35.7% of the fractures were stable, while in terms of Herbert classification all fractures were unstable (for more details see Table 2).

Table 2
| Classification | N=25 |
|---|---|
| Fracture | No =11; Yes =14 |
| Acute/subacute fracture | No =4; Yes =10 |
| Fracture localization | Distal =3; Middle =9; Proximal =2 |
| Cortical malalignment° | 0 [0–3] mm |
| Fracture morphology | Tubercle avulsion =2; Transverse =7; Long oblique =3; Longitudinal or multidirectional =2 |
| Fragment count° | n =2 [0–6] |
| Hump back deformity | No =12; Yes =2 |
| Krimmer classification | A2 =5; B1 =1; B2 =6; B3 =2 |
| Herbert classification | B1 =2; B2 =8; B3 =2; B5 =2 |
°, non-normal distribution with presentation of the median and the total range; reference standard are the results of the expert reader on the original data sets of the high quality CTs.
Effective radiation dose of high quality CT and ULD-CT
Median CTDIvol of the original high quality CT data sets was 16.92 (range, 15.04–17.03) mGy and decreased with the lowest simulated dose (5% dose level) to a median of 0.85 (range, 0.75–0.85) mGy. With a mean scan length of 7.65±1.87 cm this led to a mean calculated effective radiation dose for the patients of 11.09±3.64 µSv in high quality CT and 0.55±0.18 µSv at 5% ULD-CT (see Table 3). ULD-CT at all examined dose levels resulted in significant lower effective radiation exposure compared to high quality CT (P<0.0001) (Table 3).
Table 3
| Effective radiation dose (µSv) | DC EXP (5/4/3/2/1) | DC MOD (5/4/3/2/1) | DC BEG (5/4/3/2/1) | |
|---|---|---|---|---|
| CT (100%) | 11.09 (±3.64) | 25/0/0/0/0 | 25/0/0/0/0 | 23/2/0/0/0 |
| ULD-CT 20% | 2.22 (±0.73) | 24/1/0/0/0 | 25/0/0/0/0 | 16/8/1/0/0 |
| ULD-CT 10% | 1.11 (±0.36) | 6/18/1/0/0 | 20/5/0/0/0 | 9/14/2/0/0 |
| ULD-CT 5% | 0.55 (±0.18) | 0/5/18/2/0 | 7/17/1/0/0 | 4/9/12/0/0 |
Effective radiation dose is presented as arithmetic mean and standard deviation. ULD-CT, ultra-low dose CT; DC, subjectively diagnostic confidence rated on a 5-point Likert scale with 5/5 representing “excellent image quality” and 1/5 “non-diagnostic image quality”; EXP, expert reader; MOD, moderately experienced reader; BEG, inexperienced reader.
Results of high quality CT and ULD-CT regarding scaphoid fracture assessment
The DC of all three readers was highest in the original data sets compared to ULD-CT (P<0.0001). Thereby median DC of all readers was 5/5 at 20% dose level with a minimum of 4/5 on the 5-point-Likert-scale. There were no ratings lower than 3/5 at 10% dose level and no ratings of 1/5 (“non-diagnostic image quality”) at all in any ULD-CT (Table 3).
EXP and MOD detected nearly all scaphoid fractures in ULD-CT perfectly: with 100% sensitivity and 100% specificity on simulations up to 10 %. The sensitivity of ULD-CT with 5% dose decreased slightly to 92.86% (EXP) and 85.71% (MOD) (exemplary patient with a subtle finding of a fissural scaphoid fracture as pictured in Figure 3). Thereby specificity was still high 100% (EXP) and 90.91% (MOD).
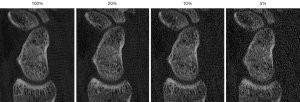
The reader at inexperienced level (BEG) showed a reduced sensitivity and specificity in scaphoid fracture detection at all dose levels of the ULD-CT compared to the original data sets (Figures 4,5).
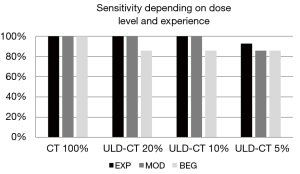
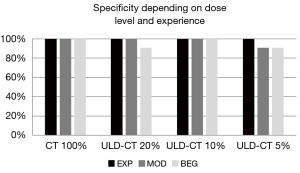
The agreement of ULD-CT results read by the EXP with the reference standard according to both Krimmer and Herbert classifications was perfect down to 10% dose level and near perfect at 5% dose level (see Table 4). If fractures were grouped in stable (A1+A2) and unstable (B1+B2+B3+B4+B5) fractures MOD also showed a perfect agreement with the reference standard down to 10% dose (1.000) and a substantial agreement at 5% dose level (0.702–0.745). The agreement of BEG with the reference standard was lower, but still substantial at all dose levels of ULD-CT and also in the high quality CT. If grouped into stable and unstable fractures the agreement of BEG with the reference standard decreased to a minimum of 0.593 (moderate) for the agreement of stable fractures at the 5% dose level.
Table 4
| Fleiss-Kappa | EXP – Krimmer | EXP – Herbert | MOD – Krimmer | MOD – Herbert | BEG – Krimmer | BEG – Herbert |
|---|---|---|---|---|---|---|
| CT 100% | – | – | 1.000 | 1.000 | 0.829 | 0.767 |
| ULD-CT 20% | 1.000 | 1.000 | 1.000 | 1.000 | 0.658 | 0.652 |
| ULD-CT 10% | 1.000 | 1.000 | 0.942 | 0.881 | 0.706 | 0.703 |
| ULD-CT 5% | 0.884 | 0.940 | 0.705 | 0.761 | 0.655 | 0.652 |
Fleiss-Kappa 0.00–0.20 = slight, 0.21–0.40 = fair, 0.41–0.60 = moderate, 0.61–0.80 = substantial, and 0.81–1.00 = almost perfect/perfect agreement. EXP, expert reader; MOD, moderately experienced reader; BEG, inexperienced reader; ULD-CT, ultra-low dose CT.
The fracture age estimation (acute/subacute vs. old/pseudarthrosis) of EXP and MOD was perfect (100%) in ULD-CT till the dose level of 10% and decreased at 5% slightly. The presence/absent of hump back deformity and the absent of perilunate displacement was recognized by all readers at all dose levels.
Discussion
Modifying CT acquisition parameters according to the ALARA principle is an elegant, practical approach to reduce ionizing radiation, which does not require additional hardware or post-processing software. It can be performed on any CT scanner of any generation. To our knowledge, this is the first study to report on ULD-CT imaging for the assessment of carpal fractures.
Our findings indicate that a radiation dose of up to 10% of the original data is viable for readers of all experiences, and that a radiation reduction as low as 5% is viable for the intermediate and advanced radiologist in comparison to conventionally used CT. There was a high level of agreement and inter-observer reliability in diagnostic accuracy of fracture detection and classification.
The increased image noise—a consequence of dose reduction—was not inhibitory to the readers. These findings indicate that ULD-CT imaging in the detection and classification of scaphoid fractures is a viable and safer option.
Over the last few years, there have been several studies focusing on radiation dose reduction in MSK imaging with ULD protocols (24-27). Konda et al. reduced radiation nearly 14-fold by lowering the current, voltage and scan time; this ULD-CT imaging for detecting limb fractures still showed good diagnostic accuracy with a sensitivity of 98% (24). These fractures, however, were situated in the lower limb (foot, ankle, knee, hip) and elbow. At the wrist, Neubauer et al. (28) were able to show that a dose optimization has even greater potential in multi-detector CT imaging in comparison to cone beam CT, which permits a detailed bony architecture but is limited due to a smaller FOV and increased scattered radiation (29). More recently, an excellent cortical outline of bone structures in simulated ULD-CT imaging was achieved in torsion measurements of the lower limbs and the results were confirmed in real ULD-CT imaging (14,15).
These findings are consistent with the results of our study indicating that ULD-CT imaging could be as accurate as high quality CT imaging of the wrist.
Radiation dose can also be reduced through various reconstruction algorithms such as iterative reconstruction as mentioned previously (30-33). At the same time reduction of tube current using low kV protocols is another way to reduce radiation dose but metallic implants cause artefacts and the BMI of the patient also affects results (24,27). Other techniques like automatic tube voltage adjust and use of tin filters are reliable for dose reduction and limiting artefacts, but they are limited to special scanners and software (34-36).
By reducing radiation dose in accordance with the ALARA principle, the image quality is reduced due to increased noise and should only be decreased to a level that still provides sufficient diagnostic image quality. In this study, the radiation dose was reduced to 10 % of the initial dose with sufficient diagnostic accuracy of all readers, resulting in a potential effective exposure of 1.11 µSv. Depending on the readers’ experience, diagnostic accuracy was still high when radiation dose was further reduced to 5% of the initial dose, with a calculated mean effective exposure of 0.55 µSv.
Standard practice to image a suspected scaphoid fracture is the use of conventional X-ray imaging. Assuming this requires three X-rays (anterior-posterior, lateral and Stecher view) with each approximately 1 µSv (see www.xrayrisk.com), then initial conventional radiography results in multiples of the radiation exposure of ULD-CT.
Accurate diagnosis of fracture morphology is paramount to therapy management, i.e., many institutions choose conservative treatment (cast immobilization) for none-displaced, stable fractures and minimally invasive surgery with cannulated screws for displaced/unstable fractures, and if not treated these fracture types show a greater risk for nonunion (8). The proximal pole fracture requires special attention to reduce complications such as avascular necrosis, secondary arthrosis and persistent nonunion due to nutritional undersupply (3). It was shown that fragment position, displacement (of more than 3 mm according to Hovius et al.) and instability of the carpus are equally important when it comes to complications (37,38). In persistent nonunions of the scaphoid, more complicated surgical approaches are required such as the dorsal and volar inlay techniques (2).
Subtle fractures without gap or displacement or comminution might, however, be less conspicuous in ULD-CT and detection might be challenging due to reduced spatial resolution. If in doubt, we would recommend further imaging like with MRI that has shown to have a high sensitivity and specificity (7).
The importance of radiation sparing is always of interest to the radiologist as part of the risk/benefit assessment for CT imaging. The ALARA principle is applied and the scan range is kept as short as reasonably possible. The patients’ risk of malignancy and germ cell damage is increased with a high cumulative radiation dose, such as from repetitive X-rays, CT imaging and interventional procedures, which are used to assess healing stages and complications (39,40). The linear, no-threshold theory is a widely accepted model for calculating the risk of cancer from low-dose radiation, however, a widely used figure is 5% excess risk of death from cancer with a dose of 1 Sv (41).
Even though the subsequent risk from radiation of an isolated wrist CT is very low, the extrapolated lifetime cancer risk of an individual from repeated imaging can become significant. An ULD-CT reduces this risk.
According to the results of our study, we confidently can recommend a decrease of radiation dose for the assessment and qualification of scaphoid fractures. At our institution, we have consequently reduced the radiation dose to 10%.
Limitations
(I) This retrospective study design was used to evaluate a hypothesis of ULD simulations with preliminary results, which is why there is a need for a prospective study to confirm the results above using CT actually acquired in ULD technique and adjustment for radiation sensitive groups like pediatric patients is needed. (II) Selection bias is possible since data is from a single center. (III) Low-dose simulations are achieved by adding noise to the raw data sets, thereby the effect of adjusted tube current, low tube voltage settings or tin filtration cannot be simulated adequately. (IV) Patients with implants at the wrist and thereby metallic artefacts were not assessed so low-dose CT protocols cannot be concluded to such patients. (V) We have not separately assessed the limitation in spatial resolution of ULD-CT on comminution and complex fractures; this could be the scope of future projects.
In conclusion, a significant radiation dose reduction in CT imaging of the scaphoid to 10% provides sufficient image quality to precisely detect and classify scaphoid fractures by radiologists with moderate experience. ULD-CT with approximately 1 µSv might compete with conventional radiography as primary imaging modality clarifying suspected scaphoid fractures.
Acknowledgments
The results of this study were presented at the annual DGMSR (Deutsche Gesellschaft für muskuloskelettale Radiologie) congress 2022 and an abstract of this study was published in the journal Skeletal Radiology.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-21-1196/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-21-1196/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the institutional review board of the Eberhard Karls University of Tuebingen (No. 644/2020BO) and the need for written informed consent was waived for this retrospective analysis of clinically acquired CT data. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tada K, Ikeda K, Okamoto S, Hachinota A, Yamamoto D, Tsuchiya H. Scaphoid Fracture--Overview and Conservative Treatment. Hand Surg 2015;20:204-9. [Crossref] [PubMed]
- Cooney WP 3rd, Dobyns JH, Linscheid RL. Nonunion of the scaphoid: analysis of the results from bone grafting. J Hand Surg Am 1980;5:343-54. [Crossref] [PubMed]
- Asmus A, Lautenbach M, Schacher B, Kim S, Eisenschenk A. Scaphoid pseudarthrosis: Indications for avascular iliac crest or radius bone grafts. Orthopade 2016;45:951-65. [Crossref] [PubMed]
- Bervian MR, Ribak S, Livani B. Scaphoid fracture nonunion: correlation of radiographic imaging, proximal fragment histologic viability evaluation, and estimation of viability at surgery: diagnosis of scaphoid pseudarthrosis. Int Orthop 2015;39:67-72. [Crossref] [PubMed]
- Koivisto J, van Eijnatten M, Kiljunen T, Shi XQ, Wolff J. Effective Radiation Dose in the Wrist Resulting from a Radiographic Device, Two CBCT Devices and One MSCT Device: A Comparative Study. Radiat Prot Dosimetry 2018;179:58-68. [Crossref] [PubMed]
- Clementson M, Björkman A, Thomsen NOB. Acute scaphoid fractures: guidelines for diagnosis and treatment. EFORT Open Rev 2020;5:96-103. [Crossref] [PubMed]
- Carpenter CR, Pines JM, Schuur JD, Muir M, Calfee RP, Raja AS. Adult scaphoid fracture. Acad Emerg Med 2014;21:101-21. [Crossref] [PubMed]
- Arsalan-Werner A, Sauerbier M, Mehling IM. Current concepts for the treatment of acute scaphoid fractures. Eur J Trauma Emerg Surg 2016;42:3-10. [Crossref] [PubMed]
- Cole RJ, Bindra RR, Evanoff BA, Gilula LA, Yamaguchi K, Gelberman RH. Radiographic evaluation of osseous displacement following intra-articular fractures of the distal radius: reliability of plain radiography versus computed tomography. J Hand Surg Am 1997;22:792-800. [Crossref] [PubMed]
- Ahlawat S, Corl FM, Fishman EK, Fayad LM. MDCT of the hand and wrist: beyond trauma. Emerg Radiol 2015;22:307-14. [Crossref] [PubMed]
- Welling RD, Jacobson JA, Jamadar DA, Chong S, Caoili EM, Jebson PJ. MDCT and radiography of wrist fractures: radiographic sensitivity and fracture patterns. AJR Am J Roentgenol 2008;190:10-6. [Crossref] [PubMed]
- Kalra MK, Maher MM, Toth TL, Hamberg LM, Blake MA, Shepard JA, Saini S. Strategies for CT radiation dose optimization. Radiology 2004;230:619-28. [Crossref] [PubMed]
- Mettler FA Jr, Wiest PW, Locken JA, Kelsey CA. CT scanning: patterns of use and dose. J Radiol Prot 2000;20:353-9. [Crossref] [PubMed]
- Keller G, Afat S, Ahrend MD, Springer F. Diagnostic accuracy of ultra-low-dose CT for torsion measurement of the lower limb. Eur Radiol 2021;31:3574-81. [Crossref] [PubMed]
- Keller G, Götz S, Kraus MS, Grünwald L, Springer F, Afat S. Radiation Dose Reduction in CT Torsion Measurement of the Lower Limb: Introduction of a New Ultra-Low Dose Protocol. Diagnostics (Basel) 2021;11:1209. [Crossref] [PubMed]
- Ellmann S, Kammerer F, Allmendinger T, Brand M, Janka R, Hammon M, Lell MM, Uder M, Kramer M. Dose reduction potential of iterative reconstruction algorithms in neck CTA-a simulation study. Dentomaxillofac Radiol 2016;45:20160228. [Crossref] [PubMed]
- Kramer M, Ellmann S, Allmendinger T, Eller A, Kammerer F, May MS, Baigger JF, Uder M, Lell MM. Computed Tomography Angiography of Carotid Arteries and Vertebrobasilar System: A Simulation Study for Radiation Dose Reduction. Medicine (Baltimore) 2015;94:e1058. [Crossref] [PubMed]
- DGU, DGOOC, DGH, DGPRÄC, DRG & DGMSR. S3-Leitlinie Skaphoidfraktur. AWMF-Leitlinien-Register. 2015(012-016). Available online: https://docplayer.org/32828865-Leitlinie-skaphoidfraktur.html
- Boone JM, Strauss KJ, Cody DD, et al. Size-Specific Dose Estimates (SSDE) in Pediatric and Adult Body CT Examinations - Report of AAPM Task Group 2042011. Accessed on April 20th, 2022. Available online: https://www.aapm.org/pubs/reports/rpt_204.pdf
- Saltybaeva N, Jafari ME, Hupfer M, Kalender WA. Estimates of effective dose for CT scans of the lower extremities. Radiology 2014;273:153-9. [Crossref] [PubMed]
- Ten Berg PW, Drijkoningen T, Strackee SD, Buijze GA. Classifications of Acute Scaphoid Fractures: A Systematic Literature Review. J Wrist Surg 2016;5:152-9. [Crossref] [PubMed]
- Krimmer H, Schmitt R, Herbert T. Scaphoid fractures--diagnosis, classification and therapy. Unfallchirurg 2000;103:812-9. [Crossref] [PubMed]
- Kundel HL, Polansky M. Measurement of observer agreement. Radiology 2003;228:303-8. [Crossref] [PubMed]
- Konda SR, Goch AM, Leucht P, Christiano A, Gyftopoulos S, Yoeli G, Egol KA. The use of ultra-low-dose CT scans for the evaluation of limb fractures: is the reduced effective dose using ct in orthopaedic injury (REDUCTION) protocol effective? Bone Joint J 2016;98-B:1668-73. [Crossref] [PubMed]
- Mansfield C, Ali S, Komperda K, Zhao H, Rehman S. Optimizing Radiation Dose in Computed Tomography of Articular Fractures. J Orthop Trauma 2017;31:401-6. [Crossref] [PubMed]
- Alagic Z, Bujila R, Enocson A, Srivastava S, Koskinen SK. Ultra-low-dose CT for extremities in an acute setting: initial experience with 203 subjects. Skeletal Radiol 2020;49:531-9. [Crossref] [PubMed]
- Yi JW, Park HJ, Lee SY, Rho MH, Hong HP, Choi YJ, Kim MS. Radiation dose reduction in multidetector CT in fracture evaluation. Br J Radiol 2017;90:20170240. [Crossref] [PubMed]
- Neubauer J, Neubauer C, Gerstmair A, Krauss T, Reising K, Zajonc H, Kotter E, Langer M, Fiebich M, Voigt J. Comparison of the Radiation Dose from Cone Beam Computed Tomography and Multidetector Computed Tomography in Examinations of the Hand. Rofo 2016;188:488-93. [Crossref] [PubMed]
- Farracho LC, Moutinot B, Neroladaki A, Hamard M, Gorican K, Poletti PA, Beaulieu JY, Bouvet C, Boudabbous S. Determining diagnosis of scaphoid healing: Comparison of cone beam CT and X-ray after six weeks of immobilization. Eur J Radiol Open 2020;7:100251. [Crossref] [PubMed]
- Stiller W. Basics of iterative reconstruction methods in computed tomography: A vendor-independent overview. Eur J Radiol 2018;109:147-54. [Crossref] [PubMed]
- Ellmann S, Kammerer F, Allmendinger T, Hammon M, Janka R, Lell M, Uder M, Kramer M. Advanced Modeled Iterative Reconstruction (ADMIRE) Facilitates Radiation Dose Reduction in Abdominal CT. Acad Radiol 2018;25:1277-84. [Crossref] [PubMed]
- Arapakis I, Efstathopoulos E, Tsitsia V, Kordolaimi S, Economopoulos N, Argentos S, Ploussi A, Alexopoulou E. Using "iDose4" iterative reconstruction algorithm in adults' chest-abdomen-pelvis CT examinations: effect on image quality in relation to patient radiation exposure. Br J Radiol 2014;87:20130613. [Crossref] [PubMed]
- Winkelmann MT, Walter SS, Stock E, Brendlin A, Kolb M, Othman AE, Afat S. Effects of radiation dose reduction on diagnostic performance of 3rd generation Dual Source CT pulmonary angiography. Eur J Radiol 2021;134:109426. [Crossref] [PubMed]
- O'Hora L, Foley SJ. Iterative reconstruction and automatic tube voltage selection reduce clinical CT radiation doses and image noise. Radiography (Lond) 2018;24:28-32. [Crossref] [PubMed]
- Frellesen C, Stock W, Kerl JM, Lehnert T, Wichmann JL, Nau C, Geiger E, Wutzler S, Beeres M, Schulz B, Bodelle B, Ackermann H, Vogl TJ, Bauer RW. Topogram-based automated selection of the tube potential and current in thoraco-abdominal trauma CT - a comparison to fixed kV with mAs modulation alone. Eur Radiol 2014;24:1725-34. [Crossref] [PubMed]
- Park EH, Yoo WH, Song YS, Byon JH, Pak J, Choi Y. Not All Green Is Tophi: The Importance of Optimizing Minimum Attenuation and Using a Tin Filter to Minimize Clumpy Artifacts on Foot and Ankle Dual-Energy CT. AJR Am J Roentgenol 2020;214:1335-42. [Crossref] [PubMed]
- Amirfeyz R, Bebbington A, Downing ND, Oni JA, Davis TR. Displaced scaphoid waist fractures: the use of a week 4 CT scan to predict the likelihood of union with nonoperative treatment. J Hand Surg Eur Vol 2011;36:498-502. [Crossref] [PubMed]
- Hovius SE, de Jong T. Bone Grafts for Scaphoid Nonunion: An Overview. Hand Surg 2015;20:222-7. [Crossref] [PubMed]
- Brenner DJ, Doll R, Goodhead DT, Hall EJ, Land CE, Little JB, Lubin JH, Preston DL, Preston RJ, Puskin JS, Ron E, Sachs RK, Samet JM, Setlow RB, Zaider M. Cancer risks attributable to low doses of ionizing radiation: assessing what we really know. Proc Natl Acad Sci U S A 2003;100:13761-6. [Crossref] [PubMed]
- Berrington de González A, Mahesh M, Kim KP, Bhargavan M, Lewis R, Mettler F, Land C. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med 2009;169:2071-7. [Crossref] [PubMed]
- Lin EC. Radiation risk from medical imaging. Mayo Clin Proc 2010;85:1142-6; quiz 1146. [Crossref] [PubMed]


